Key Points
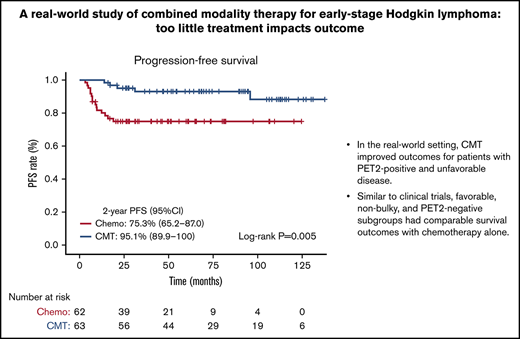
In the real-world setting, CMT led to improved outcomes for patients with PET2-positive and unfavorable disease.
Similar to clinical trials, favorable, non-bulky, and PET2-negative subgroups had comparable survival outcomes with chemotherapy-alone.
Abstract
Multiple clinical trials have assessed de-escalation strategies from combined modality therapy (CMT) to chemotherapy-alone for the treatment of early-stage classical Hodgkin lymphoma (cHL), confirming similar outcomes. The application of these data to the real-world is limited, however. We conducted a retrospective, multicenter cohort study comparing CMT vs chemotherapy-alone in patients with early-stage cHL (stage IA-IIB) treated between January 2010 and December 2020. Positron emission tomography (PET) scans after chemotherapy cycle 2 (PET2) were independently reviewed by a nuclear radiologist (Deauville score ≥4, positive; ≤3, negative). Patient outcomes were compared by using an intention-to-treat analysis. Among 125 patients (CMT, n = 63; chemotherapy-alone, n = 62) with a median follow-up of 59.8 months (95% CI, 48.6-71.0), no differences in overall survival were observed (5-year overall survival, CMT 98.0% vs chemotherapy-alone 95.1%; log-rank test, P = .38). However, there was reduced progression-free survival (PFS) with chemotherapy-alone among all patients (2-year PFS, CMT 95.1% vs chemotherapy-alone 75.3%; log-rank test, P = .005) and in those with bulky (n = 43; log-rank test, P < .001), unfavorable (n = 81; log-rank test, P = .002), or PET2-positive (n = 15; log-rank test, P = .02) disease. No significant differences in PFS were seen for patients with non-bulky (log-rank test, P = .35), favorable (log-rank test, P = .62), or PET2-negative (log-rank test, P = .19) disease. Based on our real-world experience, CMT seems beneficial for patients with early-stage cHL, especially those with PET2-positive and unfavorable disease. Chemotherapy-alone regimens can lead to comparable outcomes for patients with favorable, non-bulky, or PET2-negative disease. We conclude that although results seen in clinical trials are replicated in certain patient subgroups, other subgroups not fitting trial criteria do poorly when radiotherapy is excluded.
Introduction
Classical Hodgkin lymphoma (cHL) is a B-cell lymphoid malignancy associated with a high degree of cure, especially among patients with early-stage (I-II) disease.1,2 Traditionally, treatment for patients with early-stage cHL has been combined modality therapy (CMT), with both chemotherapy and radiotherapy (RT). Multiple studies have shown that CMT leads to improved disease control; however, there are notable long-term toxicities related to RT, including cardiac toxicities and secondary malignancies.3-5 Conversely, while the exclusion of RT may lead to a reduction in these long-term toxicities, if greater rates of relapse occur with chemotherapy-alone, patients face increased toxicity with salvage treatment and transplant down the road.6,7
Given this controversial risk–benefit scenario, many major clinical trials have focused on investigating treatment de-escalation strategies with the exclusion of RT. The positron emission tomography (PET) scan after the second cycle of chemotherapy (PET2) is an important prognostic factor that recent clinical trials have used to assess response-adapted strategies.8,9 Multiple trials have failed to show noninferiority of chemotherapy-alone compared with CMT; however, patients with interim PET-negative disease have displayed a very good prognosis with RT omitted.8-10 In patients with early-stage bulky disease, treatment is additionally controversial as the definition for bulky disease has been inconsistent, and many international trials have either excluded or grouped these patients into advanced disease cohorts.11,12
Translating clinical trial data to clinical practice is difficult, as the strict inclusion and exclusion criteria often used in trials are not representative of the heterogeneous patient populations seen in practice.13 Currently, there is limited real-world, institution-based data assessing the implications of these recent clinical trials on optimal disease management in early-stage disease. The primary objective of the current study was to assess institution-based patient outcomes of treatment with CMT compared with chemotherapy-alone in patients with early-stage cHL. Our secondary objectives were to assess treatment-related toxicities as well as differences in patient outcomes based on the number of chemotherapy cycles, PET2-response, bulky or non-bulky disease, and favorable or unfavorable disease prognosis.
Methods
Study design and participants
This retrospective, multicenter, single-institution–based cohort study was conducted in 125 consecutive adult patients (aged ≥18 years) with previously untreated early-stage cHL (stage IA-IIB) who received treatment at the Mayo Clinic (Rochester, Arizona, and Florida) between January 2010 and December 2020. Our real-world analysis stems from our institutional experience observing all patients treated in the 10-year period to provide a representation of the general patient population seen in clinical practice. We included patients regardless of clinical trial participation and excluded patients who did not have a pretreatment PET scan or who had missing treatment-related data (supplemental Figure 1). Patient demographic and clinical characteristics, along with detailed treatment-related data and outcomes, were abstracted through an electronic chart review. The study was determined to be exempt by the Mayo Clinic Institutional Review Board (IRB-21-008273).
Covariates, treatment regimens, and complications
Patient baseline characteristics, including age, sex, presence of B symptoms (fevers, drenching night sweats, or unexplained weight loss >10% of body weight), pretreatment erythrocyte sedimentation rate, and oncologic history, were abstracted. All cases had a biopsy-confirmed pathologic diagnosis of cHL. The disease subtype (nodular sclerosis, mixed cellularity, lymphocyte depleted, and lymphocyte-rich) was captured from the final pathology report. Disease stage, as well as favorable or unfavorable disease prognosis, was abstracted from chart review through pretreatment clinical notes. The standard at our institution is to use the Ann Arbor staging system and German Hodgkin Study Group (GHSG) criteria.14,15 Treatment intention, including the chemotherapy regimen, number of cycles, and the inclusion or exclusion of RT, was collected through abstraction of pretreatment clinical notes. At our institution, a plan for a treatment strategy with chemotherapy-alone or CMT is made before therapy initiation and can be adjusted based on interim PET scan. Treatment groups in the current study were stratified into a novel therapy group, ≤4 cycles of chemotherapy, and 6 cycles of chemotherapy based on treatment intention. The novel therapy group included all patients with treatment intention for novel agents in the frontline setting. The chemotherapy groups included only those planned to receive traditional chemotherapy regimens. For survival analysis based on treatment groups, 2 patients who received novel agents and further RT were assessed as part of the CMT group.
All treatments received by patients were captured, including the chemotherapy regimen, number of cycles, changes in therapy, RT inclusion, radiation field, radiation dose, number of fractions, and salvage therapy regimens for relapsed/refractory disease cases. Treatment complications, including bleomycin pulmonary toxicity (BPT), dose delays, omission of chemotherapeutic agents, hospitalizations, and any acute treatment-related toxicities, were recorded. BPT was defined as hospitalization or omission of bleomycin from the treatment regimen due to respiratory symptoms or new pulmonary changes seen on imaging. Secondary malignancies, cardiac events, and any other long-term toxicities were recorded when available.
PET scan data and treatment response
Pretreatment staging PET, PET2, end-of-treatment (EOT) scan (PET or computed tomography imaging), and relapsed/refractory scans were reviewed. PET2 and EOT scans were independently assessed by a nuclear radiologist blinded to patient treatment modality and outcomes. Due to studies using both Deauville score (DS) ≥3 and DS ≥4 as positive criteria, separate analysis was conducted by using both these cutoffs.16 Bulky disease was characterized as ≥7.0 cm (maximal diameter), per previous reports highlighting the prognostic benefit of this cutoff.12,17
Statistical analysis
Analysis was conducted by using intention-to-treat principles based on CMT or chemotherapy-alone pretreatment intention. Continuous and categorical variables were described by using median (range), frequencies (n), and percentages where applicable. Subgroup analyses were conducted to assess patients based on the presence of disease bulk, favorable or unfavorable disease, and PET2 response. The differences between CMT and chemotherapy-alone groups were evaluated by using descriptive statistics, independent-sample tests, and Pearson’s χ2 tests where applicable. Univariate and multivariate Cox proportional hazards models were used to determine hazard ratios (HRs). HRs were not calculated for subgroups given the low number of events. Kaplan-Meier analyses compared by using log-rank testing were used to determine overall survival (OS) and progression-free survival (PFS) differences between treatment groups. Events within the PFS analysis included both progression events and death from all causes. Patients who were alive or had not progressed at the time of last follow-up were censored from OS analysis and PFS analysis, respectively. Time to event was calculated by comparing date of event from date of pathologic diagnosis. For PET-based survival analysis, PFS and OS were determined from the date of PET scan to the date of event. The 2-year PFS and 5-year OS were determined by using Kaplan-Meier estimates, with a corresponding 95% confidence interval (CI). A reverse Kaplan-Meier–based method was used to estimate median follow-up time. A P value <.05 was considered statistically significant for all analyses.
All statistics were performed with the support of a biostatistician and were conducted by using IBM SPSS version 27 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and BlueSky version 7.40 (BlueSky Statistics, Chicago, IL).
Results
Study population and outcomes
The study included a total of 125 consecutive patients with early-stage cHL treated between January 2010 and December 2020, with 73 (58%) male subjects and a median age of 34 years (range, 18-78 years). Thirteen (10%) patients participated in clinical trials during their treatment course, and the remaining did not participate in trials. Baseline clinical characteristics and treatment-related characteristics are included in Table 1.
Based on intention-to-treat, there were 63 (50%) patients in the CMT group and 62 (50%) in the chemotherapy-alone group. Significant treatment differences were associated with the stage of disease (P = .01). Patients with stage IIB disease were more likely to be intended to receive chemotherapy-alone (70% vs 40%, P = .002) compared with patients of other stages (IA-IIA). In terms of adherence to intention, 4 patients in the CMT group did not receive RT: 1 patient refused RT, 1 patient developed progressive disease, 1 patient became too unwell due to pulmonary toxicity and infection, and 1 patient became ineligible due to underlying cardiopulmonary disease. Two patients in the chemotherapy-alone group received RT due to a change in treatment plan based on interim PET scan results. All patients received chemotherapy, and 124 (99%) received doxorubicin-bleomycin-vinblastine-dacarbazine (ABVD) or doxorubicin-vinblastine-dacarbazine (AVD)-based regimens. Fourteen (11%) patients were intended to receive novel agents in the frontline setting. For those receiving standard chemotherapy, 59 (47%) patients were planned to receive ≤4 cycles of chemotherapy and 52 (42%) to receive 6 cycles. Among patients in the CMT group, a greater proportion of patients were intended to receive ≤4 cycles (79%) compared with 6 cycles (18%) of chemotherapy. All treatment regimens administered are listed in supplemental Table 1.
PET2 scanning was performed in 116 (93%) patients; an independent radiologic review was conducted in 110 patients. The remaining 6 scans were from outside centers and could not be accessed for review. There were no statistical differences between treatment intention and PET2 response using a cutoff of DS ≥3 as positive; however, with DS ≥4 as positive criteria, patients in the chemotherapy-alone group had a greater degree of PET2-positive disease (20% vs 7%; P = .04) compared with CMT.
The median radiation dose delivered was 30 Gy (range, 20-40 Gy), with a median of 15 fractions (range, 5-22). In those receiving bleomycin (n = 119), 25 (21%) patients had bleomycin discontinued due to good clinical response; 22 of these had bleomycin discontinued after cycle 2. During treatment, BPT was observed in 32 (27%) patients receiving bleomycin, with BPT-associated hospitalizations in 9 patients. There was no significant difference observed in the development of BPT in older (aged ≥60 years) patients (33.3% vs 26.0%; P = .55) compared with those who were younger (aged <60 years). In addition, there was no statistical difference in BPT between those who received ≤2 cycles of bleomycin (28.1% vs 25.8%; P = .78) compared with ≥3 cycles. BPT was not associated with OS (HR, 0.57; 95% CI, 0.10-3.43; P = .54), and those who received ≥3 cycles of bleomycin had no significant difference in OS (HR, 0.54; 95% CI, 0.09-3.27; P = .50) and PFS (HR, 1.26; 95% CI, 0.51-3.14; P = .62) compared with those who received ≤2 cycles. Dose delays occurred in 26 (21%) patients and hospital admissions in 27 (22%) patients. No confirmed cases of secondary malignancies were observed in the follow-up period; however, cases of melanoma (n = 1), hepatocellular carcinoma (n = 1), and low-grade B-cell lymphoma (n = 1) were observed posttreatment.
Favorable and unfavorable disease
Unfavorable disease was characterized in 81 (69%) patients, compared with 36 (31%) who had favorable disease. Due to incomplete notes, 8 patients did not have this prognostic factor available. Baseline characteristics and treatment-based outcomes for both unfavorable and favorable disease are presented in supplemental Table 2. There was no significant difference between plan for CMT in patients with favorable (20 patients [56%]) and unfavorable (39 patients [48%]) disease. At PET2, patients with unfavorable disease had a higher proportion of PET2-positive disease (26% vs 7%; P = .04) compared with those with favorable disease when using a more conservative cutoff of DS ≥3 as positive.
Disease bulk
Bulky disease was present in 43 (34%) patients. CMT was intended in approximately one-half of the patients with bulky (23 patients [54%]) and non-bulky (40 patients [49%]) disease. Baseline characteristics and outcomes for patients with both bulky and non-bulky disease are shown in supplemental Table 3. Almost all patients with bulky disease were classified as having unfavorable disease (98%), apart from 1 patient who had <10 cm disease and no other unfavorable characteristics. There were no significant differences between PET2 responses comparing bulky vs non-bulky disease. However, more PET2-negative disease was seen among patients with non-bulky disease intended for CMT compared with chemotherapy-alone using both DS 1-2 (P = .02) and DS 1-3 (P = .006) as cutoffs.
PET-based results
Of 110 patients with PET2 scans independently assessed, PET2-positive disease was seen in 24 (22%) patients using a positive cutoff of DS ≥3 and 15 (14%) patients using a cutoff of DS ≥4. Among PET2-positive (DS ≥3) patients, 9 (38%) patients received RT, 5 (21%) received additional cycles of chemotherapy compared with the original treatment plan, and 4 (17%) patients were determined to have refractory disease and proceeded to salvage therapy and autologous stem cell transplant. EOT PET scans were available in 94 patients; 14 (15%) of these patients had positive disease based on DS ≥3, and 10 (11%) patients had positive disease based on a DS ≥4 criteria, Differences between baseline characteristics and treatment outcomes comparing PET2-positive and negative patients are shown in supplemental Table 4 (DS 3 as positive) and supplemental Table 5 (DS 3 as negative). No significant differences were seen in age, sex, histology, and stage comparing patients who were PET2-positive vs PET2-negative with both DS cutoffs.
Survival and long-term outcomes
With a median follow-up time of 59.8 months (95% CI, 48.6-71.0), there were 5 (4%) deaths, 3 of which were HL related. Seventeen (14%) patients had relapsed/refractory disease; of these, 12 (10%) patients went on to receive an autologous stem cell transplant, and 1 patient received an allogeneic stem cell transplant. Univariate survival analysis for the whole cohort based on key covariates is included in Table 2. Notably, higher age conferred a greater risk of mortality (HR, 1.14; 95% CI, 1.05-1.24; P = .002), CMT resulted in reduced risk of progression (HR, 0.26; 95% CI, 0.09-0.71; P = .009), and PET2-positive disease was associated with a higher risk of progression using both DS ≥3 (HR, 5.12; 95% CI, 1.97-13.31; P < .001) and DS ≥4 (HR, 11.53; 95% CI, 4.40-30.21; P < .001) as positive cutoffs. Assessing CMT compared with chemotherapy-alone adjusted for PET2 status, CMT continued to result in a significantly decreased risk of progression using both DS ≥3 (HR, 0.18; 95% CI, 0.05-0.65; P = .009) and DS ≥4 (HR, 0.18; 95% CI, 0.05-0.67; P = .01) in the multivariable model. No univariate differences in PFS were seen with bulky (P = .54) or unfavorable (P = .20) disease. On PFS analysis, those with PET response of DS 1-2 (n = 86 [92%]) and DS 3 (n = 9 [100%]) had excellent 2-year PFS outcomes compared with those who were DS 4-5 (n = 15 [40.0%]) (supplemental Figure 2). Assessing EOT PET, those who were PET-positive (DS 4-5) were found to have a significantly increased risk for progression (HR, 12.54; 95% CI, 4.46-35.27; P < .001) compared with those who were EOT PET-negative (DS 1-3).
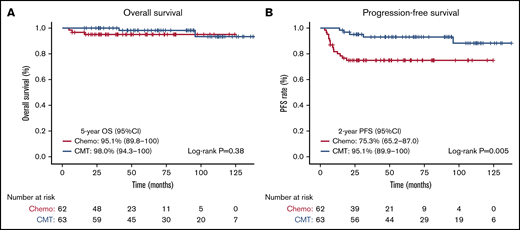
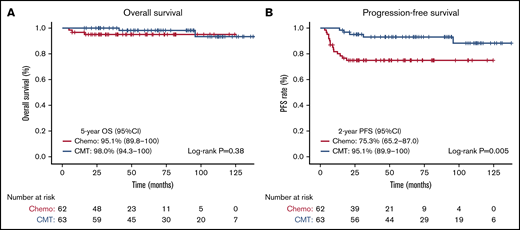
On Kaplan-Meier analysis, no OS differences were seen based on treatment intention, with the 5-year OS being 95.1% (95% CI, 89.8-100) for patients receiving chemotherapy-alone, compared with 98.0% (95% CI, 94.3-100) in patients receiving CMT (log-rank test, P = .38) (Figure 1A). PFS was substantially reduced for patients receiving chemotherapy-alone, with the 2-year PFS for chemotherapy-alone being 75.3% (95% CI, 65.2-87.0), compared with 95.1% (95% CI, 89.9-100) in patients receiving CMT (log-rank test, P = .005) (Figure 1B). Assessing CMT compared with chemotherapy-alone and novel therapies, significant differences were observed in OS (log-rank test, P = .005) (supplemental Figure 3A) and PFS (log-rank test, P = .009) (supplemental Figure 3B). The 2-year PFS was significantly lower and comparable in both the chemotherapy-alone groups (≤ 4 cycles, 67%; 6 cycles, 72%), and significantly higher in the novel therapy (92%) and CMT (95%) groups (log-rank test, P = .009).
OS and PFS based on treatment intention. Kaplan-Meier survival analysis assessed from date of diagnosis in all patients comparing chemotherapy-alone (chemo) vs CMT. (A) OS in all patients. (B) PFS in all patients.
OS and PFS based on treatment intention. Kaplan-Meier survival analysis assessed from date of diagnosis in all patients comparing chemotherapy-alone (chemo) vs CMT. (A) OS in all patients. (B) PFS in all patients.
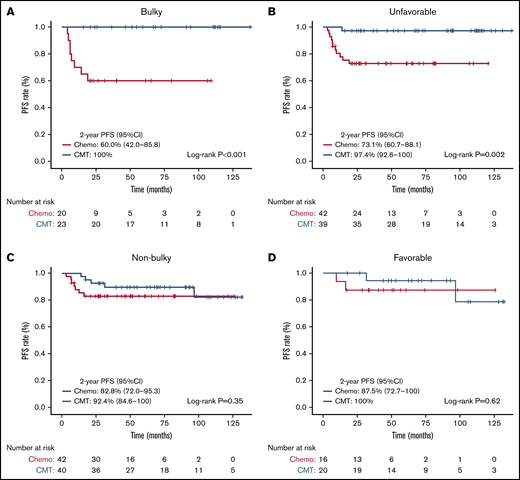
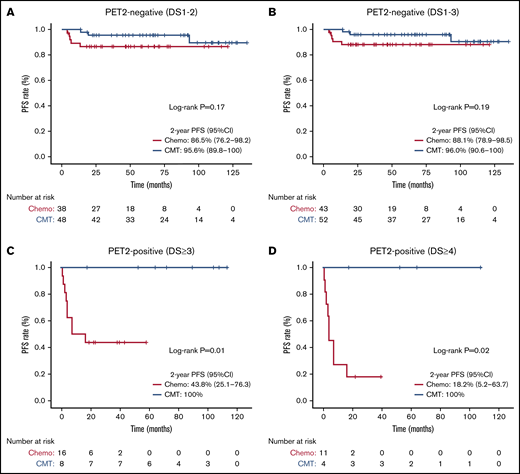
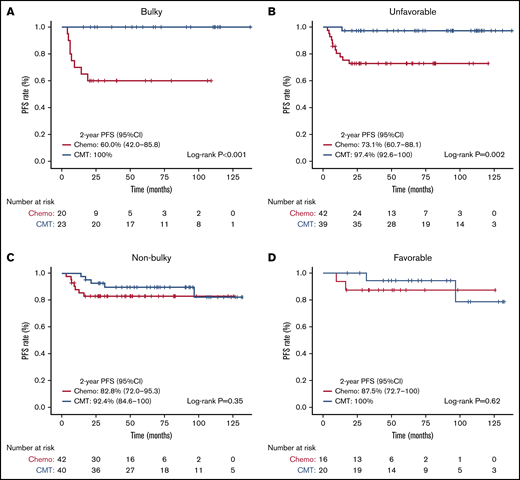
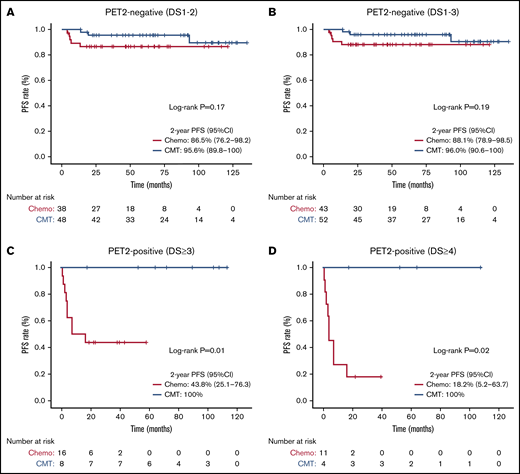
Subgroup PFS analyses with corresponding Kaplan-Meier curves are shown in Figures 2 and 3, respectively. The 2-year PFS was substantially reduced with chemotherapy-alone for patients with bulky (log-rank test, P < .001), unfavorable (log-rank test, P = .002), and PET2-positive (DS ≥3 log-rank test, P = .01; DS ≥4, P = .02) disease. Compared with CMT, similar PFS was seen with chemotherapy-alone for patients with non-bulky (log-rank test, P = .35), favorable (log-rank test, P = .62) or PET2-negative (DS 1-2, log-rank test, P = .17; DS 1-3, P = .19) disease. We next assessed patients with PET2-negative bulky disease (DS ≤3) and found that the 2-year PFS was inferior among patients who received chemotherapy-alone (80% [95% CI, 62.1-100] vs 100%; log-rank test, P = .049) compared with CMT. Kaplan-Meier curves assessing OS in subgroups are shown in supplemental Figures 4 and 5; no OS differences were seen based on treatment intention.
Subgroup PFS analysis based on treatment intention. Kaplan-Meier subgroup PFS analysis assessed from date of diagnosis comparing chemotherapy-alone (chemo) vs CMT. (A) Bulky disease. (B) Unfavorable disease. (C) Non-bulky disease. (D) Favorable disease.
Subgroup PFS analysis based on treatment intention. Kaplan-Meier subgroup PFS analysis assessed from date of diagnosis comparing chemotherapy-alone (chemo) vs CMT. (A) Bulky disease. (B) Unfavorable disease. (C) Non-bulky disease. (D) Favorable disease.
PET2-based PFS analysis comparing treatment intention. Kaplan-Meier PFS analysis assessed from date of PET2 scan comparing chemotherapy-alone (chemo) vs CMT based on PET2 response. (A) PET2-negative (DS 1-2). (B) PET2-negative (DS 1-3). (C) PET2-positive (DS ≥3). (D) PET2-positive (DS ≥4).
PET2-based PFS analysis comparing treatment intention. Kaplan-Meier PFS analysis assessed from date of PET2 scan comparing chemotherapy-alone (chemo) vs CMT based on PET2 response. (A) PET2-negative (DS 1-2). (B) PET2-negative (DS 1-3). (C) PET2-positive (DS ≥3). (D) PET2-positive (DS ≥4).
Discussion
Although multiple clinical trials have assessed treatment strategies in early-stage cHL, there is limited real-world data to evaluate the clinical implications of treatment with CMT compared with chemotherapy-alone. Based on our multicenter, single-institution, real-world study, we found that in patients with favorable, non-bulky, or PET2-negative disease, omission of RT did not lead to a significant reduction in survival. However, in patients with unfavorable, bulky, or PET2-positive disease, CMT resulted in improved outcomes.
The interim PET scan is a crucial prognostic marker within HL, and in our study, PET2-positive patients were at a significantly higher risk of progression.18 Assessing an interim PET DS 3 response, investigators from the CALGB (Cancer and Leukemia Group B) 50604 study found that the 3-year PFS of DS 3 (77%) approached that of DS 4-5 (67%)19 ; however, in our study, we observed excellent PFS outcomes in those with DS 3 response. These conflicting results could be partially explained by the small number of patients with DS 3 in our study but inform us that the patients with DS 4-5 were driving the poor PFS when using a positive cutoff of DS ≥3. Given the high risk associated with residual disease on the interim scan, the major PET-adapted treatment studies, including the UK NCRI RAPID (United Kingdom National Cancer Research Institute Randomised Phase III Trial to Determine the Role of FDG-PET Imaging in Clinical Stages IA/IIA Hodgkin’s Disease), EORTC (European Organization for Research and Treatment of Cancer)/LYSA (Lymphoma Study Association)/FIL (Fondazione Italiana Linfomi) H10, and GHSG H16 trials, assigned interim PET-positive patients to CMT-based regimens.8-10 Among the group of patients with PET2-positive disease in the current study, we observed that chemotherapy-alone regimens led to a significantly reduced PFS, especially when using DS ≥4 criteria for positive disease. However, among PET2-negative patients, chemotherapy-alone was comparable to CMT. In key clinical trials, such as the RAPID and H16 study, noninferiority of chemotherapy-alone was not observed in PET-negative patients; however, good outcomes were observed with RT exclusion in both studies.9,10 Within the RAPID trial, patients received 3 cycles of ABVD followed by PET scan evaluation; those who were PET-negative (DS 1-2) were randomly assigned to receive 30 Gy of involved-field RT or no further treatment. For patients who received chemotherapy-alone, the 3-year PFS of chemotherapy-alone (90.8%) compared with CMT (94.6%) revealed an absolute risk difference of −3.8% (95% CI, −8.8 to 1.3).10 Similarly, the phase 3 H16 trial assigned patients to standard CMT (2 × ABVD and 20-Gy involved-field RT) or PET-guided treatment, in which RT was excluded in those who were PET2-negative (DS <3). Among 628 patients with PET2-negative disease, a high 5-year PFS was seen with both CMT (93.4%; 95% CI, 90.4-96.5) and ABVD-alone (86.1%; 95% CI, 81.4-90.9).9 Overall, our real-world data yielded similar outcomes to those seen in both the UK RAPID and GHSG H16 clinic trials for PET2-negative patients. For PET2-positive patients, RT remains an integral component of treatment; however, for those with PET2-negative disease, chemotherapy-alone can lead to good outcomes.
Another important disease marker is the presence of favorable or unfavorable prognostic factors. In patients pursuing a strategy with chemotherapy-alone, the 2022 National Comprehensive Cancer Network guidelines recommend 6 cycles of chemotherapy for unfavorable disease with DS 1-3 on PET2, and between 3 and 6 cycles depending on PET2 response for favorable disease.20 From our work, although the sample size was small, we did not observe significantly improved outcomes with 6 cycles of chemotherapy-alone compared with ≤4 cycles. However, those who received novel agents had excellent PFS outcomes, which corresponds with the recent literature outlining the potential benefit of these therapies in the frontline setting.21-23 When further stratifying our cohort based on prognostic factors, we saw reduced PFS with chemotherapy-alone in patients with unfavorable disease but a lack of significant PFS differences for those with favorable disease. In the H10 trial, patients with unfavorable or favorable disease were separately randomized to upfront standard CMT or experimental PET response-adapted treatment.8 In the response-adapted treatment, patients with PET2-negative disease received 6 (unfavorable) or 4 (favorable) total cycles of ABVD, and PET2-positive patients received bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPesc) and involved-node RT. Among those who were PET2-negative, compared with AVBD + involved-node RT, chemotherapy-alone resulted in a lower 5-year PFS for both favorable and unfavorable disease, with an HR of 15.8 (95% CI, 3.8-66.1) and 1.45 (95% CI, 0.8-2.5), respectively. For unfavorable disease, we saw accordance of our results with this previously demonstrated inferiority of chemotherapy-alone. We observed that patients with unfavorable disease had a significantly higher degree of PET2-positive disease compared with those with favorable disease using DS ≥3 as a positive cutoff; this may explain the benefit they derived from further RT consolidation. However, for patients with favorable disease, we observed that most patients had a complete response at PET2 scan; thus, many patients were likely already cured and gained little benefit from additional RT.
Assessing disease bulk, about one-third of patients had bulky disease and a significantly improved prognosis with CMT compared with chemotherapy-alone. Comparing our results vs those of previous studies, a large retrospective analysis similarly found improved outcomes with CMT compared with chemotherapy-alone among patients with early-stage bulky disease.11 In 149 patients with stage IIB bulky disease, treatment with a radiation dose ≥30.1 Gy was associated with improved OS (HR, 0.25; 95% CI, 0.11-0.65).11 In another retrospective study assessing patients with early-stage bulky disease (>7 cm), treatment with chemotherapy-alone led to a worse prognosis, with a 4-year relapse-free survival of 55.2%.12 Compared with this study, our results revealed a comparable PFS (2-year PFS, 60%) with chemotherapy-alone. Recently, the CALGB 50801 trial assessed PET-adapted therapy for patients with early-stage bulky disease (>10 cm or >0.33 maximum intrathoracic diameter on chest radiograph).24 In this study, patients received 2 cycles of ABVD, those who were PET2-negative (DS 1-3) received 4 additional cycles of ABVD, and those with PET-2 positive disease received BEACOPPesc plus 30 Gy involved-site RT. The investigation revealed excellent 3-year PFS outcomes for those with PET2-negative disease (93.1%) receiving ABVD alone. Compared with the CALGB 50801 findings, we observed that chemotherapy-alone led to inferior outcomes for patients with bulky PET2-negative disease; however, the outcomes associated (2-year PFS, 80%) may be acceptable to patients and providers. It should be noted that this is a small subgroup of patients in the current study, and conclusions need to be drawn cautiously in the context of much larger clinical trials.
Assessing treatment-related complications, we observed a high degree of BPT and hospital admissions. The degree of BPT (26%) observed within our study was comparable to the previously reported rate of 37% in a retrospective study assessing 126 ABVD-treated patients with HL.25 No survival differences among those affected by BPT were seen in our study, but a previous retrospective study found that BPT was associated with a lower median 5-year OS compared with those unaffected.26 The RATHL (Response Adapted Therapy in Advanced Hodgkin Lymphoma) trial showed that for patients with PET2-negative disease, exclusion of bleomycin resulted in reduced pulmonary toxicity and good outcomes.27 Although we did not have the statistical power to compare the efficacy and toxicity associated with response-adapted bleomycin exclusion, this approach should be considered given the high prevalence of BPT and associated hospitalizations observed within our cohort. Regarding long-term toxicity, we observed no cases of secondary malignancies within our follow-up period. Previous reports of historically treated cohorts have identified the significant associations between RT and cardiac toxicity and secondary malignancies, especially breast and lung cancer.3-5 These toxicities certainly remain an important consideration during the selection of therapy; however, more recent data have suggested that modern RT techniques and targeting are associated with a lower burden of toxicity.28-30
There are several important limitations to highlight within our work. First, our study design was retrospective, and thus we are limited by the traditional biases associated with this investigation methodology. In addition, certain patients in our cohort were also followed up at outside centers, and we were unable to determine certain clinical characteristics and independently reassess imaging. Second, results from our study represent a single institution-based analysis; we therefore cannot determine the external validity of our study to other institutions. Third, we assessed a more recent cohort (2010-2020), which resulted in a shorter follow-up time available to evaluate long-term toxicities associated with treatment, which are generally reported several decades after therapy.4,5 Fourth, there was a higher number of stage IIB patients present in the chemotherapy-alone group, which may confound the proportion of PET2-positive patients included in the group. Furthermore, there was a higher proportion of stage IIB patients in the unfavorable group, and the selection of a chemotherapy-alone approach may have been due to many sites of disease, potentially confounding conclusions regarding RT. Last, compared with clinical trials that use strict treatment arms and therapies, there was significant heterogeneity in the chemotherapy regimens and RT techniques. Moreover, there were several patients who received nonstandard treatment approaches, which makes head-to-head comparisons difficult to make.
In summary, our real-world experience suggests that CMT significantly improves outcomes for patients with PET2-positive, bulky, and unfavorable disease. Chemotherapy-alone regimens can lead to comparable outcomes for patients with favorable, non-bulky, or PET2-negative disease. As clinical trial data are applied in clinical practice, careful patient selection is required when selecting a treatment strategy. Although the results seen in clinical trials are replicated in certain patient subgroups in this real-world experience, other subgroups not fitting trial criteria do less well with the exclusion of RT. Further real-world studies are certainly needed to assess the implications of recent clinical trials on the optimal treatment approach for early-stage cHL.
Acknowledgments
The authors thank Sheila A. Rushton and Matthew J. Maurer for their contributions to this study. This study was supported by the University of Iowa/Mayo Clinic Lymphoma SPORE CA97274-19.
Authorship
Contribution: K.L.C., J.R.Y., S.L., and S.M.A. designed this study, analyzed/interpreted the data, and wrote the manuscript; and M.A.M., A.R., H.W.T., B.S.H., P.B.J., I.N.M., and T.M.H. interpreted the data and assisted in writing the manuscript; and all authors provided final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosures: H.W.T. serves in a consulting/advisory role for Acrotech, Gossamer Bio, and ADC Therapeutics; and receives research funding from Acrotech. T.M.H. serves on the scientific advisory board for Eli Lilly & Co., MorphoSys, Incyte, BeiGene, and Loxo Oncology; received research funding from Genentech; and serves on the data monitoring committee for Seagen and Tess Therapeutics. S.M.A. receives research funding from Bristol Myers Squibb, Seattle Genetics, Affimed Therapeutics, Regeneron, Trillium Therapeutics, AI Therapeutics, and ADC Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology, Mayo Clinic, 200 1st St SW, Rochester, MN 55902; e-mail: ansell.stephen@mayo.edu.
References
Author notes
Requests for data sharing may be submitted to the corresponding author (e-mail: ansell.stephen@mayo.edu).
The full-text version of this article contains a data supplement.