Key Points
A hypofibrinolytic state with total PAI-1 elevation at the onset of CRS is the initial step of coagulopathy early after CAR-T infusion.
Suppressed fibrinolysis induces hypercoagulable status, which is gradually resolved after CRS remission without any organ damage in DLBCL.
Abstract
Anti-CD19 chimeric antigen receptor T (CAR-T) cell therapy has facilitated progress in treatment of refractory/relapsed diffuse large B-cell lymphoma (DLBCL). A well-known adverse event after CAR-T therapy is cytokine release syndrome(CRS). However, the etiology and pathophysiology of CRS-related coagulopathy remain unknown. Therefore, we conducted a prospective cohort study to comprehensively analyze coagulation/ fibrinolysis parameters present in peripheral blood of adult DLBCL patients treated with tisagenlecleucel in a single institution. Samples were collected from 25 patients at 3 time points: before lymphocyte-depletion chemotherapy and on days 3 and 13 after CAR-T infusion. After infusion, all patients except 1 experienced CRS, and 13 required the administration of tocilizumab. A significant elevation in the plasma level of total plasminogen activator inhibitor 1 (PAI-1), which promotes the initial step of coagulopathy (mean, 22.5 ng/mL before lymphocyte-depletion and 41.0 on day 3, P = .02), was observed at the onset of CRS. Moreover, this suppressed fibrinolysis-induced relatively hypercoagulable state was gradually resolved after CRS remission with normalization of total PAI-1 to preinfusion levels without any organ damage (mean values of soluble fibrin: 3.16 µg/mL at baseline, 8.04 on day 3, and 9.16 on day 13, P < .01; and mean PAI-1: 25.1 ng/mL on day 13). In conclusion, a hypofibrinolytic and relatively hypercoagulable state concomitant with significant total PAI-1 elevation was observed at the onset of CRS even in DLBCL patients with mild CRS. Our results will facilitate understanding of CRS-related coagulopathy, and they emphasize the importance of monitoring sequential coagulation/fibrinolysis parameters during CAR-T therapy.
Introduction
Chimeric antigen receptor T (CAR-T) cell therapy directed against CD19 has contributed to significant advancements in the treatment of refractory/relapsed (r/r) CD19+ B-cell malignances. Tisagenlecleucel (tisa-cel, Kymriah, CTL019) is the first CAR-T therapy approved in Japan, as well as in the United States and European countries, for r/r acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).1-3
In contrast to the otherwise unattainable antitumor effects to r/r CD19+ B-cell malignancies, some CAR-T–related adverse events including infusion reaction, cytopenia, hypogammaglobulinemia, and cytokine release syndrome (CRS) are observed quite frequently.4 Among them, CRS is a systemic inflammatory response due to activation of CAR-T cells and involves a massive release of multiple cytokines, such as interleukin-1 (IL-1), IL-6, IL-10, monocyte chemotactic protein-1, interferon-γ, and tumor necrosis factor-α.5-7
Clinical manifestations of CRS typically include high fever, hypotension, hypoxia, and capillary leak.2,8-10 Treatment algorithms, which include tocilizumab and corticosteroids, have been universally established according to the grade of CRS.11 However, CRS-related coagulopathy, which can be clinically apparent particularly in severe CRS cases,12 has not yet been studied widely. Changes in coagulation parameters reported after CAR-T infusion include prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen,13,14 fibrin/fibrinogen degradation products (FDP), or d-dimer.15 However, previous studies included only partial analyses of thrombotic and fibrinolytic parameters with clinical and subclinical manifestations and did not describe the entire range of post–CAR-T CRS-related coagulopathy.
Therefore, we performed a comprehensive analysis of coagulation and fibrinolytic parameters in patients with r/r DLBCL treated with CAR-T cells (tisa-cel) in a single institution. Our data illustrate time-dependent changes in these parameters associated with waxing and waning of CRS. This study further illuminates overall sequential coagulation/fibrinolysis dynamics related to CAR-T infusion and the complete landscape of CRS.
Patients and methods
Inclusion criteria
From 1 November 2019 to 30 March 2021, we consecutively enrolled adult patients (age ≥16 years) with r/r DLBCL who received CAR-T cell therapy using tisa-cel at Kyoto University Hospital, Kyoto, Japan. The study protocol complied with the Declaration of Helsinki and was approved by the institutional review board of Kyoto University Hospital. Written informed consent was obtained from all patients before the study.
Data collection and clinical courses
A database was established using data from clinical records of Kyoto University Hospital along with the information provided from Novartis regarding each lot of tisa-cel. Eligibility for tisa-cel therapy was in accordance with approval in Japan (ie, conventional DLBCL refractory to at least 2 regimens of chemotherapy) or to 1 regimen after autologous peripheral blood stem cell transplantation (PBSCT). Lymphocyte-depletion chemotherapy comprised fludarabine and cyclophosphamide, and tisa-cel was infused after 1 to 2 days from the end of chemotherapy. CRS was graded according to global criteria,11 and tocilizumab was administrated according to the guideline. Disease status was evaluated using fluoro-deoxyglucose positron emission tomography immediately before lymphocyte-depletion chemotherapy and at approximately day 28 after tisa-cel infusion.
Collection of clinical and laboratory data
Peripheral blood was sampled before lymphocyte-depletion chemotherapy and on days 3 and 13 after CAR-T infusion to analyze the following parameters: complete blood counts, PT, aPTT, fibrinogen, FDP, d-dimer, elastase-derived crosslinked fibrin degradation products (E-XDP), antithrombin (AT), thrombin-antithrombin complex (TAT), soluble fibrin (SF), α2-plasmin inhibitor (α2PI), total plasminogen activator inhibitor 1 (PAI-1), plasmin-alpha2-plasmin inhibitor-complex (PIC), thrombomodulin (TM), presepsin, soluble IL-2 receptor (sIL2R), and C-reactive protein (CRP). All samples were collected early in the morning.
Reagents are as follows: PT: Coagpia PT-Liquid (Sekisui Medical Co., Ltd., Tokyo); aPTT: Coagpia APTT-N (Sekisui Medical Co., Ltd.); fibrinogen: Thrombocheck Fib(L) (Sysmex Corporation, Kobe, Japan); FDP: LPIA FDP-P (LSI Medience Corporation, Tokyo); d-dimer: LPIA Genesis d-dimer (LSI Medience Corporation); E-XDP: E-XDP (LSI Medience Corporation); AT: TESTTEAM ATIII (Sekisui Medical Co., Ltd.); TAT: HISCL TAT (Sysmex Corporation); SF: IATRO SFII (LSI Medience Corporation); α2PI: CHROMORATE α2-PI(C) (LSI Medience Corporation); total PAI-1: LPIA tPAI Test (LSI Medience Corporation); PIC: HISCL PIC (Sysmex Corporation); TM: STACIA CLEIA TM (LSI Medience Corporation); presepsin: STACIA CLEIA Presepsin (LSI Medience Corporation); sIL2R: STACIA CLEIA IL-2R (LSI Medience Corporation); and CRP: N-Assay LA CRP-T Nittobo (Nittobo Medical Co. Ltd., Tokyo).
Statistical analyses
For comparisons of laboratory data in each patient, paired t tests were used. Two-sided P values <.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism (Version 9.2.0, GraphPad Software).
Results
Patient characteristics
Patient information is summarized in Table 1. A total of 25 r/r DLBCL patients who received tisa-cel infusion were enrolled. The median patient age at the time of infusion was 59 years (range, 20-69 years). Half of the patients (n = 14) were male, and performance status scores were 0 to 1 for all patients. Disease status at the initiation of lymphocyte-depletion chemotherapy was judged as partial remission in 4 patients, stable disease in 16 patients, and progressive disease in 5 patients. The median duration from the initial diagnosis of DLBCL and the tisa-cel infusion was 515 days (range, 199-3413). The median number of preceding chemotherapy regimens was 4 (range, 3-12), 36.0% of the patients (n = 9) underwent auto-PBSCT during the clinical courses before tisa-cel infusion, and 8.0% of the patients (n = 2) underwent bridging chemotherapy by rituximab, cyclophosphamide, cytosine arabinoside, etoposide, and dexamethasone a few weeks before lymphocyte-depletion. One patient received edoxaban, an anticoagulant, due to a history of internal jugular venous thrombosis.
Patient characteristics
| Variables . | Patients . | ||
|---|---|---|---|
| No. (n = 25) . | % . | ||
| Age at infusion | Median (range) | 59 (20–69) | |
| Sex | Male | 14 | 56.0 |
| Female | 11 | 44.0 | |
| Disease | DLBCL | 25 | 100 |
| Disease status at infusion | PR | 4 | 16.0 |
| SD | 16 | 64.0 | |
| PD | 5 | 20.0 | |
| Pre–CAR-T regimens, numbers | Median (range) | 4 (3-12) | |
| History of auto-PBSCT | Yes | 9 | 36.0 |
| No | 16 | 64.0 | |
| Bridging chemotherapy before CAR-T infusion | Yes | 2 (R-CHASE) | 8.0 |
| No | 23 | 92.0 | |
| Duration | |||
| From Dx to Aph, d | Median (range) | 447 (128-3331) | |
| From Aph to infusion, d | Median (range) | 64 (47-83) | |
| From Dx to infusion, d | Median (range) | 515 (199-3413) | |
| Variables . | Patients . | ||
|---|---|---|---|
| No. (n = 25) . | % . | ||
| Age at infusion | Median (range) | 59 (20–69) | |
| Sex | Male | 14 | 56.0 |
| Female | 11 | 44.0 | |
| Disease | DLBCL | 25 | 100 |
| Disease status at infusion | PR | 4 | 16.0 |
| SD | 16 | 64.0 | |
| PD | 5 | 20.0 | |
| Pre–CAR-T regimens, numbers | Median (range) | 4 (3-12) | |
| History of auto-PBSCT | Yes | 9 | 36.0 |
| No | 16 | 64.0 | |
| Bridging chemotherapy before CAR-T infusion | Yes | 2 (R-CHASE) | 8.0 |
| No | 23 | 92.0 | |
| Duration | |||
| From Dx to Aph, d | Median (range) | 447 (128-3331) | |
| From Aph to infusion, d | Median (range) | 64 (47-83) | |
| From Dx to infusion, d | Median (range) | 515 (199-3413) | |
Aph, apheresis; Dx, diagnosis; PD, progressive disease; PR, partial remission; R-CHASE, rituximab, cyclophosphamide, cytosine arabinoside, etoposide, and dexamethasone; SD, stable disease.
Changes in postinfusion lymphocyte counts and inflammatory markers
A summary of patients infused with tisa-cel is shown in Table 2. For all patients, data on characteristics of tisa-cel provided by the company were in accordance with approved criteria, and all cells were infused. Mild fevers were the only adverse events observed in the 1- to 6-hour period following cell infusion.
Characteristics of patients infused with tisa-cel and postinfusion CRS
| Variables . | . | No. (n = 25) . |
|---|---|---|
| Infused tisa-cel | ||
| Total viable cell number | Median (range), ×109 | 1.0 (0.2-2.3) |
| Total number of tisa-cel | Median (range), ×108 | 3.0 (0.8-4.5) |
| IFN-γ expression | Median (range), fg per transduced cell | 74 (37-346) |
| CRS | ||
| Grade | 0/1/2 | 1 (4%)/20 (80%)/4 (16%) |
| Duration, d | Median (range) | 8 (5-21) |
| Tocilizumab | ||
| Administration | Yes | 13 (52%) |
| Number of doses | 1/2/3/4 | 8 (62%)/3 (23%)/0 (0%)/2 (15%) |
| Variables . | . | No. (n = 25) . |
|---|---|---|
| Infused tisa-cel | ||
| Total viable cell number | Median (range), ×109 | 1.0 (0.2-2.3) |
| Total number of tisa-cel | Median (range), ×108 | 3.0 (0.8-4.5) |
| IFN-γ expression | Median (range), fg per transduced cell | 74 (37-346) |
| CRS | ||
| Grade | 0/1/2 | 1 (4%)/20 (80%)/4 (16%) |
| Duration, d | Median (range) | 8 (5-21) |
| Tocilizumab | ||
| Administration | Yes | 13 (52%) |
| Number of doses | 1/2/3/4 | 8 (62%)/3 (23%)/0 (0%)/2 (15%) |
IFN-γ, interferon-γ.
After infusion, peripheral blood lymphocytes (mainly composed of CD3+ T cells) decreased near day 3 (mean, 377/μL; range, 200-1280) due to lymphocyte-depletion chemotherapy immediately before tisa-cel infusion and recovered or rather increased after day 7 (mean, 821/μL; range, 200-2210) (Figure 1A). In approximately half of the patients (n = 13), lymphocyte counts were higher on day 28 than at baseline before lymphocyte-depletion chemotherapy, which suggests in vivo expansion of tisa-cel after infusion. Trends of other blood counts in the early phase after tisa-cel infusion are shown in supplemental Figure 1. Accordingly, CRS was observed in all patients except 1 (CRS grade 1, 20 patients; grade 2, 4 patients). Thirteen patients required the administration of tocilizumab (Table 2; supplemental Figure 2). Median duration of CRS was 8 days (range, 5-21) (supplemental Figure 2). Grade 3 immune effector cell–associated neurotoxicity syndrome occurred in 1 patient on day 5. Tocilizumab (anti–IL-6 antibody) was administered to 13 patients, and their median number of doses was 1 (range, 1-4). No apparent organ damage was observed. None of the patients experienced bleeding or infection, including sepsis and bacteremia, or required any transfusion of fresh frozen plasma, cryoprecipitate, or steroids during the course.
Changes in postinfusion lymphocyte counts and inflammatory markers. Peripheral blood lymphocyte (PB Lym) counts (A) and levels of presepsin (B), sIL2R (C), CRP (D), fibrinogen (E), and thrombomodulin (F) were measured periodically after tisa-cel infusion. Each open dot indicates an individual value, and horizontal black bars indicate mean values. *P < .05; **P < .01. ns, not significant.
Changes in postinfusion lymphocyte counts and inflammatory markers. Peripheral blood lymphocyte (PB Lym) counts (A) and levels of presepsin (B), sIL2R (C), CRP (D), fibrinogen (E), and thrombomodulin (F) were measured periodically after tisa-cel infusion. Each open dot indicates an individual value, and horizontal black bars indicate mean values. *P < .05; **P < .01. ns, not significant.
During these postinfusion clinical courses, levels of various biomarkers increased in a time-dependent manner. These included presepsin, which reflects activation of monocytes and macrophages16 (Figure 1B), sIL2R, which indicates T-cell activation (Figure 1C), and CRP, a general inflammation biomarker that reflects production of IL-2 and IL-617 (Figure 1D). Levels of these parameters were highest on day 3 and showed a tendency to return to baseline by day 13, consistent with the clinical phenotype of CRS.
These trends in inflammatory reactions were also analyzed for correlation with coagulation markers. Fibrinogen, which reflects the potential for coagulation as well as inflammation, followed the same pattern: elevated on day 3 and recovered or decreased on day 13 (mean values [mg/dL]: baseline= 400 vs day 3 = 457 [P = .03] vs day 13 = 246 [P < .01]; Figure 1E). On the other hand, thrombomodulin, which reflects vascular endothelial damages if elevated, showed no significant differences (mean values [U/mL]: baseline= 16.5 vs day 3 = 17.2 vs day 13 = 15.8; Figure 1F). These data suggest that postinfusion responses of tisa-cel may include fluctuation of coagulation, as well as inflammatory reactions, without endotheliopathy.
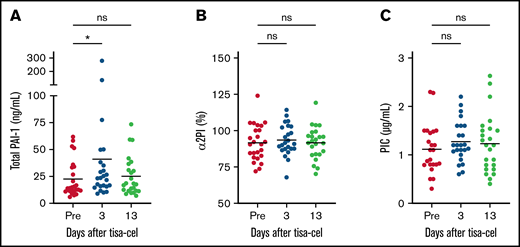
Suppressed fibrinolysis at the onset of CRS
Because fluctuation of coagulation was suspected after tisa-cel infusion, we performed comprehensive biomarker analyses for coagulopathy, with fibrinolysis biomarkers evaluated first. Significant elevation in levels of total PAI-1 was observed on day 3 (P = .02; mean, 41.0 ng/mL; range, 8.9-280.2) compared with those before lymphocyte-depletion chemotherapy (mean, 22.5; range, 5.8-61.8; Figure 2A). If assessed by the ratio on day 3 to pre–lymphocyte-depletion, this elevation was more clearly described (P = .01; mean, 2.1; range, 0.29-8.4). Elevation of PAI-1 caused impaired fibrinolysis on day 3, which was the onset of CRS in most cases. On day 13, at the end of CRS, the level of total PAI-1, which had increased on day 3, returned to preinfusion level.
Suppressed fibrinolysis at the onset of CRS. Fibrinolysis markers early after tisa-cel infusion were plotted, including total PAI-1 (A), α2PI (B), and PIC (C). *P < .05. ns, not significant.
Suppressed fibrinolysis at the onset of CRS. Fibrinolysis markers early after tisa-cel infusion were plotted, including total PAI-1 (A), α2PI (B), and PIC (C). *P < .05. ns, not significant.
In order to confirm suppressed fibrinolysis, biomarkers for enhanced fibrinolysis including α2PI and PIC were measured. No significant differences were noted among the 3 timepoints (Figure 2B-C). These data suggest that fibrinolysis was not enhanced after tisa-cel infusion and imply that fibrinolysis was suppressed at the onset of CRS.
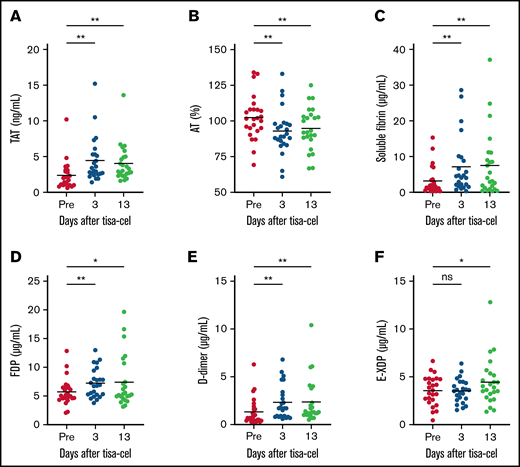
Mildly enhanced coagulation during CRS induced by suppressed fibrinolysis and corresponding enhanced fibrin degradation at the end of CRS
As fibrinolysis was moderately suppressed early after tisa-cel infusion, we hypothesized that coagulation was relatively enhanced in turn. Therefore, we attempted to characterize coagulation function at the same time points. TAT was significantly elevated on day 3 and day 13 compared with baseline (mean values [ng/mL]: baseline = 2.0 vs day 3 = 3.2 vs day 13 = 3.1 [P < .01]; Figure 3A), and AT was slightly decreased (mean values: baseline= 102.4% vs day 3 = 92.9% vs day 13 = 94.7% [P < .01]; Figure 3B), indicating enhanced coagulation after tisa-cel infusion. Increased levels of soluble fibrin can also provide direct evidence for enhanced coagulation (mean values [µg/mL]: baseline= 3.16 vs day 3 = 8.04 vs day 13 = 9.16 [P < .01]; Figure 3C). Of note, just as indicated in Figure 1F, lack of elevation in the level of thrombomodulin indicated that end-organ damage due to thrombosis was not evident in this situation, even though coagulation was systemically enhanced.
Mildly enhanced coagulation during CRS induced by suppressed fibrinolysis and corresponding enhanced fibrin degradation at the end of CRS. Fluctuation in coagulation markers is shown in TAT (A), AT (B), and soluble fibrin (C). Enhanced fibrin degradation is also indicated using FDP (D), d-dimer (E), and E-XDP (F). *P < .05; **P < .01. ns, not significant.
Mildly enhanced coagulation during CRS induced by suppressed fibrinolysis and corresponding enhanced fibrin degradation at the end of CRS. Fluctuation in coagulation markers is shown in TAT (A), AT (B), and soluble fibrin (C). Enhanced fibrin degradation is also indicated using FDP (D), d-dimer (E), and E-XDP (F). *P < .05; **P < .01. ns, not significant.
Relating to enhanced coagulation after tisa-cel infusion, fibrin degradation was also evaluated. As expected, elevations in the FDP and d-dimer levels were observed early after tisa-cel infusion (mean FDP values [μg/mL]: baseline= 5.7 vs day 3 = 7.2 [P < .01] vs day 13 = 7.4 [P = .03]; mean d-dimer values [μg/mL]: baseline = 1.3 vs day 3 = 2.3 [P < .01] vs day 13 = 2.3 [P < .01]; Figure 3D-E). In addition, E-XDP, which compensates for plasmin-induced fibrinolysis, was only elevated in the later phase of day 13 (mean values [μg/mL]: baseline= 3.5 vs day 3 = 3.5 [P = .88] vs day 13 = 4.4 [P = .01]; Figure 3F). Fluctuations of PT and aPTT were not observed during the clinical course after tisa-cel infusion in our cohort (data not shown). There were no differences in coagulation or fibrinolytic parameters in those who received tocilizumab compared with those who did not (data not shown).
Relative hypercoagulation induced by suppressed fibrinolysis after tisa-cel infusion
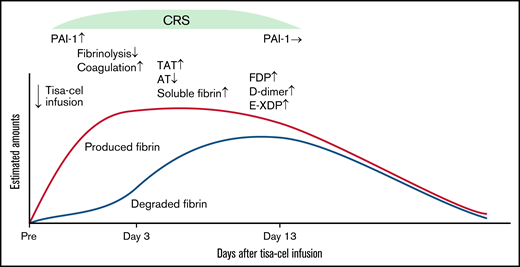
Considering that levels of coagulation-related biomarkers fluctuated, a schematic view of relative hypercoagulation by unbalanced coagulation and fibrinolysis after tisa-cel infusion is shown in Figure 4. As an initial step, inflammation and cytokines released early after tisa-cel infusion (CRS onset) can induce total PAI-1 elevation, which can suppress fibrinolysis. This instantly induces relative hypercoagulation, as suggested by the elevation of soluble fibrin and TAT and slight decrease in AT. Even under suppressed fibrinolysis, fibrin produced can slowly undergo degradation, which can be observed through the elevation of FDP and d-dimer, as well as the later increase in E-XDP. After remission of CRS, fibrinolysis is gradually restored, concomitant with total PAI-1 normalization and the later increase of E-XDP, and finally, the above-described relative hypercoagulation is presumably resolved (Figure 4).
Schematic view of the relatively hypercoagulable state after tisa-cel infusion. Trends in amounts of fibrin produced (red line) and degraded (blue line) are superimposed. In DLBCL patients with mild CRS, suppressed fibrinolysis and a relatively hypercoagulable state concomitant with significant elevation in total PAI-1 was observed at the onset of CRS. Subsequently, this status was recovered at the later stage of CRS, corresponding to normalization of total PAI-1 levels without any sequalae.
Schematic view of the relatively hypercoagulable state after tisa-cel infusion. Trends in amounts of fibrin produced (red line) and degraded (blue line) are superimposed. In DLBCL patients with mild CRS, suppressed fibrinolysis and a relatively hypercoagulable state concomitant with significant elevation in total PAI-1 was observed at the onset of CRS. Subsequently, this status was recovered at the later stage of CRS, corresponding to normalization of total PAI-1 levels without any sequalae.
Discussion
In the present study, analysis of the fluctuation in coagulation after CAR-T infusion in a time-dependent manner has revealed the following 2 major findings: (1) a significant elevation in total PAI-1 was observed at the onset of CRS, which is the initial step of coagulopathy (hypofibrinolytic state) soon after CAR-T infusion, and (2) suppressed fibrinolysis induces a relative hypercoagulable state, which is gradually resolved after remission of CRS, without any organ damage in tisa-cel therapy for r/r DLBCL.
After comprehensive analyses of coagulation and fibrinolysis, we observed a significant elevation of total PAI-1 in DLBCL patients at the onset of CAR-T–related CRS. This finding is in consistent with the other coagulopathies which are related to cytokine storms in various clinical settings. It has recently been reported that COVID-19–related cytokine storms, as well as bacterial infections, acute respiratory distress syndrome, and burns, induce PAI-1 release from vascular endothelial cells through enhanced IL-6 transsignaling. PAI-1 is positively correlated with serum IL-6,7 and particularly in sepsis patients, PAI-1 level can be the indicator associated with mortality or risk of later multiple organ failure.18
Likewise, we speculated that the trigger for PAI-1 elevation after tisa-cel infusion at the onset of CRS was also closely related to IL-6 transsignaling. In cytokine storms related to CAR-T (ie, CAR-T–induced CRS), IL-6 is one of the main determinants for the manifestations of CRS; thus, tocilizumab is the key drug used to manage CRS together with corticosteroids.11 Therefore, PAI-1 elevation soon after tisa-cel infusion at the onset of CRS may result from IL-6 production from CAR-T or activated macrophages. In our study, instead of monitoring IL-6 fluctuation, we alternatively monitored CRP, which has a well-documented association with IL-6.19 We found that PAI-1, CRP, and fibrinogen levels parallel each other during the clinical course after tisa-cel infusion.
Elevation of PAI-1 is well known to suppress fibrinolysis through inhibition of tissue-type and urokinase-type plasmin activators, which convert plasminogen to plasmin.20 Therefore, a hypofibrinolytic state occurs shortly after tisa-cel infusion at the onset of CRS. This pathophysiology is shared by other diseases, including septic disseminated intravascular coagulation21 and veno-occlusive disease, in which induced hypofibrinolysis can enhance coagulation and thrombus formation22 . Moreover, a hypofibrinolytic state by elevated PAI-1 levels is reportedly associated with disease severity in COVID-19 patients.23,24 Similar to what happens in these conditions, we revealed a hypofibrinolytic and relatively hypercoagulable state in the early phase of CRS by demonstrating a significant elevation of soluble fibrin and TAT corresponding to total PAI-1 elevation on day 3. Notably, thrombus formation was not actually confirmed in our cohort because the hypercoagulable state was within the mild and subclinical ranges. The correlation between the degree of PAI-1 elevation and CRS severity or the incidence of clinical thrombosis as well as the mechanistical analyses is subject of future studies.
This subclinical coagulopathy required no further treatment maneuvers in our cohort, which may explain why coagulopathy in tisa-cel treatment in mild CRS patients is sometimes overlooked.15 On the other hand, in tisa-cel treatment for B-ALL, in which CRS is generally more severe,13 coagulopathy induced by the same cascade (IL-6–PAI-1 axis) can sometimes be more apparent and clinically devastating because such hypercoagulable states can actually enhance micro- or macrothrombus formations, leading to end-organ damage.25 These states can be induced even in DLBCL patients with severe CRS after CAR-T treatment.26
Furthermore, inflammatory cytokines released during severe CRS in B-ALL patients cause endothelial damage and overexpression of tissue factors,12 which in turn activates the extrinsic coagulation pathway. This additional pathogenesis will further exacerbate the coagulopathy and end-organ damage.15 In contrast, in the present study, in which severe CRS patients were not observed, elevation of PAI-1 levels was relatively mild, and the following imbalance of coagulation and fibrinolysis (hypofibrinolysis/hypercoagulation) did not result in endotheliopathy, which can be explained by the lack of thrombomodulin elevation. However, in severe CRS, clinically significant coagulopathy, along with endotheliopathy followed by organ failure, can be induced even in DLBCL patients after CAR-T treatment.26
In conclusion, we found a hypofibrinolytic and relatively hypercoagulable state concomitant with significant elevation of total PAI-1 in DLBCL patients at the onset of mild CRS. Subsequent recovery in the later stage of CRS corresponded to normalization of the total PAI-1 level without any sequelae. In our cohort, coagulopathy was within subclinical levels and required no therapeutic interventions, which enabled unbiased observation of the unmanipulated clinical courses for waxing and waning of CRS. Similar observations cannot be made in the B-ALL cohort because of the complicated therapeutic interventions needed to manage the coagulopathy. Our results will facilitate understanding of coagulopathy in CRS of CAR-T–treated patients, both in DLBCL and B-ALL, and emphasize the importance of monitoring coagulation parameter during CAR-T therapy.
Acknowledgments
The authors would like to express their sincere gratitude to all the staff at Kyoto University Hospital engaged in this study. They also appreciate the feedback offered by Yutaka Nagahama (LSI Medience Co. Ltd.) on reagent characteristics.
This work was supported, in part, by research funding from the Foundation for Promotion of Cancer Research and the Japanese Society for Hematology, the Program for the Development of Next-Generation Leading Scientists with Global Insight (L-INSIGHT), and sponsored by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan to Y.A. Reagents for SF, total PAI-1, presepsin, α2PI, thrombomodulin, FDP, E-XDP, and sIL2R were provided from LSI Medience Co. Ltd.
Authorship
Contribution: M.Y.-M., Y.A., and S.A. designed the study, reviewed, and analyzed data; and T.I., T.O., H.S., K. Nakanishi, K. Nogami, Y.N., T.J., H.H., T.M., C.M., J.K., M.N., T.K., A.T.-K., and M.N. contributed to data collection and provided critiques on the manuscript.
Conflict-of-interest disclosure: A.T.-K. obtained honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Yasuyuki Arai, Department of Hematology and Oncology and Department of Clinical Laboratory Medicine, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: ysykrai@kuhp.kyoto-u.ac.jp.
References
Author notes
Contact the corresponding author for data sharing: ysykrai@kuhp.kyoto-u.ac.jp.
The full-text version of this article contains a data supplement.