Abstract
Hematologic malignancies are frequently diagnosed in dogs and result in a spectrum of clinical signs associated with specific disease types. The most frequently encountered hematologic tumors in dogs include lymphoma, lymphoid and myeloid leukemias, and mast cell, plasma cell, and histiocytic neoplasias. Coupled with the heterogeneous presentations of the different categories and subtypes of canine hematologic malignancies, outcomes for these tumors are also variable. Considering this, appropriate treatment options range from active surveillance to curative intent approaches harnessing surgical, chemotherapeutic, and radiation-based modalities. The underlying pathology of many of these diseases bears remarkable resemblance to that of the corresponding diagnosis made in human patients. We introduce some of the pathogenic drivers of canine hematologic cancers alongside their clinical presentations. An overview of standard-of-care therapies for each of these diseases is also provided. As comparative oncology gains recognition as a valuable setting in which to investigate the pathogenesis of neoplasia and provide powerful, clinically relevant, immunocompetent models for the evaluation of novel therapies, the number of clinicians and scientists participating in cancer research involving dogs is expected to increase. This review aims at providing an introductory overview of canine hematologic malignancies.
Introduction
Canine hematologic malignancies are frequent and pathogenically diverse. These tumors arise spontaneously in an immunocompetent setting and share genetic features and treatment modalities with the disease counterparts in humans. As such, dogs are compelling models for studying tumorigenesis and novel therapeutic approaches.1,2 This review is intended to introduce readers unfamiliar with veterinary oncology to the principal tenets of the pathology and clinical management of commonly encountered canine hematologic malignancies and the opportunities for comparative oncology to ultimately benefit patients with similar types of hematologic disorders.
Lymphoma
Pathology and presentation
Canine lymphomas encompass a large and heterogenous group of lymphoid malignancies affecting lymphocyte subtypes at different stages of differentiation and maturation. Lymphoma is the most common hematologic malignancy in dogs, with an estimated annual incidence of 25 per 100,000.3 As in humans, lymphoma is primarily diagnosed in older dogs but may occur at any age.4,5 Certain breeds have increased relative risk, including basset hounds, Bernese mountain dogs, Bouvier des Flandres, boxers, bulldogs, bullmastiffs, cocker spaniels, Doberman pinschers, German shepherds, golden retrievers, Irish wolfhounds, Labradors, rottweilers, Saint Bernards, and Scottish terriers.4,5 The relative inbreeding of dogs to generate distinct breeds has resulted in genetic predispositions to lymphoma that can be identified by genome-wide association studies (GWASs). Such GWASs performed in golden retrievers implicated candidate genes including MCC, MXD3, FGFR4, and TRPC6 as possibly involved in the development of lymphoma.6,7 These findings underscore the potential value that disease predisposition in given breeds may play in identifying lymphoma-related oncogenes, both germ line and somatic, in dogs and humans.
The most frequent presentation of lymphoma is generalized lymphadenopathy; however, involvement of virtually all types of tissue has been described.5 Diagnosis is primarily made through fine-needle aspiration of affected nodes and/or infiltrated tissues with cytologic evaluation, immunophenotyping, and/or polymerase chain reaction for T- or B-cell antigen receptor rearrangement testing. Although accurate classification of lymphomas requires assessment of tumor architecture, in practice lymph node biopsy is reserved for equivocal cases to establish a definitive diagnosis.8 Cytologically, medium to large lymphocytes often predominate, frequently displaying immunoblast-type prominent nucleoli and basophilic cytoplasm. As in humans, the mitotic rate can range from low (0-1 mitotic figures per 5 hpf) to high (>3 mitotic figures per 5 hpf), and lymphoma grade is based on cell morphology and mitotic index.9
Immunophenotyping of canine lymphomas is increasingly being performed because of its prognostic value.10,11 Antibodies directed against CD21, CD79a, CD19, and CD20 have been used to identify B-cell lymphomas and CD3, CD4, CD8, and CD45 to phenotype T-cell lymphomas.5,12-14 Similar to patients, dogs with T-cell lymphomas are generally at higher risk of early relapse (52 vs 160 days) and shorter survival (150 vs 330 days) compared with dogs with B-cell lymphomas.10 However, because prognostic differences occur within both B- and T-cell subtypes, morphologic and clinical characterization remain the gold standards for diagnostic purposes.15
Canine lymphomas have been classified according to the World Health Organization (WHO) system designed for humans.16 However, fewer subtypes have been identified using histology, morphology, and immunophenotyping in dogs. Diffuse large B-cell lymphoma (DLBCL), marginal zone lymphoma (MZL), peripheral T-cell lymphoma not otherwise specified (PTCL), T-zone lymphoma (TZL), T-cell lymphoblastic lymphoma (T-LBL), and follicular lymphoma (FL) are among the most commonly diagnosed subtypes.17 Our discussion will focus on these subsets (summarized in Table 1); further information regarding these and other subtypes can be found elsewhere.5,18
DLBCL is a high-grade lymphoma and the most common subtype, accounting for 40% of all canine lymphomas and ∼70% of canine B-cell lymphomas.5 Different molecular signatures involving distinct intracellular signaling pathways (eg, NF-κB, PI3K-AKT, and JAK-STAT) have been identified in human as well as canine DLBCL, and in some instances, these subtypes have prognostic value.19-22 Following human DLBCL classification, canine DLBCL has been subcategorized as activated B cell–like DLBCL or germinal center B-cell DLBCL, each with statistically different survival times, with the latter displaying ongoing hypermutations of the IGH gene.22 Canine and human lymphomas also share cell-signaling pathways such as NF-κB that distinguish activated B cell–like from germinal center B-cell lymphoma.22
MZLs have been classified as indolent B-cell lymphomas affecting the spleen and/or nodes.17 When MZL involves the spleen alone, it tends to follow an indolent course. In contrast, nodal involvement has been associated with clinically aggressive disease and shorter survival.23,24 Unlike in human MZL, expression of CD5 is frequently observed in canine splenic MZL.25 Transcriptomic profiling of canine nodal MZL revealed an MYC-driven activation signature associated with multiple recurrent gene copy gains in the region of chromosome 13, where MYC is encoded.26 Because genetic alterations of MYC are usually associated with aggressive B-cell neoplasia in humans, its involvement suggests that nodal canine MZL may be a distinct and more aggressive variant compared with splenic MZL; however, larger molecular studies comparing nodal and splenic MZLs in dogs are required to confirm this.27
FL is an indolent form of canine B-cell lymphoma and accounts for 1% of canine lymphomas.17 Variable expression of BCL2 is reported; however, it has not been shown to be associated with the hallmark BCL2-IGH gene translocation (t[14;18] [q32;q21]) typically seen in human FL.28 As such, alternative molecular mechanisms may contribute to the pathogenesis of FL in dogs,28 perhaps akin to human pediatric or cutaneous FL, which also does not carry BCL2-IGH translocation.
Canine PTCL is typically an aggressive disease and the most common T-cell lymphoma in dogs, with boxers and golden retrievers being the most frequently affected.17,18,29 Immunophenotyping has revealed that 82% of PTCLs are derived from CD4+ T cells, and genetically, the loss of PTEN alongside activation of the MTOR-PI3K-AKT axis is noted.29 The genetic factors of breed predisposition are unknown but may shed light on pathogenic mechanisms responsible for the canine disease, which in turn might be informative regarding human PTCL. Hypercalcemia and/or mediastinal involvement was identified frequently at presentation in histologically high-grade CD4+ CD45+ disease, which was classified as either PTCL or T-LBL.30
T-LBL, an another aggressive T-cell lymphoma,18 is less frequent than PTCL, and there is limited information regarding its genetic makeup.17,18 As in humans, phenotypically most cases are either CD4+ CD8+ or CD4− CD8−,31 reflecting their immature T-cell origin. Clinically, dogs with T-LBL often have mediastinal involvement and/or hypercalcemia.31
Although without a clear human counterpart, TZL is the most common indolent lymphoma in dogs.17,18 Dogs with TZL are usually older and often present with lymphadenopathy and lymphocytosis.14 Morphologically, these cells have a small nucleus and clear cytoplasm. They lack CD45 expression but express CD5, major histocompatibility complex II, and CD25 and also frequently display aberrant CD21 expression.14 Golden retrievers seem to be at increased risk for TZL, and recent evidence shows that older retrievers can have circulating CD45− TZ cells in the absence of lymphoma, suggesting the presence of a relatively common premalignant stage of this disorder.14,32 A recent GWAS found associations of TZL with single-nucleotide polymorphisms near hyaluronidase genes (SPAM1, HYAL4, and HYALP1) as well as nonsynonymous mutations in these genes, suggesting that hyaluronan breakdown might play a role in TZL pathogenesis.33 Associated single-nucleotide polymorphisms were also found near genes involved in thyroid hormone regulation (DIO2 and TSHR), suggesting thyroid dysregulation may also play a role in TZL.33
Canine lymphomas are staged using the WHO scheme (Table 2).34 As in humans, staging can be valuable in prognostication, with stage V disease having shortened progression-free and overall survival.5,11,35 Notably, although a majority of dogs with TZL present with circulating lymphocytosis, these dogs exhibit prolonged survival irrespective of the designation of stage V disease; therefore, prognostication based on staging should be taken in the context of WHO disease classification.8,14 Substage is also prognostic, and dogs with substage A disease tend to have longer median survival times compared with those with substage B.5,35 As in humans, decreased major histocompatibility complex II expression predicts a poor outcome in canine B-cell lymphoma.36
Treatment and outcomes
Numerous therapeutic approaches, including chemotherapy, radiotherapy, surgery, and immunotherapy, are described for type/stage-specific canine lymphomas.5 Standard-of-care treatment for high-stage (multicentric) high-grade lymphomas consists of multiagent chemotherapy containing CHOP. CHOP-based protocols induce remission in up to 94% of dogs.5,37 However, a majority of dogs relapse with resistant lymphoma, and median survival of 10 to 13 months has been reported.5,37 Although CHOP-based protocols are routinely used for B- and T-cell disease, there is some evidence that more alkylator-heavy protocols are more efficacious against T-cell lymphoma; however, appropriate randomized trials to test this have yet to be performed.38-40 Because responses to chemotherapy for high-grade T-cell lymphoma are generally worse than those for high-grade B-cell lymphoma, protocol optimization for T-cell disease is ongoing.5 Currently, there is no readily available clinically effective canine anti-CD20 monoclonal antibody for combination with CHOP chemotherapy; however, early preclinical data revealing B-cell depletion in healthy beagles treated with a canine anti-CD20 antibody seem promising.41 Multiple second-line rescue protocols are described for relapsed disease, but responses tend to be less durable than those to first-line treatments.5 Rabacfosadine, a nucleotide analog with described activity as both a first- and second-line agent and particular activity against B-cell disease, is notable as the first fully US Food and Drug Administration–approved lymphoma treatment for dogs.42-44 Subsequently, in 2021, the US Food and Drug Administration conditionally approved verdinexor, an oral selective inhibitor of nuclear export, after demonstration of safety and efficacy in canine lymphoma.45 With regard to indolent lymphomas, active surveillance is often used initially, with chlorambucil and prednisone usually reserved for progressive or advanced disease.5
Lymphoid and myeloid leukemias
Pathology and presentation
The incidence of acute leukemia in dogs is unknown. Dogs diagnosed with acute leukemia have a median age of 7 years and may present with multilineage cytopenias.46,47 The clinical course is usually rapidly progressive. Standard immunophenotypic analysis usually includes CD34, which is expressed by some but not all acute leukemias.48 Acute leukemias may be of lymphoid (ALL) or myeloid (AML) lineage, and whereas T-ALL is relatively well characterized, B-ALL is poorly defined, with the current belief that true B-ALL is rarely, if ever, encountered in dogs.46,47,49 The genetics of acute canine leukemia have not been extensively investigated; however, a single study found mutations in N/K-RAS, FLT-3, or C-KIT in 61% of dogs.50
Chronic lymphocytic leukemia (CLL) is typically an indolent disease that tends to affect elderly dogs.51,52 In contrast to humans, T-CLL is most frequently diagnosed followed by B-CLL and atypical variants.51,53 In canine B-CLL, IGHV genes are frequently mutated, with the exception of boxers that carry unmutated variants, which have been associated with more aggressive disease in humans.54 In regard to myeloproliferative disorders, polycythemia vera is rare but has been described in dogs presenting with erythrocytosis.55 Although there are case reports of canine chronic myeloid leukemia, it is also rarely encountered.56,57
Treatment and outcomes
CHOP-based chemotherapy is usually recommended for acute leukemias, but the prognosis is poor, with a median survival time of only 55 days.47 T-CLL has been associated with favorable survival compared with B-CLL and atypical forms (median survival time of 930, 480, and 22 days, respectively).53 In the initial stages of CLL, active surveillance may be pursued in the absence of marked lymphocytosis, cytopenias, or clinical signs. Upon progression, oral chlorambucil-based regimens are administered.53
Mast cell neoplasia
Clinical presentation and pathologic findings
Similar to humans, canine mast cell (MC) disease can affect multiple organs as a systemic mastocytosis; however, the most common manifestation is cutaneous MC tumor (MCT).58,59 Although a majority of cutaneous MCTs are solitary, presentation of multiple cutaneous lesions was observed in 14% of 300 dogs diagnosed with MCTs.60 Diverse breeds, including boxers, English bulldogs, Boston terriers, Labrador retrievers, golden retrievers, cocker spaniels, schnauzers, and Chinese shar-pei, are predisposed to developing MCTs.61 A mean age of 8 to 9 years at diagnosis is reported, but younger animals can also develop MCTs.61 Dogs presenting with systemic or cutaneous MCTs can show signs of MC degranulation, manifesting either in the vicinity of the tumor, such as erythema, or systemically and most notably involving gastrointestinal (GI) ulceration, vomiting, and abdominal pain presumed secondary to the interaction of histamine released from MCTs on H2 receptors located in gastric parietal cells.61
Multiple pathologic grading schemes have been applied to cutaneous MCTs, because the clinical course and prognosis of canine MCTs are highly variable. Two of the more commonly used grading systems are a 3-tier scheme described by Patnaik et al62 and a 2-tier scheme by Kiupel et al,63 with both applying histologic criteria including mitotic index to assign grade. It is common practice for pathologists to provide grades using both systems, because consensus as to which classification scheme is clinically most useful has not been reached.
Staging of cutaneous MCTs based on the WHO system uses a combination of histologic assessment regarding degree of skin invasion as well as presence of metastases (Table 3).34 Although the presence of multiple dermal masses confers stage III disease, it should be noted that the prognosis for these dogs is not necessarily worse than that for those presenting with individual tumors.61 When feasible, aspiration of draining lymph nodes should be performed following diagnosis of cutaneous MCTs, because these tumors most frequently disseminate through the lymphatics.64
Beyond cutaneous canine MCTs, other presentations are noted. Subcutaneous MCTs are also encountered and are reportedly indolent, with prolonged survival times; however, anecdotal experience within canine oncology suggests the these tumors can exhibit more aggressive behaviors, so further study of the biology of subcutaneous MCTs is warranted.65 Mucocutaneous MCTs affecting the oral cavity are biologically aggressive.61 Systemic disease usually manifests with infiltration of viscera, including the spleen and liver, and is associated with a poor prognosis.61 This presentation usually represents metastatic spread from a primary site but occasionally is considered to be a primary disease. MC leukemia is rarely reported in dogs.61
As in humans, KIT mutations are a hallmark of canine MCTs, with 8% to 29% of canine MCTs exhibiting mutations in exon 8, 9, or 11, which can result in unregulated downstream signal transduction.59 In 1 report, a predominance of cytoplasmic compared with membranous KIT staining was associated with poor clinical outcomes in cutaneous MCTs.66 Numerous other molecular and histologic prognostic factors have been described and are reviewed elsewhere.61,67
Treatment and outcomes
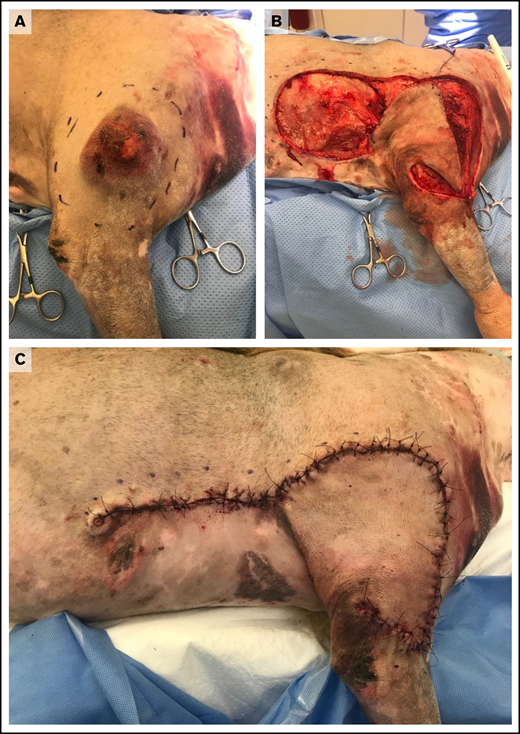
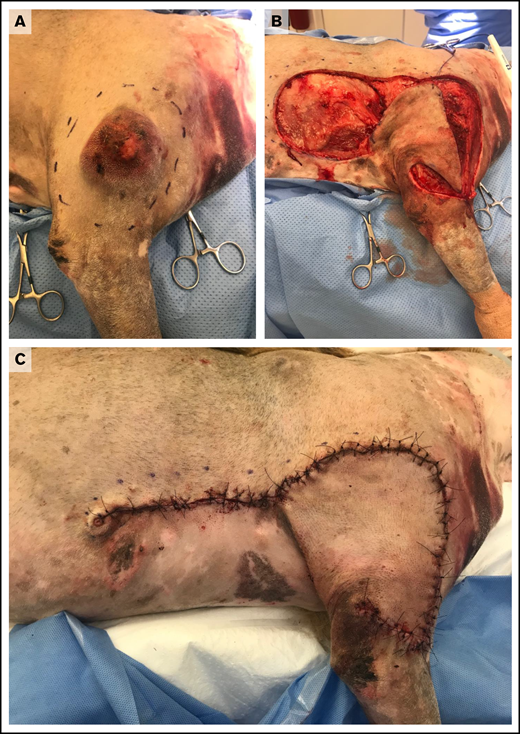
Treatment of MCTs is usually determined by the stage and predicted biologic behavior of the tumor. Surgical excision is recommended for cutaneous MCTs, and cure is expected following complete resection of low-grade tumors.67 Complete resection may be achieved using wide and aggressive margins for all tumors (Figure 1) or by tailoring surgical dose in proportion to the size of the mass.68 For incompletely resected tumors, various options, including repeat excision, adjuvant external-beam radiotherapy, adjuvant medical therapy, or active surveillance, may be recommended based upon defined prognostic indicators.61,67
Wide surgical excision of a degranulating canine cutaneous MCT. (A) Preoperative photograph after initial surgical preparation and planning for wide excision. (B) Intraoperative photograph after wide resection of the tumor and elevation of a subdermal plexus skin fold flap. (C) Postoperative photograph revealing skin closure using a subdermal plexus flap. Images provided by Maureen Griffin and Ingrid Balsa.
Wide surgical excision of a degranulating canine cutaneous MCT. (A) Preoperative photograph after initial surgical preparation and planning for wide excision. (B) Intraoperative photograph after wide resection of the tumor and elevation of a subdermal plexus skin fold flap. (C) Postoperative photograph revealing skin closure using a subdermal plexus flap. Images provided by Maureen Griffin and Ingrid Balsa.
In cases of MCTs that harbor more worrisome prognostic indicators, surgical excision to remove gross disease is still frequently performed; however, adjuvant therapy is also routinely recommended. Supportive medications in the form of antihistamines and gastroprotectants are often prescribed for these dogs.58 Resection of a primary tumor and infiltrated local lymph nodes is often performed in the absence of overt distant disease, and at our institution, adjuvant chemotherapy would be recommended for such cases. In 61 dogs with high risk for metastasis, treatment with adjuvant prednisone and vinblastine resulted in a median disease-free interval of 1305 days.69 Combining chloroethylcyclo-hexylnitrosourea (CCNU) with vinblastine in 37 dogs with macroscopic disease resulted in an overall response rate (ORR) of 57%, for a median overall survival time of 35 weeks.70 The same protocol resulted in a median overall survival of 48 weeks for 20 dogs with microscopic disease.
Because the tyrosine kinase receptor KIT is often mutated in canine MCTs, treating dogs with tyrosine kinase inhibitors (TKIs) is now common practice. Toceranib (Palladia) is the most frequently used TKI for canine MCTs. A multicenter trial using toceranib to treat recurrent canine MCTs in 145 dogs resulted in an ORR of 42.8% (including 21 complete responses) and a median time to progression of 18.1 weeks.71 Beneficial responses of some dogs treated with another semispecific KIT inhibitor, imatinib (Gleevec), have also be observed72 ; however, there are few data to support the use of this agent compared with toceranib. Comparatively, various TKIs have been appraised for human MC disease; however, it should be noted that the D816V KIT mutation that frequently occurs in human MC disease confers resistance to imatinib.73
Plasma cell neoplasia
Pathology and presentation
Canine plasma cell neoplasms have varied presentations, with plasma cell/multiple myeloma (MM) being the most notable disease clinically.74 Unlike in humans, cytogenetic abnormalities in canine plasma cell disease have not been well characterized. Waldenström’s macroglobulinemia, monoclonal gammopathy of unknown significance, solitary osseous plasmacytoma, extramedullary plasmacytoma (EMP), and plasma cell leukemia have all been described in dogs, but apart from MM and EMP, these diseases are rarely encountered.74-80 Older dogs age 8 to 10 years are typically affected by MM and EMP, with no clear breed predispositions for MM; however, cocker spaniels, West Highland white terriers, Yorkshire terriers, Airedale terriers, boxers, and golden retrievers are overrepresented when EMP is diagnosed.79
Presenting clinical signs and clinicopathologic abnormalities in dogs with MM are ascribed either to direct tissue infiltration by neoplastic cells or to associated paraneoplastic syndromes. Historically, the diagnosis of MM is established by documenting the presence of at least 2 of the following: neoplastic marrow/organ/nodal infiltration, lytic skeletal lesions, and monoclonal gammopathy (M proteins) in the serum or urine.81 Although 1 small series of dogs presenting with monoclonal gammopathy revealed MM to be the most frequent cause, other diseases, including leishmaniasis and ehrlichiosis, should be excluded.80 Monoclonal gammopathies have also been documented secondary to GI EMP.75 Blood hyperviscosity secondary to hypergammaglobulinemia can affect neurologic, ocular, and cardiovascular systems, leading to diverse clinical presentations.74 Hemostatic disorders secondary to paraproteinemia-associated platelet dysfunction or dysregulation of the coagulation cascade may also be evident.74 Renal disease is also encountered and may be secondary to nephrotoxicity associated with hyperproteinemia or hypercalcemia as a separate paraneoplastic syndrome.74 Cytopenias may result from hemorrhage, chronic disease, or myelophthisis, with the latter contributing to increased susceptibility to infectious diseases.74 If significant osteolytic activity has occurred, skeletal pain may be reported, which can be severe and in extreme cases may present with pathologic fracture.74 As such, MM poses many challenges for the veterinarian in terms of supportive care and direct therapy against the neoplastic cells.
EMPs usually present as solitary masses and are most frequently cutaneous, followed by involvement of the mucus membranes of the oral cavity or lips, with the more distal GI tract being less frequently involved (Figure 2).79 Cutaneous and oral EMPs are usually benign.79 Multiple cutaneous EMPs (cutaneous plasmacytosis) have been described in dogs, and these have the potential to display malignant behavior reminiscent of MM.82 Similarly, distal GI EMPs also have a tendency to metastasize.75,79
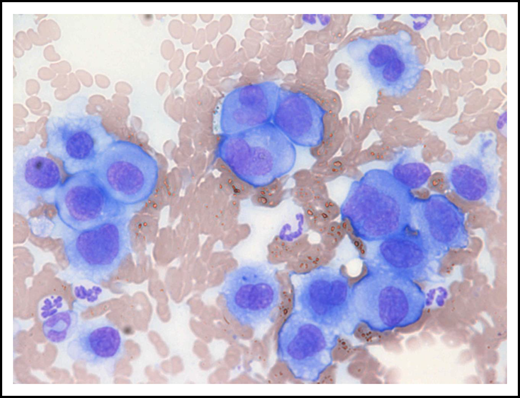
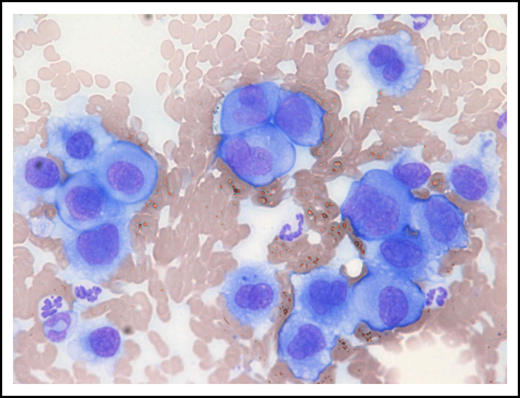
Metastatic gastric EMP in a 10-year-old female neutered cross-breed dog. Cytologic smear of fine-needle aspirate taken from a mesenteric lymph node revealing a pleomorphic population of malignant round cells with erythrocytes and neutrophils noted in the background (modified Wright-Giemsa stain; ×40 objective). Reprinted with permission.75
Metastatic gastric EMP in a 10-year-old female neutered cross-breed dog. Cytologic smear of fine-needle aspirate taken from a mesenteric lymph node revealing a pleomorphic population of malignant round cells with erythrocytes and neutrophils noted in the background (modified Wright-Giemsa stain; ×40 objective). Reprinted with permission.75
Treatment and outcomes
Canine MM is responsive to medical management; however, cure is not anticipated.74 Melphalan is the current cytotoxic treatment of choice for canine MM and is often combined with prednisone.74 In 1 study, 2 melphalan protocols were described, and both were well tolerated and led to a median overall survival of 930 days.83 An earlier study of 60 dogs treated with melphalan, cyclophosphamide, and prednisone resulted in a median survival of 540 days.84 A majority of dogs will become refractory to melphalan and various other cytotoxics, including CCNU, vincristine, doxorubicin, chlorambucil, and rabacfosadine have then been prescribed as second-line rescue agents.83 Along with long-term medical therapy, supportive measures, including antibiotics, analgesia, and fluid therapy, may be indicated, depending on the clinical findings.74 In specific clinical settings, bisphosphonates can be prescribed for hypercalcemia. Bisphosphonates and palliative radiotherapy can also provide relief for bone pain, and plasmapheresis is sometimes performed to manage severe cases of hyperglobulinemia.74 Although usually an ultimately fatal condition, the high response rates and prolonged durations of remission make MM a rewarding disease to treat.
Solitary cutaneous or oral EMPs carry a favorable prognosis, and when complete excision is achievable, cure is usually attained.79 Exceptions to this include GI EMPs distal to the oral cavity and extensive cutaneous plasmacytosis; in such cases, melphalan and other cytotoxics have been described.75,82 Nonetheless, even in these aggressive tumors, favorable responses to therapy can be achieved, with ORRs >70% and an overall survival of 542 days, as shown in 21 dogs treated with either melphalan- or CCNU-based protocols for cutaneous plasmacytosis.82
Histiocytic neoplasia
Pathology and presentation
Several neoplastic histiocytic diseases are clinically recognized in dogs, and the most frequently encountered variants are summarized in Table 4. Histiocytomas are typically benign tumors that usually occur in dogs age <3 years, with predispositions reported in boxers and dachshunds.85 These tumors are derived from Langerhans cell (LCs) and express CD1a, CD11c/CD18, IBA1, and E-cadherin; the presence of a florid cytotoxic T-cell intralesional infiltrate is frequently noted and can be associated with spontaneous regression.85,86 Notably, in canine LCs, the absence of Birbeck granules may be ascribed to a nonfunctional langerin variant.85 Additional studies are required to define what role, if any, langerin plays in canine LC function. Currently, langerin expression is not widely used in canine oncology to interrogate histiocytic cell lineage. Histiocytomas are usually solitary cutaneous tumors and may occur anywhere but are most common on the head; involvement of local lymph nodes is infrequent.85 Although less commonly observed, multiple cutaneous histiocytomas resembling cutaneous Langerhans cell histiocytosis (LCH) have also been recognized in dogs and follow a more aggressive course compared with solitary lesions, particularly if the disease extends systemically.85
Canine HS is a malignancy that is broadly classified as either localized or disseminated depending upon disease extent.85 Canine HS usually occurs in adults of various breeds, with Bernese mountain dogs, retrievers, rottweilers, and miniature schnauzers being predisposed.85,87,88 Primary disease can be localized to the periarticular tissue, lymph node, skin, lung, bone marrow, and spleen (Figure 3), with other sites less frequently affected, whereas disseminated disease involves multiple sites and organs.85,87 Clinical signs may be vague, such as weight loss or lethargy, or may be specific to infiltrated tissue, such as joint pain and swelling.85,87 Canine HS is derived from interstitial dendritic cells that express CD1a, CD11c/CD18, and IBA1.85,86 Although E-cadherin expression is not classically associated with HS, E-cadherin+ cutaneous HS has been described.85,89 Localized HSs are variably infiltrated by immune T cells, with increased T-cell density being associated with favorable outcomes.90 Genetic analyses of canine HS have revealed association between mutations affecting PTEN, PTPN11, and KRAS with activation of MAPK pathway signaling.91-93 Hemophagocytic HS follows an aggressive course; dogs frequently present with Coombs− anemias, often accompanied by thrombocytopenia, hyperbilirubinemia, hypoalbuminemia, and hypocholesterolemia.85,94 Immunophenotypically, hemophagocytic HS expresses markers of the macrophage lineage (CD11d/CD18+, CD1a low).85,94 Dendritic cell leukemia is rarely reported in dogs and manifests as marked leukocytosis with potential for effacement of pulmonary, splenic, and hepatic tissue.85,95
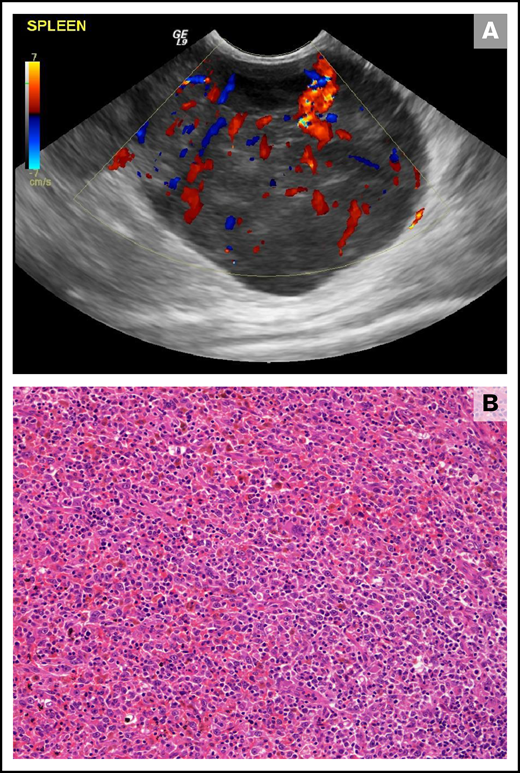
Splenic HS in a 10-year-old female neutered cross-breed dog. (A) Ultrasonographic still of a large (maximum diameter, 59 mm) well-vascularized (as demonstrated by Doppler color flow) mass lesion of mixed echogenicity within the tail of the spleen. (B) Histopathology of the splenic mass revealed a poorly encapsulated neoplasm showing both round and spindle morphologies of large tumor cells forming sheets and streams. Diagnosis of HS was confirmed using immunohistochemical stains (not shown; hematoxylin and eosin stain; ×20 objective).
Splenic HS in a 10-year-old female neutered cross-breed dog. (A) Ultrasonographic still of a large (maximum diameter, 59 mm) well-vascularized (as demonstrated by Doppler color flow) mass lesion of mixed echogenicity within the tail of the spleen. (B) Histopathology of the splenic mass revealed a poorly encapsulated neoplasm showing both round and spindle morphologies of large tumor cells forming sheets and streams. Diagnosis of HS was confirmed using immunohistochemical stains (not shown; hematoxylin and eosin stain; ×20 objective).
Treatment and outcomes
Because cutaneous histiocytomas usually regress spontaneously, no therapy is typically required. The prognosis is usually excellent; however, surgery can be performed for tumors that do not regress.87 Dogs with cutaneous LCH may also have delayed regression, but approximately 50% of them are euthanized because of lack of regression or complications with skin lesions. Systemic involvement usually results in death from rapid disease progression.85 Complete resolution of multiple cutaneous histiocytomas was reported after the treatment of 1 dog with CCNU; however, standard-of-care treatment is not defined for canine LCH.96
Dogs with localized HS can exhibit prolonged survival times after adequate local control with surgery and/or radiotherapy and adjuvant CCNU; the median overall survival for 16 dogs receiving such treatment was found to be 568 days.97 A recent report describing the treatment of 49 dogs with periarticular HS reported no significant differences in outcome between the use of external-beam radiotherapy and surgical excision for local control when adjuvant chemotherapy was prescribed in all cases.98 Activity of CCNU in canine HS has also been documented outside the adjuvant setting, where an ORR of 46% was observed in 56 dogs with bulky disease.99 Various other cytotoxic agents have been prescribed for dogs with HS, but CCNU is considered the treatment of choice based on the reported responses rates.87 Prognostically, a separate study revealed that overall survival in disseminated HS was significantly shorter than that in localized HS (78 vs 398 days).100 The outlook for hemophagocytic HS is particularly bleak, with a reported mean survival of only 7 weeks.94 Although prolonged survival can be achieved in localized HS, it is usually a terminal diagnosis in dogs, despite therapy.
Concluding remarks
As veterinary oncologists and scientists have gained access to advanced technologies, and our understanding of the pathology of hematologic malignancies has increased, some key similarities as well as significant differences between these diseases in dogs and in their human counterparts have been revealed. Such discoveries have shaped how we best manage these conditions, but the poor outlook for some of the more aggressive hematologic cancers encourages application of novel therapies in these malignancies (eg, the ongoing development of chimeric antigen receptor T-cell therapies for B-cell malignancies in dogs).101-103 Immunotherapy has cemented itself as the fourth pillar in human oncology; however, despite the remarkable successes achieved within this field, cure is only achieved in a minority of patients.104 Lack of translationally relevant preclinical models has been cited as a top-10 challenge for cancer immunotherapy, and because pet dogs develop disease in the context of an outbred immune system with complex and orchestrated responses, they are uniquely poised to help overcome this challenge.104 Similarly, the value of spontaneous over transplanted tumor models is increasingly being recognized.105 Pet dogs present to the oncology clinic with spontaneous tumors in the most organic sense and therefore represent a significant advantage over many other preclinical models. As the science of veterinary oncology continues to develop, there is significant scope to advance next-generation therapeutic approaches that will be of future benefit to both dogs and humans with hematologic cancers.
Acknowledgments
M.J.A. is supported by National Cancer Institute (NCI), National Institutes of Health (NIH), grant K08CA252619. N.J.M. is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, and NCI, NIH grants (R01AR075337, U24CA224122 and U54CA244711).
Authorship
Contribution: N.J.M. and M.J.A. performed the review and wrote the paper.
Conflict-of-interest disclosure: N.J.M. is a cofounder of Vetigenics, a startup biopharmaceutical company that generates fully canine antibodies for veterinary therapeutics and comparative research. M.J.A. declares no competing financial interests.
Correspondence: Matthew J. Atherton, University of Pennsylvania, 310 Hill Pavilion, 380 South University Ave, Philadelphia, PA 19104; e-mail: mattath@upenn.edu.