Key Points
HexaBody-DR5/DR5 induces potent cytotoxicity of primary MM cells, especially in samples from recently treated relapsed/refractory patients.
HexaBody-DR5/DR5–mediated cytotoxicity can be enhanced via combination with bortezomib, daratumumab, or lenalidomide.
Abstract
Apoptosis induction by death receptor (DR)-specific agonistic antibodies is a potentially effective antitumor therapy. Nonetheless, to date, all conventional DR-targeting antibodies that induce apoptosis via FcγR-dependent DR clustering failed to show clinical efficacy. HexaBody-DR5/DR5 (GEN1029) has been developed to overcome full FcγR dependence. HexaBody-DR5/DR5 is a mixture of 2 noncompeting DR5-specific immunoglobulin G1 (IgG1) antibodies, each with an E430G mutation in the Fc domain. This mutation enhances Fc-Fc interactions, resulting in antibody hexamerization, followed by FcγR-independent clustering of DR5 molecules. This unique combination of dual epitope targeting and increased IgG hexamerization resulted in potent preclinical antitumor activity in various solid cancers. In this study, we explored the preclinical activity of HexaBody-DR5/DR5 in multiple myeloma (MM), because MM cells are known to express DR5. In bone marrow samples from 48 MM patients, HexaBody-DR5/DR5 induced potent cytotoxicity of primary MM cells. Importantly, HexaBody-DR5/DR5 mediated the highest cytotoxic activity in samples from relapsed/refractory MM patients, including those who are refractory to daratumumab. This improved cytotoxic activity was observed only in patients who received their last anti-MM treatment <1 month ago, suggesting that anti-MM drugs sensitized MM cells to HexaBody-DR5/DR5. Supporting this, bortezomib combined with HexaBody-DR5/DR5 synergistically increased cytotoxicity in MM cells in 7 of 11 newly diagnosed patients. Lenalidomide also synergized with HexaBody-DR5/DR5, but only via its immunomodulatory effects, presumably by enhancing the antibody-dependent cellular cytotoxicity activity of HexaBody-DR5/DR5. Daratumumab showed additive effects when combined with HexaBody-DR5/DR5. In conclusion, the results of this preclinical study indicate a therapeutic potential for HexaBody-DR5/DR5, especially in recently treated relapsed/refractory MM patients.
Introduction
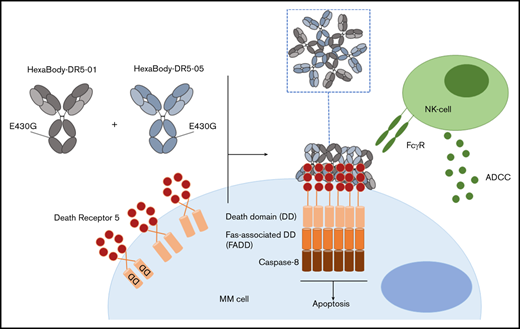
Over the past 20 years, the available treatment modalities for multiple myeloma (MM), the malignant disease of antibody-producing plasma cells in the bone marrow (BM), have expanded rapidly. Starting with the introduction of immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs), and followed by the implementation of monoclonal antibodies, the overall survival of MM patients has improved significantly.1 Nonetheless, MM remains difficult to cure. Few patients achieve long-term remissions, and most of them eventually develop refractory disease, requiring alternative and more effective therapies. Various attempts have been undertaken to develop an effective therapy for MM and several solid malignancies by targeting death receptor 4 (DR4) and death receptor 5 (DR5). Triggering of these receptors via tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) or agonistic antibodies selectively induced apoptosis in malignant cells derived from a variety of tumors, including MM.2-4 Despite promising preclinical studies, recombinant TRAIL, as well as DR-targeting antibodies, failed to show a therapeutic benefit in clinical trials.5,6 Primary resistance of cancer cells, as well as unfavorable pharmacokinetics or insufficient agonist activity, has been considered responsible for the limited clinical efficacy of these agents. Importantly, it was shown that DR di- or trimerization requires subsequent higher-order clustering for proper activation of the DR pathway.7 The agonist activity of conventional DR-targeting immunoglobulin G (IgG) antibodies is largely dependent on Fc tail–mediated antibody crosslinking via FcγR+ cells to provide sufficient target clustering.8 This is probably a substantial limitation for their potency in clinical settings, because the frequency of intratumoral FcγR+ cells may be too low to achieve efficient DR crosslinking.
To overcome this limitation, HexaBody-DR5/DR5 (GEN1029) was developed through the application of the novel HexaBody platform to DR5-targeting antibodies. This platform is based on the natural concept that, upon binding to antigens on a cell surface, IgG molecules can organize into ordered hexamers through intermolecular Fc-Fc interactions.9 HexaBody molecules are IgG1 molecules with a single point mutation in the Fc domain that facilitates intermolecular Fc-Fc interactions, thereby improving hexamer formation upon binding to the target antigen.9,10 HexaBody-DR5/DR5 is a 1:1 mixture of 2 noncompeting DR5 antibodies (clone 01 and 05), both harboring the specific E430G mutation for hexamerization; it was established that, in addition to hexamerization, agonist activity of DR5 antibodies was enhanced by dual-epitope targeting of DR5.11 HexaBody-DR5/DR5 effectively induced apoptosis in a variety of preclinical solid tumor models, which could be achieved independently of FcγR-mediated antibody crosslinking.11 Encouraged by these results, we investigated the preclinical efficacy of HexaBody-DR5/DR5 in MM, because MM cells are known to express DR5.12 To this end, we tested a large set of primary BM samples (n = 48) derived from newly diagnosed (ND; n = 13) and relapsed/refractory (RR; n = 35) MM patients.
Material and methods
Ethical approval
According to the Dutch Central Committee on Research involving Human Subjects, this type of study does not require approval from an ethics committee. Hence, all patient material and clinical data used in this project have been collected according to the code of conduct for medical research developed by The Council of the Federation of Medical Scientific Societies, the Netherlands in 1995, which was revised in 2002-2003 on the basis of the European Data Protection Directive and its implementation in the Dutch Act on the Protection of Personal Data. The study was conducted in accordance with the Declaration of Helsinki.
Antibodies
IgG1-DR5/DR5 is a 1:1 mixture of 2 noncompeting DR5-specific humanized wild-type IgG1 antibodies: IgG1-DR5-01 and IgG1-DR5-05. HexaBody-DR5/DR5 is a 1:1 mixture of the same 2 noncompeting DR5-specific humanized IgG1 antibodies with an E430G hexamerization-enhancing mutation in their Fc domains: Hx-DR5-01 and Hx-DR5-05. Additional technical details were described by Overdijk et al.11 The anti–HIV-1 gp120 antibody IgG1-b12 with E430G mutation was used as an IgG1 isotype control (IgG1-Ctrl).
Cell lines
The indicated MM cell lines were cultured in RPMI 1640 medium (Invitrogen), supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and 1% penicillin-streptomycin (Invitrogen) at 37°C in 5% CO2. All MM cell lines were authenticated by short-tandem repeat profiling at a maximum of 3 months before testing.
BM and peripheral blood mononuclear cells
BM-derived mononuclear cells (BMMCs) or peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation (Ficoll-Paque Plus; Cytiva) from BM aspirates or peripheral blood samples of patients with MM. Cells were used directly or cryopreserved in liquid nitrogen until further use.
Incucyte ZOOM imaging
NCI-H929 cells incubated with caspase-3/7 green detection reagent (Essen Bioscience) were pelleted by centrifugation in a microplate image lock (Incucyte) (2 × 103 cells per well) and incubated with HexaBody-DR5/DR5 and IgG1-Ctrl (20 µg/mL) in triplicates. Images were obtained by Incucyte ZOOM imaging with a 10× objective every 5 minutes for 24 hours and then every 30 minutes once a decrease in signal was detected. Apoptosis was detected by total green object area (µm2) per image.
Western blotting
Cells were incubated with the indicated concentrations of HexaBody-DR5/DR5 or IgG1-DR5/DR5 or with medium (negative control) for 6 hours in 12-well plates. To prepare cell lysates, cells were washed with ice-cold phosphate-buffered saline and incubated for 45 minutes with ice-cold 1% NP40 lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing complete protease inhibitor cocktail (Roche). Total cell lysates were loaded onto a 4% to 20% gradient polyacrylamide gel (Bio-Rad) for electrophoresis and were blotted onto polyvinylidene difluoride membranes (Merck Millipore). Membranes were incubated with primary antibodies (rabbit anti–caspase-3, rabbit anti-PARP, and mouse anti–caspase-8 [Cell Signaling Technology] and mouse anti-actin [Merck Millipore]) and bands were detected with infrared dye–labeled antibodies p680 Goat anti-Rabbit IgG or 800Cw Goat anti-Mouse IgG and visualized using an Odyssey imager (all from LI-COR Biosciences). Actin was used as protein loading control.
Flow cytometry–based cytotoxicity assays
Cryopreserved BMMCs from MM patients containing plasma cells (range, 2-80%) and other mononuclear immune cells were incubated at a concentration of 1 × 106 total BMMCs per milliliter in 96-well U-bottom plates with serial dilutions of HexaBody-DR5/DR5 or IgG1-DR5/DR5 or with a fixed concentration of IgG1-Ctrl in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin at 37°C for 24 hours. The optimum length of the assay was determined to be 24 hours. In the samples, quantitative flow cytometry (BD LSRFortessa or Celesta) was used to enumerate viable (7AADneg) plasma cells (CD38+CD138+), monocytes (CD14+), T cells (CD3+), B cells (CD19+/CD20+), and natural killer (NK) cells (CD3−CD56+ lymphocytes). Absolute live cell counts in the samples were normalized to untreated negative control to calculate the percentage viability [100 × (plasma cell counts in the test sample/average plasma cell counts in the negative control samples)] and the percentage kill [(100 − % viable)]. An overview of the antibodies used in these flow cytometry–based cytotoxicity assays is shown in Table 1.
Cell surface expression of DR5 in median fluoresence intensity (MFI) was determined using an anti-human DR5 APC-labeled antibody (BioLegend). ΔMFI was determined by subtracting the MFI of an isotype control APC-labeled antibody from that of the DR5-specific antibody. MM and immune cell populations were included for analysis only if >500 events were counted in untreated samples. A similar procedure was followed for cytotoxicity assays with cell lines. Where indicated, violet tracer (Thermo Fisher)–labeled PBMCs were added at an effector-to-target ratio of 40:1.
To explore the direct effect of lenalidomide, bortezomib, or daratumumab combined with HexaBody-DR5/DR5 on primary patient samples, all agents were simultaneously added to the 96-well plates. Given that, in preliminary assays, the immunomodulatory and tumor-sensitizing effects of lenalidomide were optimally detectable after an incubation period of 5 days, which typically exceeds the survival time of primary MM cells ex vivo, we explored the combinatorial effects of lenalidomide with HexaBody-DR5/DR5 on MM cell lines. Either the MM cell line or the effector PBMCs were preincubated with lenalidomide for 5 days; thereafter, the effector cells and MM cells were coincubated in the presence of HexaBody-DR5/DR5 for an additional 24 hours. Combination with daratumumab was tested in the presence of 20% pooled normal human serum (NHS) that contains active complement. When daratumumab was used as a control for antibody-dependent cellular cytotoxicity (ADCC), heat-inactivated human serum was used.
Statistical analysis
Flow cytometry data were analyzed using FACSDiva software. Graphs were plotted using GraphPad Prism 8.2. for Windows. Statistical analysis was performed with the appropriate parametric or nonparametric test, as indicated in the figure legends. Two-sided P values < .05 were considered statistically significant. Synergy was calculated according to the Bliss synergy model: combination treatment is considered “synergistic” if the observed effect significantly exceeded the expected effect.
Results
HexaBody-DR5/DR5 induces high and specific MM cell kill in BM samples from MM patients
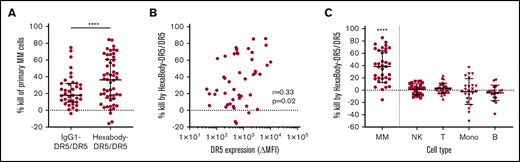
We tested the capacity of HexaBody-DR5/DR5 to kill malignant MM cells using BMMCs from MM patients (n = 48) that contained 2% to 80% MM cells. The clinical characteristics of the MM patients are shown in Table 2. HexaBody-DR5/DR5 induced cytotoxicity of primary MM cells with a median cell kill of 36% (range, 0-86). A 1:1 mixture of the same DR5-antibodies without the hexamerization-enhancing E430G mutation (IgG1-DR5/DR5) induced significantly lower cytotoxicity compared with HexaBody-DR5/DR5 (Figure 1A). The cytotoxic activity of IgG1-DR5/DR5 may be attributable, at least in part, to the presence of FcγR+ cells (eg, NK cells) in the BM suspensions that can induce FcγR-dependent ADCC and/or crosslinking of membrane-bound antibodies. However, enhanced tumor cytotoxicity in the presence of HexaBody-DR5/DR5 supports the relevance of enhanced hexamerization in addition to dual-epitope targeting for DR5 agonist activity.
HexaBody-DR5/DR5 induces selective MM cell kill in BM samples of MM patients. (A) Kill (%) by HexaBody-DR5/DR5 (20 µg/mL; n = 48) and IgG1-DR5/DR5 (n = 39) in a 24-hour assay with BMMCs from MM patients. Tumor cells were identified as CD38+CD138+. ****P < .0001, Wilcoxon matched-pairs signed-rank test. (B) Pearson’s correlation of DR5 surface expression level on MM cells with HexaBody-DR5/DR5–induced cytotoxicity (n = 46). (C) MM cell kill (%) by HexaBody-DR5/DR5 compared with kill of immune cell populations present in the BM of MM patients. n = 35. ****P < .0001, MM vs all subsets, Wilcoxon matched-pairs signed-rank test. Horizontal lines denote the median, and error bars indicate the interquartile range. B, B cells; Mono, monocytes; NK, NK cells; T, T cells.
HexaBody-DR5/DR5 induces selective MM cell kill in BM samples of MM patients. (A) Kill (%) by HexaBody-DR5/DR5 (20 µg/mL; n = 48) and IgG1-DR5/DR5 (n = 39) in a 24-hour assay with BMMCs from MM patients. Tumor cells were identified as CD38+CD138+. ****P < .0001, Wilcoxon matched-pairs signed-rank test. (B) Pearson’s correlation of DR5 surface expression level on MM cells with HexaBody-DR5/DR5–induced cytotoxicity (n = 46). (C) MM cell kill (%) by HexaBody-DR5/DR5 compared with kill of immune cell populations present in the BM of MM patients. n = 35. ****P < .0001, MM vs all subsets, Wilcoxon matched-pairs signed-rank test. Horizontal lines denote the median, and error bars indicate the interquartile range. B, B cells; Mono, monocytes; NK, NK cells; T, T cells.
DR5 expression, which was determined in 46 samples, was heterogeneous and showed a weak, but significant, correlation with HexaBody-DR5/DR5–induced cytotoxicity (r = +0.33; P = .02; Figure 1B). In 35 of these samples, we also evaluated the cytotoxicity of HexaBody-DR5/DR5 on monocytes and T, NK, and B cells (Figure 1C). HexaBody-DR5/DR5 induced little to no cytotoxicity in all of these nonmalignant immune cell subsets. The heterogeneity observed for monocytes was likely due to their tendency to adhere to the bottom of plates, which affected their harvesting in automated flow measurements.
RR MM patient samples are more sensitive to HexaBody-DR5/DR5
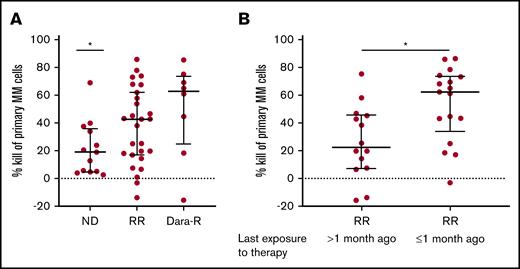
The stratification of all tested samples into ND (n = 13) vs RR (n = 35) patients revealed a significantly better efficacy of HexaBody-DR5/DR5 in the RR group (median kill, 43%; range, 0-86 vs ND: median kill, 19%; range, 3-69) (Figure 2A), which also included 8 patients who had become refractory to daratumumab monotherapy. Importantly, the MM cells of these daratumumab-refractory patients were equally susceptible to HexaBody-DR5/DR5 as were MM cells from other RR patients (median kill, 63% vs 43%; P = not significant). A similar differentiation between ND and RR patients was not observed for IgG1-DR5/DR5 (supplemental Figure 1).
RR MM patients who received anti-MM treatment <1 month prior to BM biopsy are most sensitive to HexaBody-DR5/DR5. (A) MM cell kill (%) by HexaBody-DR5/DR5 (20 µg/mL) of BM samples from ND (n = 13) and RR (n = 35) patients, including daratumumab-refractory (Dara-R) patients (n = 8). *P = .0246 (ND vs total RR group). (B) MM cell kill (%) by HexaBody-DR5/DR5 (20 µg/mL) of BM samples from RR patients who had received their last exposure to therapy >1 month (n = 14) and ≤1 month (n = 17) prior to their BM biopsy. *P = .0116. Data are shown as median; error bars indicate the interquartile range. Statistical analyses were performed with a Mann-Whitney U test.
RR MM patients who received anti-MM treatment <1 month prior to BM biopsy are most sensitive to HexaBody-DR5/DR5. (A) MM cell kill (%) by HexaBody-DR5/DR5 (20 µg/mL) of BM samples from ND (n = 13) and RR (n = 35) patients, including daratumumab-refractory (Dara-R) patients (n = 8). *P = .0246 (ND vs total RR group). (B) MM cell kill (%) by HexaBody-DR5/DR5 (20 µg/mL) of BM samples from RR patients who had received their last exposure to therapy >1 month (n = 14) and ≤1 month (n = 17) prior to their BM biopsy. *P = .0116. Data are shown as median; error bars indicate the interquartile range. Statistical analyses were performed with a Mann-Whitney U test.
Further analyses of the RR group demonstrated that the cytotoxic activity of HexaBody-DR5/DR5 was significantly higher in samples that had been obtained from patients who were still receiving an anti-MM treatment or had received their last therapy <1 month ago compared with samples obtained from patients who received their last therapy >1 month ago (median kill, 62% vs 23%; P = .0116; Figure 2B). This difference was not associated with DR5 surface expression levels of the MM cells (supplemental Figure 2).
Combination of HexaBody-DR5/DR5 with standard-of-care MM treatments increases tumor cell kill
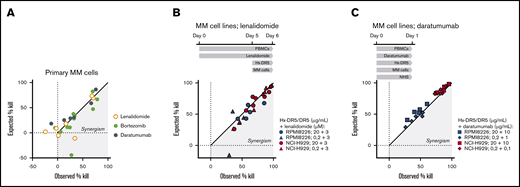
The superior activity of HexaBody-DR5/DR5 in samples from recently treated RR patients prompted us to explore whether its combination with standard-of-care MM treatments could improve cytotoxicity in MM cells. We tested BM samples from ND patients in 24-hour cytotoxicity assays without supplementing with additional FcγR+ effector cells (Figure 3A). Combination of HexaBody-DR5/DR5 with bortezomib (3 nM; n = 11) increased the MM cell kill in a modest synergistic manner in 7 of 11 samples according to the Bliss synergy model. Daratumumab (10 µg/mL; n = 6), in the presence of NHS with complement activity, did not show synergy with HexaBody-DR5/DR5. Lenalidomide (3 µM; n = 7) showed strong synergism with HexaBody-DR5/DR5 in only 1 of 7 BM samples; however, lenalidomide exerted limited single-drug activity in these ex vivo assays. Because the activity of lenalidomide may be more evident after prolonged incubation, which precludes ex vivo experiments with primary MM cells, we also performed cytotoxicity assays with MM cell lines NCI-H929 and RPMI8226. The cell lines or the effector PBMCs were incubated separately with lenalidomide for up to 5 days, before the addition of HexaBody-DR5/DR5. The longer exposure of NCI-H929 and RPMI8226 cells to lenalidomide had no effect on HexaBody-DR5/DR5–mediated kill (supplemental Figure 3), indicating that lenalidomide did not sensitize the tumor cells to HexaBody-DR5/DR5–mediated kill. However, preincubation of PBMCs with lenalidomide for 5 days synergistically increased the HexaBody-DR5/DR5–mediated kill of NCI-H929 cells (n = 7 donor PBMCs) and RPMI8226 cells (n = 5 donor PBMCs) (Figure 3B). These results indicated that lenalidomide may contribute to HexaBody-DR5/DR5–mediated cytotoxicity by its immunomodulatory effects on PBMCs, presumably via the increase in the total number of NK cells and the fraction of cytotoxic CD16+ NK cells (supplemental Figure 4). We also tested the combination of HexaBody-DR5/DR5 and daratumumab in the presence of healthy donor PBMCs at a 40:1 effector-to-target ratio and pooled NHS (20%) to allow the most optimal conditions for ADCC and complement-dependent cytotoxicity. Results demonstrated enhanced tumor cell kill of the combination, although the effects were additive rather than synergistic (Figure 3C).
HexaBody-DR5/DR5 combined with standard-of-care MM treatments increases tumor cell kill. (A) Observed vs expected MM cell kill (%) of BM samples from ND MM patients treated with HexaBody-DR5/DR5 (20 µg/mL) in combination with lenalidomide (3 µM; n = 7) bortezomib (3 nM; n = 11), or daratumumab (10 µg/mL; n = 6). (B) Observed vs expected MM cell kill (%) of the MM cell lines RPMI8226 and NCI-H929 by HexaBody-DR5/DR5 (20 and 0.2 µg/mL) and lenalidomide-pretreated healthy donor PBMCs (5 days; 3 µM) at a 40:1 effector-to-target ratio. Each point represent a single donor. (C) Observed vs expected MM cell kill (%) of the MM cell lines RPMI8226 and NCI-H929 by HexaBody-DR5/DR5 (20 and 0.2 µg/mL) and daratumumab (10 and 1 µg/mL) in the presence of healthy donor PBMCs at a 40:1 effector-to-target ratio and NHS (20%). Each point represent a single donor.
HexaBody-DR5/DR5 combined with standard-of-care MM treatments increases tumor cell kill. (A) Observed vs expected MM cell kill (%) of BM samples from ND MM patients treated with HexaBody-DR5/DR5 (20 µg/mL) in combination with lenalidomide (3 µM; n = 7) bortezomib (3 nM; n = 11), or daratumumab (10 µg/mL; n = 6). (B) Observed vs expected MM cell kill (%) of the MM cell lines RPMI8226 and NCI-H929 by HexaBody-DR5/DR5 (20 and 0.2 µg/mL) and lenalidomide-pretreated healthy donor PBMCs (5 days; 3 µM) at a 40:1 effector-to-target ratio. Each point represent a single donor. (C) Observed vs expected MM cell kill (%) of the MM cell lines RPMI8226 and NCI-H929 by HexaBody-DR5/DR5 (20 and 0.2 µg/mL) and daratumumab (10 and 1 µg/mL) in the presence of healthy donor PBMCs at a 40:1 effector-to-target ratio and NHS (20%). Each point represent a single donor.
HexaBody-DR5/DR5 mediates cytotoxicity via extrinsic apoptotic pathway activation and induction of FcγR-mediated ADCC
The beneficial effect of combining HexaBody-DR5/DR5 with lenalidomide-treated PBMCs suggested effector mechanisms of HexaBody-DR5/DR5, in addition to FcγR-independent DR5 agonism,11 such as ADCC or FcγR-mediated crosslinking. To dissect these possible effector mechanisms, we further investigated HexaBody-DR5/DR5–mediated killing using the MM cell lines RPMI8226, NCI-H929, and MM.1s. As expected, HexaBody-DR5/DR5 activated the extrinsic apoptotic pathway in these cell lines in the absence of any accessory cells (Figure 4A-C). Interestingly, when PBMCs from healthy donors were added to the assay, the cytotoxic activity of HexaBody-DR5/DR5 was increased significantly in MM.1s cells. A similar trend was seen for NCI-H929 cells in 5 of 6 donors, although statistical significance was not reached (Figure 4D). These data suggest that HexaBody-DR5/DR5 is capable of inducing FcγR-independent apoptosis, as well as FcγR-mediated cytotoxicity, in vitro. To confirm the ADCC activity of HexaBody-DR5/DR5 ex vivo, we tested 4 MM patient samples with the HexaBody-DR5/DR5 variant carrying the L234F/L235E/D265A triple mutation, which abrogates FcγR binding.11 In accordance with the cell line data, the L234F/L235E/D265A variant could still induce cytotoxicity but at a reduced level compared with HexaBody-DR5/DR5 (supplemental Figure 5). To discriminate whether the increase in HexaBody-DR5/DR5–mediated cytotoxicity in the presence of PBMCs was due to ADCC or additional FcγR-mediated crosslinking, we fixed PBMCs with 1% paraformaldehyde, which is known to inhibit FcγR-mediated ADCC or phagocytosis but not FcγR-mediated crosslinking of membrane-bound antibodies.13 The anti-CD38 antibody daratumumab was used as a control antibody for ADCC, and the wild-type IgG1-DR5/DR5 mixture was used as a control for FcγR-mediated crosslinking. Indeed, IgG1-DR5/DR5 could still induce some cytotoxicity, especially on NCI-H929 cells, suggesting that fixed PBMCs could still mediate DR5 agonism by crosslinking membrane-bound antibody, whereas fixation of PBMCs completely abolished daratumumab-mediated ADCC, as expected. Fixation of PBMCs also largely neutralized the increased cytotoxic activity of HexaBody-DR5/DR5, revealing that ADCC, rather than FcγR-mediated clustering of DR5 antibodies, is the primary mechanism for the additional increase in MM cell kill by HexaBody-DR5/DR5 in the presence of PBMCs (Figure 4E).
HexaBody-DR5/DR5 mediates extrinsic apoptotic pathway activation and induces low levels of ADCC in MM cell lines. (A) Representative dose-response curve of HexaBody-DR5/DR5 in RPMI8226 (left panel), NCI-H929 (middle panel), and MM.1s (right panel) cell lines after a 24-hour incubation compared with IgG1-Ctrl (20 µg/mL) (n = 3 or 4). (B) Time-lapse representation of apoptosis induction in NCI-H929 cells by HexaBody-DR5/DR5 and IgG1-Ctrl (20 µg/mL). NCI-H929 cells were labeled with caspase-3 green label, and images were taken using Incucyte for 24 hours. Each point represents the average total green object area per image of 3 replicates. (C) Expression of extrinsic apoptotic pathway–related (cleaved) proteins for RPMI8226, NCI-H929, and MM.1s cell lines, as detected by western blot after a 6-hour incubation with HexaBody-DR5/DR5 (1 µg/mL [Hx1] and 4 µg/mL [Hx4]). Actin was incorporated as loading control. A representative image is shown of 2 independent experiments. (D) Kill (%) of RPMI8226, NCI-H929, and MM.1s cell lines with HexaBody-DR5/DR5 (20 µg/mL) after a 24-hour incubation in the presence of healthy donor PBMCs in a 40:1 effector-to-target ratio. Each point represents a single donor. Data are shown as mean and SD. **P = .009, paired Student t test. (E) Kill (%) of NCI-H929 (left panel) and MM.1s (right panel) cell lines by HexaBody-DR5/DR5 and IgG1-Ctrl in the absence of PBMCs, as well as in the presence of paraformaldehyde-fixed or nonfixed PBMCs in a 40:1 effector-to-target ratio. IgG1-DR5/DR5 and the anti-CD38 antibody daratumumab (Dara) are incorporated to control for cytotoxicity that is dependent on FcγR-mediated crosslinking and cytotoxicity that is dependent on ADCC, respectively. Data are shown as mean and standard deviation of 2 (NCI-H929) or 3 (MM.1s) independent experiments. *P < .05, **P < .01, 1-way analysis of variance and Tukey’s multiple-comparisons test.
HexaBody-DR5/DR5 mediates extrinsic apoptotic pathway activation and induces low levels of ADCC in MM cell lines. (A) Representative dose-response curve of HexaBody-DR5/DR5 in RPMI8226 (left panel), NCI-H929 (middle panel), and MM.1s (right panel) cell lines after a 24-hour incubation compared with IgG1-Ctrl (20 µg/mL) (n = 3 or 4). (B) Time-lapse representation of apoptosis induction in NCI-H929 cells by HexaBody-DR5/DR5 and IgG1-Ctrl (20 µg/mL). NCI-H929 cells were labeled with caspase-3 green label, and images were taken using Incucyte for 24 hours. Each point represents the average total green object area per image of 3 replicates. (C) Expression of extrinsic apoptotic pathway–related (cleaved) proteins for RPMI8226, NCI-H929, and MM.1s cell lines, as detected by western blot after a 6-hour incubation with HexaBody-DR5/DR5 (1 µg/mL [Hx1] and 4 µg/mL [Hx4]). Actin was incorporated as loading control. A representative image is shown of 2 independent experiments. (D) Kill (%) of RPMI8226, NCI-H929, and MM.1s cell lines with HexaBody-DR5/DR5 (20 µg/mL) after a 24-hour incubation in the presence of healthy donor PBMCs in a 40:1 effector-to-target ratio. Each point represents a single donor. Data are shown as mean and SD. **P = .009, paired Student t test. (E) Kill (%) of NCI-H929 (left panel) and MM.1s (right panel) cell lines by HexaBody-DR5/DR5 and IgG1-Ctrl in the absence of PBMCs, as well as in the presence of paraformaldehyde-fixed or nonfixed PBMCs in a 40:1 effector-to-target ratio. IgG1-DR5/DR5 and the anti-CD38 antibody daratumumab (Dara) are incorporated to control for cytotoxicity that is dependent on FcγR-mediated crosslinking and cytotoxicity that is dependent on ADCC, respectively. Data are shown as mean and standard deviation of 2 (NCI-H929) or 3 (MM.1s) independent experiments. *P < .05, **P < .01, 1-way analysis of variance and Tukey’s multiple-comparisons test.
Discussion
Over the past decade, several conventional monoclonal antibodies targeting DRs have failed to induce a therapeutic response in clinical trials. These conventional antibodies require FcγR-mediated crosslinking to induce apoptosis in tumor cells, posing a potential and important limitation for the induction of DR5 agonist activity in the clinical setting.6,14 HexaBody-DR5/DR5 is an equimolar mixture of 2 DR5-specific IgG1 antibodies with an Fc-domain mutation that augments antibody hexamerization after cell surface target binding.11 Here, we explored the preclinical efficacy of HexaBody-DR5/DR5 in MM. We demonstrate that HexaBody-DR5/DR5 induced potent MM cell cytotoxicity in BM samples obtained from MM patients. HexaBody-DR5/DR5 is effective in samples obtained from ND patients and RR patients, including PI- and IMiD-refractory patients, as well as daratumumab-refractory patients, that were used for these patients as end-stage of disease therapy. Remarkably, samples from RR patients recently treated with these anti-MM treatments showed significantly increased sensitivity to HexaBody-DR5/DR5 compared with samples from ND and RR MM patients who had not received treatment in the month prior to the BM biopsy. This difference in HexaBody-DR5/DR5 sensitivity was not related to expression levels of DR5 on these patient MM cells. The lack of a strong correlation between DR5 expression and tumor cytotoxicity has also been observed in a previous study with HexaBody-DR5/DR5 in solid cancers and in other studies of DR-targeting antibodies and TRAIL-based therapeutics.11,15,16
The increased HexaBody-DR5/DR5 sensitivity in recently-treated RR patients suggested that several standard anti-MM treatments could sensitize MM cells to HexaBody-DR5/DR5. To investigate this possibility in our preclinical setting, we tested the efficacy of HexaBody-DR5/DR5 combined with standard of care anti-MM agents using samples of ND patients and MM cell lines. The anti-MM activity of HexaBody-DR5/DR5 was readily enhanced by bortezomib, in line with previous studies which described bortezomib as a potent enhancer of TRAIL-based therapeutics.16-18 Interestingly, we also observed increased activity of HexaBody-DR5/DR5 in combination with lenalidomide, which only occurred in the presence of PBMCs which were preincubated with lenalidomide for 5 days. Based on the findings that HexaBody-DR5/DR5 can mediate ADCC in MM cells, these results suggest that lenalidomide synergizes with HexaBody-DR5/DR5 by improving its FcγR-mediated ADCC activity. This is in agreement with the well-known activating effects of lenalidomide on NK cells, the main cell population mediating the ADCC.19,20 Although more research is needed to elucidate the exact contribution of NK cells or other FcγR+ cells, such as monocytes, in Hexabody-DR5/DR5 mediated cytotoxicity, the observation that lenalidomide, and possibly other IMiDs can enhance HexaBody-DR5/DR5–mediated cytotoxicity is highly relevant for the design of future clinical trials. In this respect, it is also worth mentioning the additive effects of daratumumab, which also has immunomodulatory effects by activating NK cells,21 and HexaBody-DR5/DR5 in our ex vivo assays. Thus, taken together, our results indicate that HexaBody-DR5/DR5 can be an excellent partner for PIs, as well as IMiDs, and perhaps also for daratumumab. These findings will be of great importance in the design of future clinical treatment strategies for MM patients.
In conclusion, HexaBody-DR5/DR5 represents a promising therapeutic option for MM patients with RR disease, including daratumumab-refractory patients. Furthermore, our data suggest that HexaBody-DR5/DR5 benefits from effects of prior antimyeloma agents or in combination with other antimyeloma agents.
Data sharing requests should be sent to Tuna Mutis (t.mutis@amsterdamumc.nl).
Acknowledgments
The authors thank Henk Lokhorst for contributions in the early stage of the study and Klaas de Lint for assistance with the Incucyte experiments.
This work was supported by research funding from Genmab.
Authorship
Contribution: H.J.v.d.H. and A.T.G. performed experiments; H.J.v.d.H., A.T.G., M.E.D.C., E.C.W.B., S.Z., I.S.N., M.B.O., and T.M., designed experiments and discussed results; H.J.v.d.H. wrote the manuscript; and all authors critically revised and modified the manuscript and approved the final version.
Conflict-of-interest disclosure: E.C.W.B. and M.B.O. are employees of Genmab and own Genmab warrants and/or stock. H.J.H., M.E.D.C., E.C.W.B., M.B.O., and T.M. are inventors on Genmab patent applications. M.E.D.C. has received research support from Gilead Sciences, Genmab, and Celgene. S.Z. has received research support from Celgene, Janssen Pharmaceuticals, and Takeda and serves on advisory boards for Celgene, Janssen Pharmaceuticals, Takeda, Amgen, and Sanofi. T.M. has received research support from Janssen Pharmaceuticals, Genmab, Takeda, Onkimmune, and Gadeta. The remaining authors declare no competing financial interests.
Correspondence: Tuna Mutis, Department of Hematology, Cancer Center Amsterdam, Amsterdam UMC, Location VUmc, Boelelaan 1177, 1081 HV Amsterdam, The Netherlands; e-mail: t.mutis@amsterdamumc.nl.
References
Author notes
The full-text version of this article contains a data supplement.

![HexaBody-DR5/DR5 mediates extrinsic apoptotic pathway activation and induces low levels of ADCC in MM cell lines. (A) Representative dose-response curve of HexaBody-DR5/DR5 in RPMI8226 (left panel), NCI-H929 (middle panel), and MM.1s (right panel) cell lines after a 24-hour incubation compared with IgG1-Ctrl (20 µg/mL) (n = 3 or 4). (B) Time-lapse representation of apoptosis induction in NCI-H929 cells by HexaBody-DR5/DR5 and IgG1-Ctrl (20 µg/mL). NCI-H929 cells were labeled with caspase-3 green label, and images were taken using Incucyte for 24 hours. Each point represents the average total green object area per image of 3 replicates. (C) Expression of extrinsic apoptotic pathway–related (cleaved) proteins for RPMI8226, NCI-H929, and MM.1s cell lines, as detected by western blot after a 6-hour incubation with HexaBody-DR5/DR5 (1 µg/mL [Hx1] and 4 µg/mL [Hx4]). Actin was incorporated as loading control. A representative image is shown of 2 independent experiments. (D) Kill (%) of RPMI8226, NCI-H929, and MM.1s cell lines with HexaBody-DR5/DR5 (20 µg/mL) after a 24-hour incubation in the presence of healthy donor PBMCs in a 40:1 effector-to-target ratio. Each point represents a single donor. Data are shown as mean and SD. **P = .009, paired Student t test. (E) Kill (%) of NCI-H929 (left panel) and MM.1s (right panel) cell lines by HexaBody-DR5/DR5 and IgG1-Ctrl in the absence of PBMCs, as well as in the presence of paraformaldehyde-fixed or nonfixed PBMCs in a 40:1 effector-to-target ratio. IgG1-DR5/DR5 and the anti-CD38 antibody daratumumab (Dara) are incorporated to control for cytotoxicity that is dependent on FcγR-mediated crosslinking and cytotoxicity that is dependent on ADCC, respectively. Data are shown as mean and standard deviation of 2 (NCI-H929) or 3 (MM.1s) independent experiments. *P < .05, **P < .01, 1-way analysis of variance and Tukey’s multiple-comparisons test.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/8/10.1182_bloodadvances.2020003731/1/m_advancesadv2020003731f4.png?Expires=1769496898&Signature=lpvbrZFQn015hS0UMMwzFyWujkU4mLzh6ioZknBwrdH02Tdeo5Ric1TVyXeHs17uGhlag50cTIAAaoqzhAgcXlWucwXfBLAIaTHWblgN5NsISbKI2gxzyu97UTNbB4t-5cxT59XlAhQGU0DhcV2XeNoNCdZvTa9vthv0RCfVyz20RoJ77Jv6ZNvWGo-vwMdL~FLFDprMztHXcHTCdGbFZn3vjLTcUehPI9kWay2ajECb3VYyrREls7H0~IHKnh1bsEegmOl3P1OxPM9Ji-xst45QpUNmrq5ScLFN7o-jhtlvs~RMt~qgYFFa10UUhhQvrw1mQzQmLfyA2e~IcY--Ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
