Key Points
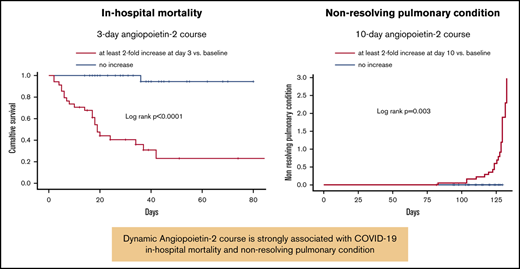
Three-day change in angiopoietin-2 levels predicts COVID-19 in-hospital mortality, whereas the 10-day trend is associated with chronic lung disability.
Angiopoietin-2 may play an important pathogenic role in patients with COVID-19, and it could be a target for new treatments.
Abstract
This study examined the association between dynamic angiopoietin-2 assessment and COVID-19 short- and long-term clinical course. We included consecutive hospitalized patients from 1 February to 31 May 2020 with laboratory-confirmed COVID-19 from 2 Italian tertiary referral centers (derivation cohort, n = 187 patients; validation cohort, n = 62 patients). Serum biomarker levels were measured by sandwich enzyme-linked immunosorbent assay. Lung tissue from 9 patients was stained for angiopoietin-2, Tie2, CD68, and CD34. Cox model was used to identify risk factors for mortality and nonresolving pulmonary condition. Area under the receiver operating characteristic curve (AUROC) was used to assess the accuracy of 3- and 10-day angiopoietin-2 for in-hospital mortality and nonresolving pulmonary condition, respectively. Three-day angiopoietin-2 increase of at least twofold from baseline was significantly associated with in-hospital mortality by multivariate analysis (hazard ratio [HR], 6.69; 95% confidence interval [CI], 1.85-24.19; P = .004) with AUROC = 0.845 (95% CI, 0.725-0.940). Ten-day angiopoietin-2 of at least twofold from baseline was instead significantly associated with nonresolving pulmonary condition by multivariate analysis (HR, 5.33; 95% CI, 1.34-11.77; P ≤ .0001) with AUROC = 0.969 (95% CI, 0.919-1.000). Patients with persistent elevation of 10-day angiopoietin-2 levels showed severe reticular interstitial thickening and fibrous changes on follow-up computed tomography scans. Angiopoietin-2 and Tie2 were diffusely colocalized in small-vessel endothelia and alveolar new vessels and macrophages. Angiopoietin-2 course is strongly associated with COVID-19 in-hospital mortality and nonresolving pulmonary condition. Angiopoietin-2 may be an early and useful predictor of COVID-19 clinical course, and it could be a relevant part of disease pathogenesis. Angiopoietin-2 blockade may be a COVID-19 treatment option.
Introduction
Pneumonia associated with SARS-CoV-2 is a life-threatening condition characterized by diffuse alveolar damage, with the formation of hyaline membranes and interstitial thickening. Histologic autopsy data from the severe acute respiratory syndrome epidemic in 2003 showed varying degrees of exudative- and proliferative-phase acute lung injury, as indicated by the presence of edema, inflammatory infiltrates, pneumocyte hyperplasia, and fibrinous exudates. Presentations had in common the presence of vascular fibrin thrombi, often associated with pulmonary infarcts, and vascular endothelial damage in small and medium pulmonary vessels.1
Recently, Ackermann et al2 reported similar pulmonary findings from an autopsy series of 7 patients who died of COVID-19 pneumonia. Apart from the expected pathologic findings, the authors reported severe endothelial injury associated with the intracellular presence of the virus, with intravascular fibrin deposition, edema, and red blood cell extravasation in areas of diffuse acute alveolar damage.2 These aspects are shared with acute respiratory distress syndrome (ARDS), a highly fatal condition that is associated in many cases with severe virus- and sepsis-associated pneumonia.
Angiopoietins are endothelium-derived angiogenic factors with potent effects on the vascular endothelium.3,4 The binding of angiopoietin-2 to its tyrosine kinase with immunoglobulin-like and Epidermal Growth Factor–like domains 1 (Tie2) receptor is associated with impairment of vessel integrity, and it enhances endothelial inflammation.5,6 Interestingly, the angiopoietin-2/1 ratio and circulating levels of angiopoietin-2 have been shown to be closely related to prognosis of patients with ARDS.7
The purpose of this study was to correlate the dynamic course of angiopoietin-2 with in-hospital mortality and nonresolving pulmonary condition in COVID-19. The hypothesis was that the rapid increase in circulating angiopoietin-2 levels could be associated with in-hospital mortality and that its persistent elevation could be a risk factor for chronic lung function disability.
Methods
Patients
The demographic, clinical, and biochemical characteristic of all consecutive patients admitted with COVID-19 to the Units of Medicine and Intensive Care of the Ospedale Civile Baggiovara and Policlinico, AOU Modena, Modena, Italy, between 1 March and 31 May 2020 (derivation cohort), were studied. COVID-19 infection was confirmed by nasopharyngeal swab reverse-transcriptase polymerase chain reaction assay positivity. Depending on their clinical conditions, patients were admitted to the internal medicine (n = 71), subintensive care (n = 70), or intensive care unit (n = 46). Patients showing clinical deterioration (ie, ARDS onset, defined as acute-onset hypoxemia [partial pressure of arterial blood oxygen (PaO2)/fraction of inspired air consisting of oxygen (FiO2) ratio < 300]) with bilateral pulmonary opacities evident on chest imaging were transferred to the subintensive or intensive care unit. Coexisting conditions (eg, chronic obstructive pulmonary disease, history of hypertension, diabetes mellitus, hyperlipidemia, underlying cardiovascular disease, immunologic or immunosuppressed condition) were identified from existing physician documentation. The patients were treated according to the standard of care at the time of admission (ie, hydroxychloroquine, with enoxaparin and/or tocilizumab in some subgroups). After discharge, patients were followed up for clinical and biochemical evaluation on a monthly basis or more frequently depending on clinical conditions. Nonresolving pulmonary condition was defined as PaO2/FiO2 ratio lower than 300 at months 1 and 3 of follow-up and/or abnormal chest computed tomography (CT) scan.
A consecutive series of patients admitted to the Division of Infectious Diseases I, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, between 1 March and 15 May 2020, was used as an external cohort for validation of the prognostic scores. The same demographic, clinical, and biochemical characteristics as in the derivation cohort were collected.
The study protocol was approved by the Area Vasta Emilia Nord ethics committee (535/2020/OSS/AOUMO).
Data collection and analysis
Blood samples were prospectively obtained daily for the first 3 days after admission and every second day thereafter. White blood cell counts and D-dimer, C-reactive protein (CRP), serum creatinine, bilirubin, aminotransferase, and lactate dehydrogenase levels from patients of the derivation and validation cohorts were measured using routine techniques at admission and subsequently, as indicated by their clinical conditions. In cases of clinical deterioration and transfer to the intensive care unit, daily blood sampling was resumed.
Sandwich enzyme-linked immunosorbent assay.
The angiopoietin-1, angiopoietin-2, Tie2, Endoglin, von Willebrand factor (vWF), and interleukin (IL)-6 levels were measured in serum samples by sandwich enzyme-linked immunosorbent assay using the respective kits’ reagents and protocols (R&D Systems, Minneapolis, MN). The baseline levels were evaluated at the first day of admission in each patient. The dynamic course of angiopoietin-2 and IL-6 levels after admission (3-day course) was categorized at 3 days as an increase of at least twofold from baseline throughout the first 3 days or no increase. The dynamic course of angiopoietin-2 and IL-6 levels at 10 days after admission (10-day course) was categorized as an increase of at least twofold from baseline maintained throughout the 10-day period or no increase.
Immunohistochemistry.
Formalin-fixed paraffin-embedded samples from postmortem lung tissue from 9 patients were subjected to routine histologic and immunohistochemical analysis for angiopoietin-2, Tie2, CD68, and CD34. Briefly, after deparaffinization and rehydration, antigen unmasking was performed with 1 mM EDTA buffer, pH 8, at 98°C for 15 minutes. The sections were then incubated in methanol 5% and H2O2 1% for 5 minutes for blocking endogenous peroxidases; nonspecific sites were blocked using a blocking solution reagent with bovine serum albumin 3% for 30 minutes at room temperature. Sections were then incubated with goat anti-ANGPT2 (AF623; R&D Systems) primary antibody at working dilution of 1:50, or with goat anti-Tie2 (AF313; R&D Systems) primary antibody at working dilution of 1:150, both at 4°C overnight in humidity chamber, or with prediluted CD34 (clone QBEnd-10; Roche-Ventana) or CD68 (clone PG-M1; Roche-Ventana) primary antibodies. Sections were then incubated with prediluted OmniMap anti-goat horseradish peroxidase conjugated secondary antibody (Ventana Medical Systems, Tucson, AZ), for 20 minutes in humidity chamber and then with detection kit reagents (ultra-view universal horseradish peroxidase multimer and diaminobenzidine [DAB] chromogen, Ventana Medical Systems) following the manufacturer’s instructions. The sections were then counterstained with hematoxylin, dehydrated, and permanently mounted for microscopic examination. Images of stained liver tissue were processed with ImageJ software (http://rsbweb.nih.gov/) to obtain the intensity value of DAB signal.
To verify an ANGPT2 and Tie2 colocalization, we merged 2 consecutive sections stained, respectively, with antibody goat anti-ANGPT2 (AF623) and goat anti-Tie2 (AF313) after artificial conversion of brown DAB staining. Briefly, for each section, we acquired the images of DAB immunostaining on the same region of interest at the same magnifications (20× and 63×). The brown DAB signal was converted with Adobe Photoshop into a different color to subsequently perform a merge (ANGPT2 signal into blue color, Tie2 signal into red color).
Imaging.
All chest CT scans were performed during single full inspiratory breath hold in the supine position on a 128-slice CT scanner (GE Lightspeed VC, GE Healthcare). The technical average parameters for the scan were as follows: tube voltage, 120 kV; slice thickness, 2.5 mm; collimation width, 1.25. The scanner automatically adjusted the tube current modulation based on the scout image to administer an adequate dose amount. Nonresolving pulmonary condition was defined as pO2/FiO2 ratio lower than 300 at 1 and 3 months of follow-up and/or chest CT scan demonstrating persistence of abnormal lung appearance, such as airspace opacity and/or presence of reticular shadows.
Statistical analysis
Dichotomous and continuous variables were compared using Fisher’s exact test and the nonparametric Mann-Whitney U test, respectively. Strength of the association between circulating biomarkers was determined by the Pearson correlation coefficient. The Kaplan-Meier method was used to estimate the cumulative probability of overall survival. Patient data were censored at the time of death, discharge, or the end of the study period (31 May 2020). Differences in observed probability were assessed using the log-rank test.
The Cox proportional hazards model was used to identify risk factors for in-hospital mortality and nonresolving pulmonary condition. Candidate risk factors for in-hospital mortality were sex, age, presence of comorbidities, and treatment applied; PaO2/FiO2 ratio and international normalized ratio; platelet count; bilirubin, CRP, and D-dimer levels; and angiopoietin-2, endoglin, Tie2, and IL-6 3-day course. For nonresolving pulmonary condition, a 3-day course of angiopoietin-2 and IL-6 was substituted with a 10-day course. Variables with P ≤ .10 in the univariate analyses were included in the final multivariate model.
The accuracy of risk factors for in-hospital mortality and chronic lung disability, respectively, was assessed by the area under the receiver operating characteristic curve (AUROC). Validation was performed by comparing the AUROC of the derivation and validation cohorts.
Predictive Analytics SoftWare statistics (version 26; IBM Corporation, Armonk, NY) were used for the statistical analyses. P < .05 was considered to indicate statistical significance.
Results
Demographic, clinical, and imaging characteristics
The derivation and validation cohorts comprised 187 and 62 patients, respectively. Their demographic and clinical characteristics are shown in supplemental Table 1.
The only difference observed between the 2 cohorts was in the therapeutic regimen administered. For all but 4 (2.1%) patients of the derivation cohort who received only supportive care, treatment was started at admission. All patients received hydroxychloroquine as a single agent in 28 (15.0%) patients or in association with other agents in the remaining patients: with enoxaparin in 75 (40.1%) patients, with enoxaparin and tocilizumab in 55 (29.4%) patients, and with darunavir/cobicistat in 15 (8.0%) patients. Ten patients (5.34%) received intravenous steroids only (supplemental Table 1). Thirty-two (17.1%) patients also received intravenous steroids (associated with only hydroxychloroquine in 6 [3.2%] patients, with hydroxychloroquine and enoxaparin in 9 [4.8%] patients, with hydroxychloroquine, enoxaparin, and tocilizumab in 7 [3.7%] patients).
Fifty-four (28.9%) patients in the derivation cohort underwent CT examination at admission. Twenty-eight (51.8%) patients underwent follow-up CT examinations because of nonresolution of respiratory conditions or suspicion of thromboembolism or when clinically indicated.
In-hospital mortality
As of 31 May 2020, 53 patients (28.3%) in the derivation cohort and 18 patients (29.0%) in the validation cohort died.
The baseline levels of circulating angiopoietin-1, angiopoietin-2, Tie2, vWF, endoglin, and IL-6 did not significantly differ between COVID-19 survivors and nonsurvivors both in the derivation and validation cohorts (Table 1). By contrast, the angiopoietin-2/angiopoietin-1 ratio differed significantly (P = .007) between survivors (0.084 ± 0.015) and nonsurvivors (0.383 ± 0.210) both in the derivation and validation cohorts (survivors: 0.159 ± 0.128; nonsurvivors: 0.298 ± 0.260, P = .040). In the derivation cohort, the angiopoietin-2/angiopoietin-1 ratio differed significantly (P = .007) between survivors (0.084 ± 0.015) and nonsurvivors (0.383 ± 0.210). Similar results were observed in the validation cohorts (survivors: 0.159 ± 0.128; nonsurvivors: 0.298 ± 0.260, P = .040). In the derivation cohort, angiopoietin-2 (–0.398 [P = .001]) and Tie2 (–0.299 [P = .041]) were significantly related to the PaO2/FiO2 ratio, whereas IL-6 levels (–0.199 [P = .626]) and angiopoietin-1 (–0.137 [P = .705) did not. The circulating angiopoietin-2, but not angiopoietin-1, level was strongly related to the circulating Tie2 level (derivation cohort: r = .424, P = .028; validation cohort: r = .294; P = .026). Hematocrit levels were not related with circulating angiopoietin-2. However, patients who had increased angiopoietin-2 at day 3 had significantly higher hematocrit than those with nonincreased angiopoietin-2 (41.2% vs 38.6%, respectively; P = .029).
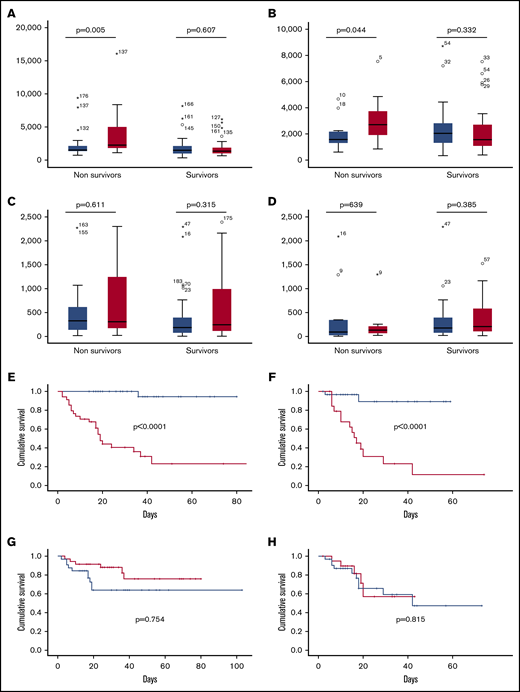
Among nonsurvivors, but not among survivors, the 3-day levels of angiopoietin-2 were significantly higher than 1-day levels in both derivation and validation cohorts, while IL-6 level was not significantly different between survivors and nonsurvivors in both the derivation and validation cohorts (Figure 1A-D). By contrast, the 3-day course of angiopoietin-2, but not the 3-day course of IL-6, was significantly associated with in-hospital mortality both in the derivation and validation cohort (Figure 1E-H).
Relationship between angiopoietin-2 and IL-6 and in-hospital mortality in the derivation and validation cohorts in patients with COVID-19. Day 1 (blue columns) and day 3 (red columns) angiopoietin-2 levels according to survivors and nonsurvivors in the derivation (A) and validation (B) cohorts. Day 1 (blue columns) and day 3 (red columns) IL-6 levels according to survivors and nonsurvivors in the derivation (C) and validation (D) cohorts. In-hospital mortality according to 3-day course of angiopoietin-2 in the derivation (E) and validation (F) cohorts. In-hospital mortality according to 3-day course of IL-6 in the derivation (G) and validation (H) cohorts. Red line, increase of at least twofold from baseline; blue line, no increase.
Relationship between angiopoietin-2 and IL-6 and in-hospital mortality in the derivation and validation cohorts in patients with COVID-19. Day 1 (blue columns) and day 3 (red columns) angiopoietin-2 levels according to survivors and nonsurvivors in the derivation (A) and validation (B) cohorts. Day 1 (blue columns) and day 3 (red columns) IL-6 levels according to survivors and nonsurvivors in the derivation (C) and validation (D) cohorts. In-hospital mortality according to 3-day course of angiopoietin-2 in the derivation (E) and validation (F) cohorts. In-hospital mortality according to 3-day course of IL-6 in the derivation (G) and validation (H) cohorts. Red line, increase of at least twofold from baseline; blue line, no increase.
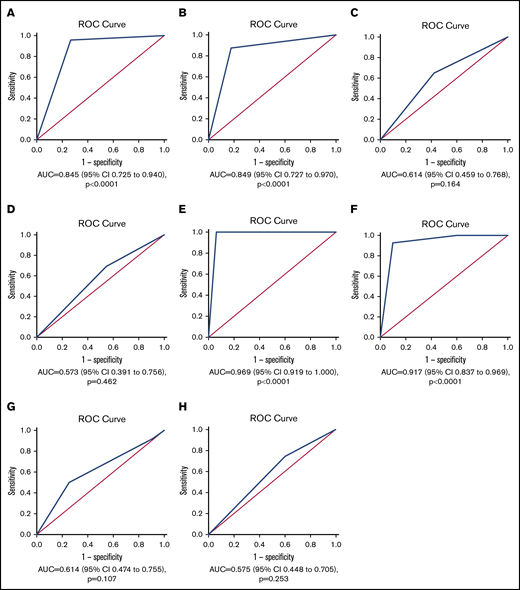
The 3-day angiopoietin-2 course showed excellent discrimination for in-hospital mortality (derivation cohort: AUROC = 0.845; 95% confidence interval [CI], 0.725-0.940; validation cohort: area under the curve [AUC] = 0.949; 95% CI, 0.727-0.970; Figure 2A-B). Angiopoietin-2/angiopoietin-1 ratio displayed lower accuracy than the 3-day angiopoietin-2 course for in-hospital mortality (AUC = 0.660; 95% CI, 0.489-0.831). The accuracy of the 3-day IL-6 course for in-hospital mortality was also low (AUC = 0.504; 95% CI, 0.355-0.652; Figure 2C-D, derivation and validation cohorts, respectively).
ROC curves of angiopoietin-2 and IL-6 courses for in-hospital mortality and nonresolving pulmonary condition in patients with COVID-19. Three-day course for in-hospital mortality of angiopoietin-2 in the (A) derivation and (B) validation cohorts. Three-day course for in-hospital mortality of IL-6 in the (C) derivation and (D) validation cohorts. Ten-day course for nonresolving pulmonary condition of angiopoietin-2 in the (E) derivation and (F) validation cohorts. Ten-day course for nonresolving pulmonary condition of IL-6 in the (G) derivation and (H) validation cohorts.
ROC curves of angiopoietin-2 and IL-6 courses for in-hospital mortality and nonresolving pulmonary condition in patients with COVID-19. Three-day course for in-hospital mortality of angiopoietin-2 in the (A) derivation and (B) validation cohorts. Three-day course for in-hospital mortality of IL-6 in the (C) derivation and (D) validation cohorts. Ten-day course for nonresolving pulmonary condition of angiopoietin-2 in the (E) derivation and (F) validation cohorts. Ten-day course for nonresolving pulmonary condition of IL-6 in the (G) derivation and (H) validation cohorts.
Univariate Cox regression analysis identified older age, use of combined therapies (data not shown), lower platelet levels, and the 3-day angiopoietin-2 course as risk factors for in-hospital mortality. In the derivation cohort, 3-day angiopoietin-2 course (hazard ratio [HR], 6.692; 95% CI, 1.852-24.188; P = .004) and older age (HR, 2.991; 95% CI, 1.052-8.500; P = .040) were independent risk factors for in-hospital mortality by multivariate analysis. In the validation cohort, 3-day angiopoietin-2 course (HR, 4.447; 95% CI, 1.345-13.777; P = .010) was the only independent risk factor for in-hospital mortality (Table 2).
Nonresolving pulmonary condition
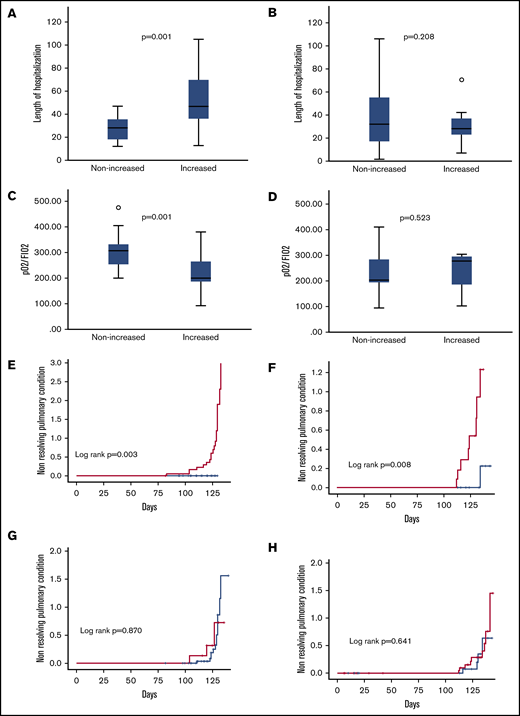
Twenty patients (10.7% of the derivation cohort) showed evidence of chronic lung disability after a median follow-up of 3 months. All patients fulfilled the criteria defined in the Methods section for nonresolving pulmonary condition (ie, PaO2/FiO2 < 300, which was associated in 15 patients with radiologic abnormalities). Three of them died 1, 2.5, and 3 months after discharge. Nineteen of these 20 patients (95.0%) had increased angiopoietin-2 levels at 10-day evaluation. All these patients also had increased angiopoietin-2 at day 3. Patients with elevated 10-day angiopoietin-2 levels had significantly longer duration of hospitalization than those who had not (P = .013; Figure 3A). IL-6 course was not equally predictive (Figure 3B). The 10-day angiopoietin-2 course was also significantly associated with a lower pulmonary function assessed by PaO2/FiO2 at 1 month (233 ± 70 mm Hg in patients with stably increased 10-day angiopoietin-2 levels vs 335 ± 100 mm Hg in patients without increased levels; P = .001; Figure 3C).
Outcomes according to 10-day angiopoietin-2 and IL-6 course. Length of hospitalization according to 10-day angiopoietin-2 (A) and IL-6 (B) courses. PaO2/FiO2 ratio levels at 1 month according to 10-day angopoietin-2 (C) and IL-6 (D) courses. Cumulative incidence of nonresolving pulmonary condition according to 10-day angiopoietin-2 course in derivation (E) and validation (F) cohorts. Cumulative incidence of nonresolving pulmonary condition according to 10-day IL-6 course in derivation (G) and validation (H) cohorts. Red line, increase of at least twofold from baseline; blue line, no increase.
Outcomes according to 10-day angiopoietin-2 and IL-6 course. Length of hospitalization according to 10-day angiopoietin-2 (A) and IL-6 (B) courses. PaO2/FiO2 ratio levels at 1 month according to 10-day angopoietin-2 (C) and IL-6 (D) courses. Cumulative incidence of nonresolving pulmonary condition according to 10-day angiopoietin-2 course in derivation (E) and validation (F) cohorts. Cumulative incidence of nonresolving pulmonary condition according to 10-day IL-6 course in derivation (G) and validation (H) cohorts. Red line, increase of at least twofold from baseline; blue line, no increase.
All patients but 2 with nonresolving pulmonary condition had a plain lung x-ray, and 10 (75.0%) also had follow-up CT scans after a median follow-up time of 10 weeks from admission. Patients with follow-up shorter than 10 weeks (n = 9) showed persistence of interstitial pneumonia and of ground glass opacity, whereas those with longer follow-up (n = 9) showed initial thickening of the interlobular and intralobular septa, progressive reticular interstitial thickening, and fibrous changes (P = .020, χ2 test; supplemental Figure 1).
The 10-day angiopoietin-2 course was significantly associated with a greater probability of developing chronic lung disability (derivation cohort: P = .003; Figure 3E; validation cohort: P = .008; Figure 3F), whereas IL-6 did not (P = .870; Figure 3G).
AUROC revealed that the 10-day angiopoietin-2 course but not IL-6 had an excellent discriminative ability for nonresolving pulmonary condition (derivation cohort: AUC = 0.969; 95% CI, 0.919-1.000; validation cohort: AUC = 0.917; 95% CI, 0.837-0.969; Figure 2D-F).
Univariate Cox analysis also revealed that the use of combined therapies, median baseline PaO2/FiO2 ratio, and 10-day angiopoietin-2 course were associated with nonresolving pulmonary condition (Table 3). In both derivation (HR, 6.861; 95% CI, 1.342-11.769; P = .001) and validation cohorts (HR, 3.085; 95% CI, 1.138-8.363; P = .027), 10-day angiopoietin-2 course was the only independent risk factor for nonresolving pulmonary condition by multivariate analysis (Table 3).
Pulmonary histologic and immunohistochemical findings
The histologic features of lung tissues from 9 patients with COVID-19 were consistent with various stages of diffuse alveolar disease, depending on the time since disease onset. In all patients, the damage (exudative and/or reparative) involved 50% to 100% of both lungs. Morphologic alterations observed in the acute exudative phase were edema, hemorrhage, and hyaline membrane development in alveolar spaces. In the subacute proliferative phase, the predominant feature consisted of multiple foci of fibroblastic proliferation with many thin bands of fibrotic tissue. In the advanced proliferative phase, areas of dense fibrosis were observed (Figure 4A).
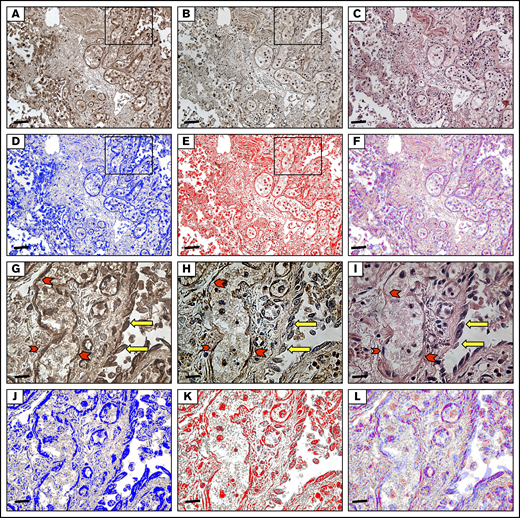
Immunohistochemical analysis of autoptic lung tissue from a patient with COVID-19. (A) Immunostaining for angiopoietin-2 (20×): immunohistochemistry shows immunoreactivity in the endothelia, pneumocytes, and macrophages. Scale bar, 6.7 μm. (B) Immunostaining for Tie2 (20×): the same field as in Figure 1. Tie2 showed the same immunoreactivity observed for angiopoietin-2 immunoreaction. (C) Hematoxylin and eosin staining (20×): diffuse alveolar damage in COVID-19, interstitial inflammation, and dilated capillaries in the alveolar wall. The alveolar spaces show fibrin and macrophages. (D-F) The brown DAB signal for angiopoietin-2 shown in panel A was converted into a blue color and that for Tie2 shown in panel B was converted into a red color. The images in panels D and E were merged, resulting in a violet color, where the angiopoietin-2 and Tie2 colocalize. (G-I) Higher magnification (63×) of the inset in panel A highlights angiopoietin2 and tie2 immunoreactivity both in endothelia (red arrowheads) and in pneumocytes (yellow arrows). Scale bar, 1.7 μm. (J-L) The brown DAB signal for angiopoietin-2 shown in panel G was converted into a blue color and that for Tie2 shown in panel H was converted into a red color. The images in panels J and K were merged, resulting in a violet color, where the angiopoietin-2 and Tie2 colocalize.
Immunohistochemical analysis of autoptic lung tissue from a patient with COVID-19. (A) Immunostaining for angiopoietin-2 (20×): immunohistochemistry shows immunoreactivity in the endothelia, pneumocytes, and macrophages. Scale bar, 6.7 μm. (B) Immunostaining for Tie2 (20×): the same field as in Figure 1. Tie2 showed the same immunoreactivity observed for angiopoietin-2 immunoreaction. (C) Hematoxylin and eosin staining (20×): diffuse alveolar damage in COVID-19, interstitial inflammation, and dilated capillaries in the alveolar wall. The alveolar spaces show fibrin and macrophages. (D-F) The brown DAB signal for angiopoietin-2 shown in panel A was converted into a blue color and that for Tie2 shown in panel B was converted into a red color. The images in panels D and E were merged, resulting in a violet color, where the angiopoietin-2 and Tie2 colocalize. (G-I) Higher magnification (63×) of the inset in panel A highlights angiopoietin2 and tie2 immunoreactivity both in endothelia (red arrowheads) and in pneumocytes (yellow arrows). Scale bar, 1.7 μm. (J-L) The brown DAB signal for angiopoietin-2 shown in panel G was converted into a blue color and that for Tie2 shown in panel H was converted into a red color. The images in panels J and K were merged, resulting in a violet color, where the angiopoietin-2 and Tie2 colocalize.
Staining indicated the diffuse presence of angiopoietin-2 and Tie2 in the endothelia of small vessels in portions of lung with less severe disease, in newly formed vessels in the alveolar walls (immunoreactivity was stronger for Tie2), in pericytes, and in macrophages inside alveolar spaces (supplemental Figure 2). Desquamating alveolar cells did not show the same unequivocal immunoreactivity (Figure 4B). Angiopoietin-2 and Tie2 colocalized in all these structures (Figure 4B-C). CD68-positive activated macrophages surrounding the altered alveola, and the abundant CD34-positive new vessels in the alveolar wall were also reactive for angiopoietin-2 and Tie2 (Figure 4D).
Discussion
In a cohort of Caucasian patients with COVID-19, we demonstrated that circulating 3- and 10-day angiopoietin-2 courses can early and accurately assess the risk for in-hospital mortality and nonresolving pulmonary condition, respectively. To the best of our knowledge, this is the first study demonstrating that a single simple biomarker (ie, 10-day angiopoietin-2 course) is able to predict chronic lung disability. The robustness of these results was confirmed in an external validation cohort. Circulating angiopoietin-2 course and older age were the only 2 independent risk factors for in-hospital death, as shown by multivariate analysis. External validation of this model showed good accuracy. From a clinical point of view, 3-day angiopoietin-2 course could help physicians to improve the allocation of medical resources, potentially reducing the overcrowding that we have witnessed in health care systems, which significantly impacted mortality worldwide during the COVID-19 pandemic. Although baseline angiopoietin-2 level has been shown to predict mortality in patients with non–COVID-19-ARDS7 and intensive care unit admission in patients with COVID-19,8 we did not find such association at baseline among patients with COVID-19, likely because the baseline value resents the individual heterogeneity of patients at presentation. We did find, however, that the dynamic 3-day angiopoietin-2 course, blunting the heterogeneity of the stage of disease at admission, was an early, strong, and independent risk factor for COVID-19 mortality. Interestingly, Pine et al9 provided further evidence that angiopoietin-2 levels at intensive care unit admission were elevated in patients with critical COVID-19, strongly predicting in-hospital mortality. All these data may prove useful to guide novel therapeutic strategies, including those that protect the endothelium.
Results of our analyses confirmed previous reports from China and the United States,10-12 showing older age as a significant risk factor for in-hospital death in COVID-19. However, in-hospital mortality in our study was higher than that observed in other studies conducted both within and outside China.10,11 A possible reason could be because of the very high proportion of elderly people in Italy, the highest in Europe.13 Not surprisingly, the median age in our cohort was 69 years, which is higher than that observed in other studies.
The 10-day angiopoietin-2 course showed a similarly strong predictive power for chronic lung disability. The predictive power of angiopoietin-2 for in-hospital mortality and chronic lung disability is not surprising, given that modifications in the angiopoietin-2 level may be a relevant part of disease pathogenesis. Recently, Ackermann et al2 showed that pulmonary vascular endothelialitis and angiogenesis are distinctive COVID-19 features, with affected patients showing almost 3 times more new vessel growth in the lungs than do patients with influenza. Our findings reflect maximal activation of the angiopoietin-2/Tie2 system in patients with COVID-19, as shown by extremely high circulating levels and hyperintense staining in the lungs, with constant colocalization with CD34 demonstrating the hyperactivation of neoangiogenesis.
The angiopoietin-1/angiopoietin-2/Tie2 system is known to be extremely important in physiologic and pathologic blood vessel formation and angiogenesis.14 Several groups have evaluated endothelial damage in ARDS, identifying the angiopoietin-Tie2 pathway as a prognostic factor.7,15,16 Angiopoietins are endothelium-derived angiogenic factors with potent effects on the vascular endothelium.3,4 They interact with their receptor, Tie2, which is expressed on the luminal endothelium. Angiopoietin-1 and angiopoietin-2 are antagonist ligands that bind with similar affinity to Tie2. Angiopoietin-1/Tie2 signaling maintains vessel integrity, inhibits vascular leakage, and suppresses inflammatory gene expression.3,4 The binding of angiopoietin-2 to Tie2 interferes with this protective role and facilitates endothelial inflammation.5,6 Endothelial damage associated with inflammation and hypoxia stimulates the exocytosis of endothelial Weibel-Palade bodies and the rapid release of angiopoietin-2.5,17 High angiopoietin-2 levels directly activate Tie2, enhancing endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway.18 Tie2 is expressed almost exclusively by endothelial cells, with the greatest expression in the lung and liver under normal conditions.19 In pathologic conditions, especially those with inflammatory backgrounds (as in the experimental asthma model established with ovalbumin-sensitized and ovalbumin-challenged mice),14 Tie2 is expressed abundantly in airway epithelial cells and in a subset of macrophages in addition to its constitutive expression in pulmonary vessels. Consistently, we observed enhanced Tie2 expression not only in pulmonary vessels, but also in the bronchial epithelial cells and macrophages, in patients with COVID-19. This expression was associated with strong angiopoietin-2 expression in the lungs, extremely high circulating angiopoietin-2 levels, and significantly increased angiopoietin-2/1 ratios. This combination of factors has been associated with extensive airway remodeling, including epithelial cell shedding, subepithelial fibrosis, smooth muscle cell hyperplasia, edema, and angiogenesis,14 and appears to form the pathologic basis for acutely progressive respiratory distress in patients with COVID-19. Further evidence supported by our observations is that patients with persistently elevated angiopoietin-2 and Tie2 levels rapidly developed a nonresolving pulmonary condition, as reflected by significantly lower follow-up PaO2/FiO2 ratios and follow-up CT findings in comparison with recovering patients with normalized angiopoietin-2 levels.
The slope of circulating angiopoietin-2 level elevation is of particular relevance. Angiopoietin-2 is stored in the Weibel-Palade bodies in the cytoplasm of endothelial cells. It can be released within minutes after stimulation, as in rapid vascular homeostatic reactions triggered by inflammation and coagulation.5,17 In the chain of events after angiopoietin-2 release, neoangiogenesis is a key factor. This neoangiogenic reaction, in turn, increases inflammation, starting a vicious cycle of angiogenesis–inflammation–angiogenesis associated with the formation of defective capillaries, susceptibility to microthrombosis, and increased vascular permeability. Consistently, Parikh et al20 identified in non–COVID-19 patients excess serum angiopoietin-2 as a critical factor favoring the disruption of pulmonary endothelial function and determining congestion and pulmonary vascular leakage, which in turn facilitates the recruitment of immune cells to the lung, where they participate in the development and persistence of cytokine storms.
All the evidence suggests that angiopoietin-2 blockade is an important therapeutic option, as the drugs currently used for COVID-19 achieve low survival benefit. Although this treatment approach has not been examined in a human study, many methods, including RNA aptamers,21,22 blocking antibodies and peptides,23 and angiopoieitin-1 administration24 have been used to block angiopoietin-2 in animal models. More recently, Lomas-Neira et al25 used the small interfering RNA inhibition of angiopoietin-2 protein synthesis to block its activity in an experimental mouse model of ARDS. They observed significant improvements in the PaO2/FiO2 ratio and inflammation biomarkers, improving 10-day survival. Several selective angiopoietin-2 inhibitors are under evaluation in the oncologic setting (NCT03239145, NCT01004822, NCT01248949), and an antibody against angiopoietin-2 is already under evaluation in a phase 2 randomized controlled trial in patients hospitalized with COVID-19 pneumonia, who are at higher risk for progression to ARDS.26 Their demonstrated safety and efficacy in the inhibition of angiopoietin-2 activity warrant the exploration of their efficacy for COVID-19–induced lung damage.
Our study has some limitations. First, there is a lack of a control group with similar non–COVID-19 respiratory conditions. However, it should be underlined that the mechanisms, which we have described, could not be unique to COVID-19, but nevertheless, they could have pathogenetic and therapeutic implications. Second, treatment regimens were different between derivation and validation cohort, with a greater propensity to use enoxaparin in the derivation cohort. This may have resulted in a better survival for these patients in comparison with those treated with hydroxychloroquine alone. Differently from enoxaparin, hydroxychloroquine does not have known effects on endothelial function, whereas the effect of tocilizumab on angiopoietin-2 or other markers of endothelial function is not really known. In this study, the association between different treatment regimens and in-hospital mortality was not confirmed by multivariate analysis, making us confident that differences in treatments have not affected our results. Third, retrospective studies have problems that reduce their internal and external validity and selection bias can lead to incorrect results and spurious associations. However, we included only consecutive patients with confirmed COVID-19; therefore, we are confident that selection bias could not be relevant. Fourth, to overcome the issue of the generalizability of results to different populations and settings, we performed an external validation that showed good discrimination ability, despite the significant difference in the therapeutic approach between derivation and validation cohorts. Finally, a weakness of our study is the relatively small sample size, and further data collected in different clinical settings are needed.
In conclusion, we demonstrated that circulating 3- and 10-day angiopoietin-2 courses can early and accurately assess the risk for in-hospital mortality and chronic lung disability in Caucasian patients with COVID-19. These findings could improve the triage and management of patients with COVID-19 in different health care organization settings. The key role played by angiopoietin-2 in endothelial cell disruption and associated events indicates angiopoietin-2 as an important part of disease pathogenesis and a potential target for new specific COVID-19 treatments.
For original data, please contact the corresponding author at erica.villa@unimore.it.
Acknowledgment
This study was supported by a grant from Regione Emilia-Romagna–Bankitalia.
Authorship
Contribution: E.V. performed research, analyzed data, and wrote the manuscript; G.G., T.T., G.P., E.B., A.d., and R.B. performed research, analyzed data, and revised the manuscript; R.C., A.M., I.C., E.d.S., and T.T. did the assays to measure endothelial biomarkers and cytokines, and analyzed data; S.L., M.R., F.M., and A.d. performed histologic and immunohistochemical evaluation; A.C., D.R., G.M., A.P., L.D.M., D.A., F.T., V.Z., V.B., M.B., F.S., B.L., N.D.M., L.C., C.G., S.M., M.D.B., L.B., G.P., and E.B. collected samples and clinical data collection and revised the manuscript; C. Celsa and C. Cammà did the statistical evaluation and revised the manuscript; and F.C. and P.T. did the radiologic evaluation and revised the manuscript.
Conflict-of-interest disclosure: E.V. has consulted for and received honoraria from Abbvie, MSD, and Gilead. F.S. has received travel reimbursements from Cook. C. Cammà consulted for and received honoraria from Abbvie, MSD, and Gilead. The remaining authors declare no competing financial interests.
Correspondence: Erica Villa, Department of Gastroenterology, University of Modena & Reggio Emilia and Azienda Ospedaliero-Universitaria di Modena, Via del Pozzo 71, 41124 Modena, Italy; e-mail: erica.villa@unimore.it.
References
Author notes
The full-text version of this article contains a data supplement.