Key Points
Variation in initial systemic therapy for chronic graft-versus-host disease includes varied prednisone dose and use of nonsteroid agents.
Prospective trials are needed to verify efficacy of reduced-dose prednisone or prednisone-free initial therapy approaches.
Abstract
Prior clinical trials largely considered prednisone 1 mg/kg per day with or without calcineurin inhibitor as standard initial therapy for chronic graft-versus-host disease (cGVHD), but uncertainty remains regarding the extent of practice variation and whether this affects subsequent outcomes. We assembled a cohort of 745 patients with cGVHD treated with initial systemic immune suppressive (IS) therapy from 3 prior cGVHD Consortium observational studies. Initial therapy was defined as first IS therapy started for cGVHD or prednisone increased to ≥0.4 mg/kg per day from lower doses within 30 days before cGVHD diagnosis to any time afterward. Initial therapies were nonprednisone IS therapies (n = 137, 18%), prednisone alone (n = 411, 55%), or prednisone plus other IS therapy (n = 197, 26%). In multivariate analysis, initial therapy group was not associated with failure-free survival (FFS; a composite of death, relapse, and new IS therapy), overall survival (OS), or nonrelapse mortality (NRM). Among the prednisone-based approaches, steroid dose was <0.25 (9%), 0.25 to 0.74 (36%), 0.75 to 1.25 (42%), or >1.25 mg/kg per day (13%). Prednisone dose within the patients treated with steroids was not significantly associated with FFS, OS, or NRM. No significant interactions were detected between overall cGVHD severity and either initial therapy group or prednisone dose for the outcomes of FFS, OS, or NRM. These observational data document heterogeneity in more contemporary cGVHD initial treatment practices, including prednisone dose and use of nonsteroid approaches. This variation was not associated with FFS, OS, or NRM. Prospective trials are needed to verify efficacy of reduced-dose prednisone or prednisone-free initial therapy approaches.
Introduction
Chronic graft-versus-host disease (cGVHD) is a common complication of allogeneic hematopoietic cell transplantation (HCT)1 and is associated with increased risk of death, morbidity, impaired quality of life, and prolonged immune suppressive (IS) therapy.2-7 Based on evidence arising from prior clinical trials and practice recommendations, initial therapy for cGVHD remains prednisone (generally starting at 1 mg/kg per day) with or without a calcineurin inhibitor. Previous work has demonstrated that this approach fails to provide durable control of cGVHD, as failure-free survival (FFS; a composite failure end point including death, malignancy relapse, or additional systemic IS therapy) after initial steroid therapy is 68% by 6 months and 54% by 12 months.8 Failure of initial therapy is important, because this is commonly followed by multiple lines of secondary therapy with associated adverse outcomes.9,10
One major research question has been whether combination approaches (prednisone plus other systemic IS agents) may provide improved outcomes. Several large randomized trials of combination therapy have been completed, yet none have demonstrated superiority over prednisone alone.11-14 A more recent Blood and Marrow Transplant Clinical Trials Network 0801 trial tested sirolimus/prednisone vs calcineurin inhibitor/sirolimus/prednisone15 and demonstrated comparable treatment success across these 2 approaches and improved quality of life in the 2-drug approach. This trial, published in 2018, has provided a current benchmark for initial therapy. Multiple novel approaches are being tested in smaller trials including other agents, lower-dose prednisone, or even non–prednisone-based therapy.
To date, no large survey of actual treatment practices has been conducted, although individual management of cGVHD initial therapy is anecdotally highly variable and may affect treatment response. The selection of treatment type and intensity may vary according to cGVHD features and severity and perceptions regarding individual patients’ tolerance of certain therapies. To address this gap, we gathered data from 3 prior national cGVHD Consortium observational studies to examine variation in initial cGVHD therapy and its association with subsequent FFS.
Methods
Data sources
For the purpose of this analysis, data pertinent to patients starting their initial therapy for cGVHD were aggregated from 3 separate cGVHD Consortium studies (NCT00637689, NCT01902576, and NCT01206309). It was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. All patients signed informed consent.
The first study was a prospective observational study entitled “Improving Outcomes Assessment in Chronic GVHD.” A total of 9 centers enrolled 601 patients with cGVHD from 2007 to 2012 with 2 years of active follow-up, and then long-term data were collected through a median of 5.4 years after enrollment. Incident cGVHD cases (enrolled within 3 months of cGVHD diagnosis) were studied at enrollment, 3 months, 6 months, and every 6 months thereafter. Prevalent cases (>3 months from cGVHD diagnosis but within 3 years of HCT) from this parent study were excluded from our current analysis of initial cGVHD therapy. Detailed information on cGVHD staging, functional assessments, patient-reported outcomes, medications (systemic and topical agents used for cGVHD therapy), and research samples were collected. Medication data on cGVHD treatment were captured from cGVHD diagnosis to the end of study, including maximum dose of steroid therapy during the interval since the last study visit. Otherwise, exact start/stop dates and other medication doses were not captured.
The second study was a prospective observational study entitled “Chronic GVHD Response Measures Validation.” A total of 12 centers enrolled 383 patients with chronic GVHD from 2013 to 2017 with 18 months of active follow-up and then long-term follow-up data collection through chart review. Enrolled subjects were starting a new systemic therapy for cGVHD (within a 4-week window of the start date of the index agent). Assessments occurred at enrollment and 3, 6, and 18 months and if a new systemic therapy was added after enrollment. Similarly, information on cGVHD, functional assessments, patient-reported outcomes, and medications (systemic and topical agents used for cGVHD therapy), and research samples were collected. Medication exposure was captured as a running log of medications and treatments for cGVHD from cGVHD diagnosis to end of study. Start and stop dates were captured for all agents, and comprehensive dose information was captured for steroid therapy.
The third study was a prospective observational study entitled “Longitudinal Study of Immune Mediated Disorders after Allogeneic HCT.” A total of 911 patients were enrolled from 13 total centers between 2011 and 2014; 413 developed cGVHD. Patients were enrolled before HCT or up to day 121 after HCT and followed prospectively for the development of immune-mediated disorders: cGVHD overall and cGVHD subtypes including bronchiolitis obliterans, cutaneous sclerosis, or late acute GVHD. Assessments occurred at baseline, day 100, day 180 or 365, and day 730; or if they developed immune-mediated disorders, clinical data and samples were obtained at onset and then at 3 or 6 months later. The intended study follow-up was 5 years for subjects with immune-mediated disorders. IS medications were captured as a running log from time of HCT through total study follow-up period. The indication for each (eg, initial prophylaxis, acute GVHD therapy, cGVHD therapy) was captured, as well as start/stop dates. Dose information was captured for steroid therapy. Patient-reported outcomes (PRO) were not collected in this study. Given this incomplete PRO data, we did not analyze PRO outcomes in this current analysis.
Therapy type definitions
We examined all IS therapies given throughout each patient’s history to fully characterize variation in treatment practices. The following definitions for background, initial, and failure therapies were developed for the purpose of this analysis. Background therapy was defined as any systemic IS agent that the patient was taking when they were diagnosed with cGVHD. These agents were identified with a start date range from before HCT to cGVHD onset, with an indication that was not cGVHD (included initial acute GVHD prophylaxis, acute GVHD therapy, or other), and did not have a stop date before cGVHD onset. Initial therapy was defined as first-line systemic IS therapy akin to that in therapeutic cGVHD trials. It included any systemic agent started 30 days before cGVHD diagnosis to any time afterward with an indication of cGVHD and included any new systemic IS agent or therapy including steroids newly started for cGVHD at any dose or steroids increased to ≥0.4 mg/kg per day from a lower dose that was started more than 30 days before cGVHD onset. The threshold of ≥0.4 mg/kg per day from a lower dose was selected to capture a therapeutic intent to escalate background steroid dose to nearly 0.5 mg/kg per day (with possible rounding for dose/weight considerations) or greater. Changes in doses of other background medications (eg, tacrolimus) were not considered initial therapy, as detailed information on doses of medications besides steroids was not captured. Finally, failure therapy was defined as new systemic therapy to treat inadequately responding, recurrent, or progressive cGVHD. It was defined as having an indication of cGVHD and being added ≥10 days after initial therapy, except that certain agents (extracorporeal photopheresis [ECP], rituximab, ibrutinib, ruxolitinib, sirolimus) were considered failure therapy if started ≥30 days after initial therapy, acknowledging that these agents may have more prolonged time to approval and initiation compared with others. Any systemic IS agent or therapy was eligible to be considered a failure therapy, including steroids newly started at any dose, whereas dose increases in prednisone from initial therapy dose were not considered failure.
Therapy categories
All therapy approaches were initially summarized, revealing marked heterogeneity in treatment practices. From this initial summary, therapy subgroups were defined to bring together conceptually related treatment approaches and to ensure sufficient sample sizes per treatment group for analysis. The 3 initial groups included the following: (a) prednisone only (or prednisone dose-equivalent), (b) prednisone + additional systemic IS agent(s), and (c) other systemic agent(s) without prednisone. For the prednisone + additional IS agents group, the additional IS agents had to be newly started per the initial therapy definition and did not meet the failure medication definition. Thus, these were truly an intentional combined therapy bundle. Sole use of topical agents was not considered in this analysis. For groups 2 and 3, additional subgroups were defined. For group 2, subgroups included the following: (a) prednisone ± calcineurin inhibitor (CNI), (b) prednisone + sirolimus ± CNI, (c) prednisone + mycophenolate mofetil (MMF) or methotrexate (MTX) ± CNI, (d) prednisone + ECP ± CNI, (e) prednisone + rituximab ± CNI ± other, and (f) prednisone + other/rare combinations. For group 3, subgroups included the following: (a) CNI only, (b) MMF or MTX ± CNI without sirolimus, (c) sirolimus ± CNI without MMF, and (d) other/rare combinations ± CNI. For all prednisone-containing approaches (groups 1 and 2 above), prednisone (or equivalent) doses were categorized to examine the impact of steroid dose on treatment outcome: <0.25, 0.25 to 0.74, 0.75 to 1.25, and >1.25 mg/kg per day dose, to bracket common doses of 0.5 and 1.0 mg/kg per day. The 3 parent consortium observational studies informing this analysis did not capture whether IS agents were given as part of a concurrent therapeutic clinical trial vs off-protocol therapy.
Study outcome measures and statistical methods
Descriptive statistics were used to summarize baseline demographic and cGVHD features, as well as proportions of subjects within the studied treatment categories. Major outcomes of interest included FFS (composite outcome including death, malignancy relapse, and use of additional lines of systemic IS therapy), overall survival (OS), and nonrelapse mortality. All outcomes were calculated from the start time of initial therapy, and as such, only subjects with a definable initial therapy were included. Cox regression models were used to examine the association between the major outcomes and initial therapy, as well as other potential risk factors first univariately, and then factors found to be associated (P < .1) were combined into a multivariate model. Factors considered included initial therapy, initial therapy steroid dose, sex, study site, disease, conditioning regimen, transplant source, donor age, donor match and relation, age at cGVHD diagnosis, year of cGVHD diagnosis, time from transplant to cGVHD, prior acute GVHD grade II to IV, overlap vs classic cGVHD, cGVHD organ scores, overall National Institutes of Health (NIH) severity score, Sorror comorbidity index, Karnofsky performance status (KPS), platelets, and bilirubin. Statistical analyses were performed using SAS/STAT software, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Patient characteristics
A total of 745 subjects were included in this analysis from 17 centers. Baseline patient, disease, HCT, and cGVHD features are presented in Table 1 with groups defined according to initial therapy. Acute myeloid leukemia (AML) was the predominant HCT indication, peripheral blood mobilized stem cells were most commonly used as a graft source, and there was representation of expected variation in conditioning regimen intensity and donor type. Acute GVHD prophylaxis was most commonly CNI-based (CNI/sirolimus, CNI/MMF, or CNI/MTX), with limited representation of posttransplant cyclophosphamide-based approaches. Prior grade II to IV acute GVHD was present in 48% of cases. The median time from HCT to cGVHD diagnosis was 7.5 months (interquartile range [IQR], 5.4-11.2 months). Initial therapy was largely started within 1 week of cGVHD diagnosis (71% of cases), whereas 19.7% of cases started initial therapy > 30 days from cGVHD diagnosis.
Certain baseline features differed according to initial therapy group: compared with the prednisone alone group, the nonprednisone group had more myeloablative conditioning, mismatched donors, prior acute GVHD, longer median time from HCT to cGVHD onset, and longer time between cGVHD diagnosis and initial therapy (P < .05 for each). The prednisone + other IS therapy group had more severe skin, gastrointestinal, and eye cGVHD involvement and more severe cGVHD overall (P < .001 for each).
Background therapy (Table 2) was most commonly nonsteroid agents (N = 330, 44.3% of total), predominated by CNI alone (N = 235), sirolimus ± CNI (N = 50), and MMF or MTX ± CNI (N = 24), whereas other agents were less common. No background therapy was the second most common category (N = 247, 33.2%). Other background therapy categories included prednisone + other agents (N = 125, 16.8% of total), followed by predominance of prednisone + CNI (N = 77) or prednisone + MMF or MTX ± CNI (N = 23), whereas other subcategories were less common. Prednisone alone was present in N = 40 (5.4% of total), and topical agents only were present in N = 3 (0.4% of total).
Variation in treatment practices
Detailed description of initial therapy is provided in Table 3, showing extensive heterogeneity in practice. About half of the subjects were treated with prednisone alone (n = 411, 55%), whereas a quarter received prednisone + other IS agent(s) (n = 197, 26%), and almost 1 in 5 received nonprednisone IS therapy (n = 137, 18%). Common agents to combine with prednisone were sirolimus ± CNI (N = 66, 33.5%), CNI alone (N = 63, 32%), MMF or MTX ± CNI (N = 30, 15%), rituximab ± CNI (N = 17, 8.6%), ECP ± CNI (N = 11, 5.6%), and other rare agents (N = 10, 5%). Among the combined prednisone alone group and prednisone + other agents group, steroid dose was <0.25 (9%), 0.25 to 0.74 (36%), 0.75 to 1.25 (42%), or >1.25 mg/kg per day (13%). Among those receiving prednisone alone, dose differed according to overall cGVHD severity, with higher-dose therapy overrepresented in more severe cGVHD (Table 4).
Treatment outcomes
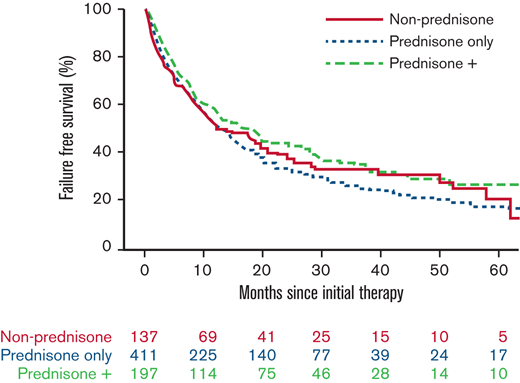
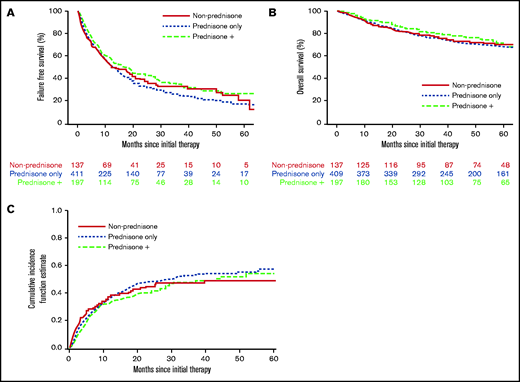
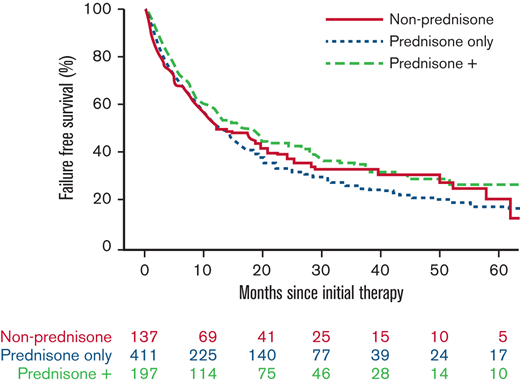
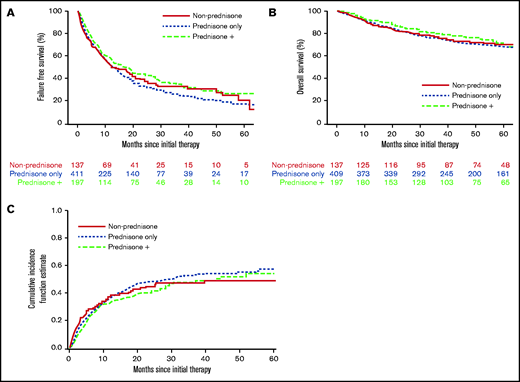
With a median follow-up time for surviving patients of 56.5 months, FFS at 6 and 12 months, respectively, was 69% and 53%. We found no evidence that the initial therapy group (prednisone alone, prednisone + other agents, nonprednisone) had a significant association with subsequent FFS, OS, or incidence of second-line systemic therapy (Figure 1; Table 5). There was no evidence that the trajectory of treatment failure differed between these initial therapy groups. Similarly (data not shown), there was no association between the initial therapy group and nonrelapse mortality. No statistical interactions were observed between the initial therapy group and NIH overall severity, as well as between prednisone dose and NIH overall severity. FFS according to initial therapy group and prednisone dose category are presented in supplemental Figures 1 and 2, respectively. Patients treated with initial therapy at a later time point from cGVHD onset had longer FFS (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.59-0.93; P = .01). Failure medications used were most commonly nonprednisone agents used after steroid failure (Table 6).
Treatment outcome according to initial cGVHD therapy group. (A) FFS. (B) OS. (C) Cumulative incidence of second-line therapy.
Treatment outcome according to initial cGVHD therapy group. (A) FFS. (B) OS. (C) Cumulative incidence of second-line therapy.
Discussion
This large, nationally representative cGVHD Consortium analysis provides new insight into key questions regarding treatment variation and its impact on subsequent outcome and highlights opportunities for future trials testing novel approaches in cGVHD initial therapy.
In keeping with expectations based on standards in the field and prior clinical trials, most cases had prednisone-based initial therapy, whereas only 18% had non–steroid-based initial therapy. As well, in keeping with 78% of the studied population having NIH moderate/severe cGVHD, most cases started initial therapy early after diagnosis. The major new insights, however, were that both initial steroid dose and use of combination therapies varied extensively. For example, among all treated with any steroid-based initial therapy, 45% were treated at doses less than 0.75 mg/kg per day, suggesting that a range of 0.5 to 1.0 mg/kg per day is the standard and not 1 mg/kg per day. Higher-dose steroid therapy was associated with higher NIH overall cGVHD severity, as anticipated. We acknowledge that we can only characterize these initial therapy group patients through the variables we have collected and that certain factors unaccounted for may have driven the varied selection of initial therapy types and intensity for given patients.
Although no randomized study has shown that combination therapy is superior to prednisone alone, 26% received prednisone plus other agents for initial therapy. The most common were prednisone + CNI, prednisone + sirolimus ± CNI, or prednisone + MMF ± CNI, although data for these approaches are mixed.13,15,16 Less common approaches were particularly diverse; numerous agents paired with prednisone (ECP, anti-CD20 antibodies including rituximab or ofatumumab, hydroxychloroquine, bortezomib, entospletinib, abatacept, and tocilizumab), some of which may have been delivered on clinical trials concurrent with enrollment on the Consortium observational studies that informed this analysis. Variation was also seen within the nonprednisone initial therapy group, where different single- or multiple-agent approaches were used. Most commonly, this included agents from the other/rare ± CNI group (with greatest representation of ECP, rituximab, and imatinib) or use of CNI alone, sirolimus, or MMF. These non–steroid-based approaches met our initial therapy definition, because they were the first systemic agent added for cGVHD within our defined initial therapy window.
Beyond characterizing variation in treatment practices, this analysis suggests that marked variation in both initial therapy group and prednisone dose among prednisone-containing treatment regimens did not significantly impact FFS, OS, or use of second-line agents. Similar conclusions were reached when examining effect of therapy type and prednisone dose within NIH overall severity categories. Overall, both the magnitude of failure and time to failure did not appear to differ according to these factors, and there was no evidence of different mortality risk according to these factors. These observational data suggest that treating clinicians were trying to integrate cGVHD- and patient-level considerations to select initial therapy, and the result was that outcomes were comparable both to other groups within this analysis and current benchmarks for expected FFS after initial cGVHD therapy. These data are hypothesis-generating only, however, and require confirmation in well-designed prospective clinical trials. One major area of focus in clinical trials could be the safety and efficacy of lower initial prednisone dose in initial therapy of cGVHD. Careful deliberation will need to go into assembly of eligibility criteria for such trials focused on lower-dose prednisone therapy. Another approach of interest is the use of nonprednisone systemic IS therapy for cGVHD initial therapy. On this point, our observational data do not provide robust guidance given there were approximately 20 to 40 total cases per each agent in the nonprednisone initial therapy group, limiting conclusions that could be drawn regarding differential outcome per agent and according to background medications. In addition, novel agents were not represented in this study population.
We note the following limitations of this analysis: First, despite the national Consortium-based approach and large study population analyzed, we acknowledge that our coverage of diverse practices is not complete and that additional variation likely exists. Second, we did not address the impact of background therapies or model cumulative steroid dose exposure in our analyses. Next, within this analysis, nonprednisone initial therapy approaches were relatively limited in total regarding the overall study population but also had limited representation in certain cGVHD subgroups such as severe skin or lung involvement. Another limitation is that we did not directly capture the medical decision making behind treatment heterogeneity, instead having to infer based on chronic GVHD characteristics. Our observations of similar outcomes between treatment groups are consistent with either initial treatment regimen and steroid dose not mattering or that clinicians are fairly good at estimating the intensity of treatment needed. Finally, although FFS and OS did not differ according to initial therapy group or prednisone dose, other important outcomes such as chronic GVHD symptom burden, disability, and quality of life were not studied in this analysis. Whether lower-dose prednisone initial approaches (or non–steroid-based approaches) can achieve similar treatment response while also decreasing prednisone-associated morbidity remains to be fully examined in future clinical trials.
Acknowledgments
This work was supported by grants CA118953 and CA163438 (principal investigator: S.J.L.).
Authorship
Contribution: J.P., S.J.L., and L.O. designed the study, performed analysis, and wrote and approved the final manuscript and all authors contributed to the analysis.
Conflict-of-interest disclosure: S.J.L. received research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda; received study medication from Janssen; and served on a steering committee for Novartis. The remaining authors declare no competing financial interests.
Correspondence: Joseph Pidala, Blood and Marrow Transplantation and Cellular Immunotherapy, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612; e-mail: joseph.pidala@moffitt.org.
References
Author notes
Requests for data sharing may be submitted to Joseph Pidala (joseph.pidala@moffitt.org).
The full-text version of this article contains a data supplement.