Key Points
MRD at time of allo-HCT was an important risk factor in Ph+ ALL patients during both CR1 and CR2.
No significant difference in relapse rate was observed between CR1 MRD− and CR2 MRD−.
Abstract
Although measurable residual disease (MRD) at the time of allogeneic hematopoietic cell transplantation (allo-HCT) has been reported to be an important prognostic factor for Philadelphia chromosome (Ph)–positive acute lymphoblastic leukemia (ALL) during first complete remission (CR1), the prognostic impact of MRD is unclear during second CR (CR2). To clarify the impact of MRD for both CR1 and CR2, we analyzed data from a registry database including 1625 adult patients with Ph+ ALL who underwent first allo-HCT during either CR1 or CR2 between 2002 and 2017. Adjusted overall and leukemia-free survival rates at 4 years were 71% and 64%, respectively, for patients undergoing allo-HCT during CR1 with MRD−, 55% and 43% during CR1 with MRD+, 51% and 49% during CR2 with MRD−, and 38% and 29% during CR2 with MRD+. Although survival rates were significantly better among patients with CR1 MRD− than among patients with CR2 MRD−, no significant difference was observed in survival rate between patients with CR1 MRD+ and CR2 MRD−. Relapse rates after 4 years were 16% in patients with CR1 MRD−, 29% in CR1 MRD+, 21% in patients with CR2 MRD−, and 46% in patients with CR2 MRD+. No significant difference was identified in relapse rate between patients with CR1 MRD− and CR2 MRD−. CR2 MRD− was not a significant risk factor for relapse in multivariate analysis (hazard ratio, 1.26; 95% confidence interval, 0.69-2.29; P = .45 vs CR1 MRD−). MRD at time of allo-HCT was an important risk factor in patients with Ph+ ALL during both CR1 and CR2.
Introduction
Measurable residual disease (MRD) has become more important in Philadelphia chromosome (Ph)–positive acute lymphoblastic leukemia (ALL) because tyrosine kinase inhibitors (TKIs) have facilitated the achievement of deep molecular responses.1,2 MRD can predict treatment responses and serve as a prognostic factor.3,4 In the development of new treatment drugs, such as blinatumomab and inotuzumab ozogamicin (Ino), MRD has become an important end point, and treatment strategies for ALL cannot be considered without thorough MRD evaluation.5-7
Allogeneic hematopoietic cell transplantation (allo-HCT) remains a popular treatment option for Ph+ ALL in the era of TKIs.8-12 Although MRD status at time of allo-HCT performed during first complete remission (CR1) has attracted attention,9,10,13-15 the prognostic value of MRD during second CR (CR2) remains unclear. Understanding how MRD at time of allo-HCT affects outcomes of patients during CR2 is critical, particularly when considering that the number of cases for which deep molecular remission can be achieved during CR2 is likely to increase with development of new drugs.16 Therefore, in this study, we evaluated the impact of MRD at time of allo-HCT in patients undergoing transplantation during either CR1 or CR2.
Patients and methods
Collection of data and data source
Patients’ clinical data were provided by the Japan Society for Hematopoietic Cell Transplantation (JSHCT) and the Japanese Data Center for Hematopoietic Cell Transplantation using the Transplant Registry Unified Management Program as described previously.17 Data regarding survival, disease status, and long-term complications, including chronic graft-versus-host disease (GVHD) and secondary malignancies, are updated annually. Opt-out is permitted for observational studies using existing data, according to current ethical guidelines. Those who declined registration were not included among patients in this study. This study was approved by the data management committees of JSHCT as a study performed by the Adult ALL Working Group of JSHCT and the Nagoya University Hospital Institutional Review Board.
Patients
Data for 1865 patients who were at least age 16 years and who had undergone first allo-HCT for Ph+ ALL during either CR1 or CR2 between 2002 and 2017 were available in the JSHCT registration database. After excluding 240 patients for whom no pretransplantation TKI administration data were available, we analyzed data for 1625 adult patients with Ph+ ALL.
Definitions
Ph+ ALL was diagnosed by presence of Ph through chromosome and/or fluorescence in situ hybridization analysis and detection of BCR-ABL fusion transcripts using real-time quantitative polymerase chain reaction (qPCR) analysis. Timing and procedure of allo-HCT, including conditioning regimen, GVHD prophylaxis, and assessment of BCR-ABL fusion transcripts, were determined by each institution. In most laboratories, BCR-ABL transcript copy numbers were normalized against copy numbers of glyceraldehyde-3-phosphate dehydrogenase and then converted into molecules per microgram of RNA. Threshold for quantification was 50 copies per microgram of RNA, which corresponded to minimal sensitivity of 10−5.8 In the present study, MRD− was defined when the MRD result was reported as not detected by real-time qPCR. Of the 110 patients whose real-time qPCR values were not registered in the database, those whose status at time of allo-HCT was registered as molecular CR by the transplantation center were also defined as MRD−. MRD at time of allo-HCT was evaluated by results of real-time qPCR a median of 21 days before transplantation (interquartile range, 14-36 days; MRD was assessed within 60 days before allo-HCT in 1572 [97%] of 1625 patients).9 Determination of whether to prescribe TKIs after allo-HCT was also made by each institution.
Acute and chronic GVHD were diagnosed and graded according to existing consensus criteria.18-20 Relapse was defined as hematological leukemia recurrence. Nonrelapse mortality (NRM) was defined as death during continuous remission. For analyses of overall survival (OS), failure was defined as death resulting from any cause, and surviving patients were censored at date of last contact. We defined a reduced-intensity regimen as having the following dose levels: <9 mg/kg busulfan, <140 mg/m2 melphalan, and total body irradiation at ≤500 cGy (single) or 500 to 800 cGy (fractionated).21 HLA matching of cord blood (CB) was performed using low-resolution typing for HLA-A, -B, and -C and high-resolution molecular typing for HLA-DRB1. HLA matching of unrelated donors was performed using high-resolution typing for HLA-A, -B, -C, and -DRB1.22-24 For unrelated donors, well matched was defined as no known disparity in HLA-A, -B, -C, or -DRB1, partially matched was defined as presenting 1 locus that demonstrated disparity with donor, and mismatched was defined as disparities in ≥2 loci. For CB, well matched was defined as no known disparity in HLA-A, -B, -C, or -DRB1, partially matched was defined as at least 4 loci matches, and mismatched was defined as ≤3 loci matches.9,25-27
Statistical analysis
A 2-sided χ2 test was used to compare categorical variables, and Mann-Whitney or Kruskal-Wallis test was used to compare continuous variables. OS and leukemia-free survival (LFS) were estimated by Kaplan-Meier method, and P values were calculated using log-rank test.28,29 Holm-Sidak test was used to compare 2 groups, for post hoc comparisons. Cumulative incidence of relapse, NRM, and GVHD was calculated by Gray’s method.30,31 For relapse, death without relapse was the competing event; for NRM, relapse was the competing event; and for GVHD, relapse and death, without GVHD, were the competing events. Cox proportional hazards models were used to perform multivariate analyses of OS and LFS.32 Fine-Gray proportional hazards models were used for multivariate analyses of events with competing risks. Adjusted probabilities of OS and LFS were estimated using Cox proportional hazards regression models, with consideration of other significant clinical variables in final multivariate models. Backward stepwise procedure was used to develop a final model based on a threshold value of P < .05. Covariates considered included disease status at allo-HCT (CR1 with MRD− vs CR1 with MRD+ vs CR2 with MRD− vs CR2 with MRD+), age at allo-HCT (<55 vs ≥55 years), performance status (PS; 0 vs 1-4), white blood cell (WBC) count at diagnosis (<30 × 103/μL vs ≥30 × 103/μL), additional chromosomal abnormalities, breakpoint cluster region (p190 vs p210 vs other), TKI use (imatinib vs dasatinib vs other), cytomegalovirus status, sex matching (match vs male to female vs female to male), donor (related vs unrelated bone marrow or peripheral blood vs unrelated CB), HLA disparity (well matched vs partially matched vs mismatched), preparative regimen (myeloablative conditioning vs reduced-intensity conditioning; etoposide + cyclophosphamide + total-body irradiation33 vs other), GVHD prophylaxis (cyclosporine A ± other vs tacrolimus ± other vs other), posttransplantation TKI (before hematological relapse, as a time-dependent covariate; no vs yes), and year of allo-HCT (2002-2012 vs 2013-2017). Significance level of P < .05 was used for all analyses.
Results
Patient characteristics
Patient characteristics, according to stage and MRD, are listed in Table 1. MRD was negative in 1111 (73%) of 1523 patients undergoing allo-HCT during CR1 and 61 (60%) of 102 patients undergoing allo-HCT during CR2. Age at allo-HCT was higher and PS worse among patients undergoing allo-HCT during CR2 compared with those undergoing allo-HCT during CR1. WBC counts were lower in patients with CR1 MRD− compared with other groups. Posttransplantation TKI was administered to 23% of patients. Although more patients with MRD received posttransplantation TKI compared with those without MRD, frequency was comparable between patients with CR1 MRD− and CR2 MRD−.
Survival
OS.
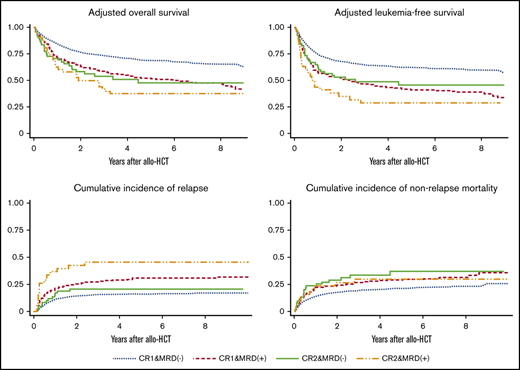
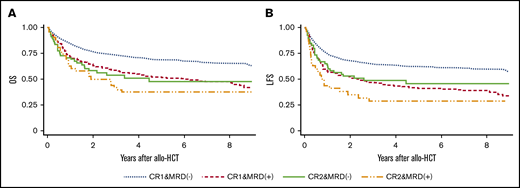
Median follow-up period for survivors was 49 months (range, 1.9-198 months). OS rates at 4 years were 70% (95% confidence interval [CI], 67-73) for patients with CR1 MRD−, 54% (95% CI, 49-59) for patients with CR1 MRD+, 47% (95% CI, 33-60) for patients with CR2 MRD−, and 31% (95% CI, 17-46) for patients with CR2 MRD+. Although OS was significantly better for CR1 MRD− patients than for CR2 MRD− patients (P < .001), no significant difference in OS was observed between patients with CR1 MRD+ and CR2 MRD− (P = .52) when post hoc analyses were performed. In multivariate analysis, disease status was a significant prognostic factor, as were age, PS, WBC count, and year of allo-HCT (Table 2). Adjusted OS rates at 4 years were 71% for patients with CR1 MRD−, 55% for patients with CR1 MRD+, 51% for patients with CR2 MRD−, and 38% for patients with CR2 MRD+ (Figure 1A).
Adjusted survival according disease status. (A) OS. Results adjusted for age at allo-HCT, PS, WBC count at diagnosis, and year of transplantation. (B) LFS. Results adjusted for age at allo-HCT, WBC count at diagnosis, preparative regimen (etoposide + cyclophosphamide + total-body irradiation vs other), and year of transplantation.
Adjusted survival according disease status. (A) OS. Results adjusted for age at allo-HCT, PS, WBC count at diagnosis, and year of transplantation. (B) LFS. Results adjusted for age at allo-HCT, WBC count at diagnosis, preparative regimen (etoposide + cyclophosphamide + total-body irradiation vs other), and year of transplantation.
LFS.
LFS rates at 4 years were 63% (95% CI, 60-66) for patients with CR1 MRD−, 42% (95% CI, 37-48) for patients with CR1 MRD+, 46% (95% CI, 32-58) for patients with CR2 MRD−, and 25% (95% CI, 12-39) for patients with CR2 MRD+. Although LFS was significantly better for patients with CR1 MRD− patients than for patients with CR2 MRD− (P = .007), no significant difference in LFS was observed between patients with CR1 MRD+ and CR2 MRD− (P = .77) when post hoc analyses were performed. In multivariate analysis, disease status was a significant prognostic factor, as were age, WBC count, preparative regimen, and year of allo-HCT (Table 2). Adjusted LFS rates at 4 years were 64% for patients with CR1 MRD−, 43% for patients with CR1 MRD+, 49% for patients with CR2 MRD−, and 29% for patients with CR2 MRD+ (Figure 1B).
Relapse.
Relapse rates after 4 years were 16% (95% CI, 14-19) for patients with CR1 MRD−, 29% (95% CI, 25-34) for patients with CR1 MRD+, 21% (95% CI, 12-32) for patients with CR2 MRD−, and 46% (95% CI, 29-61) for patients with CR2 MRD+ (Figure 2A). No significant difference in relapse rate was observed between patients with CR1 MRD− and CR2 MRD− (P = .36) when post hoc analyses were performed. In multivariate analysis, disease status was a significant prognostic factor, as were WBC count, donor, preparative regimen, and posttransplantation TKI (Table 2).
NRM, GVHD, and cause of death
NRM.
NRM rates after 4 years were 20% (95% CI, 18-23) for patients with CR1 MRD−, 29% (95% CI, 24-33) for patients with CR1 MRD+, 33% (95% CI, 21-46) for patients with CR2 MRD−, and 30% (95% CI, 16-45) for patients with CR2 MRD+ (Figure 2B). Although tendency toward lower NRM rate was observed for patients with CR1 MRD− compared with patients with CR2 MRD− (P = .07), no significant difference in NRM rate was observed between patients with CR1 MRD+ and CR2 MRD− (P = .83) when post hoc analyses were performed. In multivariate analysis, disease status was a significant prognostic factor, as were age, PS, donor, posttransplantation TKI, and year of allo-HCT (Table 2).
GVHD.
Cumulative incidence of grade 2 to 4 acute GVHD was not significantly different according to disease status (CR1 MRD−, 40%; CR1 MRD+, 40%; CR2 MRD−, 39%; and CR2 MRD+, 37% at day 100; P = .98). Similarly, cumulative incidence of grade 3 to 4 acute GVHD was not significantly different according to disease status (CR1 MRD−, 10%; CR1 MRD+, 12%; CR2 MRD−, 10%; and CR2 MRD+, 12% at day 100; P = .54).
Among evaluable patients who survived for at least 100 days after allo-HCT, no significant difference was observed in incidence of chronic GVHD according to disease status (CR1 MRD−, 37%; CR1 MRD+, 34%; CR2 MRD−, 35%; and CR2 MRD+, 41% at 2 years; P = .61).
Cause of death.
Relapse was the most common cause of death for patients undergoing allo-HCT during CR1 and those undergoing allo-HCT during CR2, and infection was the most frequent cause of nonrelapse fatality. No significant difference in any nonrelapse cause of death was observed according to disease status (Table 3).
Impact of MRD in patients undergoing allo-HCT during CR2
Among 102 patients undergoing allo-HCT during CR2, CR1 duration was longer for patients with MRD− compared with patients with MRD+ (193 vs 116 days; P = .01). Sixteen of 33 patients whose CR1 duration was <100 days (48%) were MRD−, whereas the remaining 17 (52%) were MRD+. Forty-one of 78 patients whose CR1 duration was <1 year (53%) were MRD−, whereas the remaining 37 (47%) were MRD+. In multivariate analysis, MRD was a significant risk factor for LFS, as were PS and conditioning intensity (CR2 MRD+: hazard ratio, 1.73; 95% CI, 1.03-2.91; P = .04), and MRD was also a significant risk factor for relapse, along with HLA disparity (CR2 MRD+: hazard ratio, 2.63; 95% CI, 1.26-5.49; P = .01).
Discussion
This study showed the impact of MRD at time of allo-HCT not only in patients with Ph+ ALL undergoing transplantation during CR1, but also in those undergoing transplantation during CR2. Survival rate was similar between patients with CR1 MRD+ and CR2 MRD−. In addition, no significant difference in relapse rate was observed between patients with CR1 MRD− and CR2 MRD−. CR2 MRD− was not a significant risk factor for relapse in multivariate analysis. To our knowledge, this is the first study to demonstrate that MRD is also an important risk factor for patients with Ph+ ALL who undergo allo-HCT during CR2.
Allo-HCT during CR1 remains standard treatment for Ph+ ALL in Japan. Although relatively high survival rates can be achieved, especially in patients with CR1 MRD−, long-term impairment of quality life associated with chronic GVHD is a transplantation-specific complication that we aim to avoid, in addition to NRM.34 Four-year OS was 66% without allo-HCT in CR1 in patients who achieved complete molecular remission at 3 months in a previous study,35 similar to our results for patients with MRD− undergoing allo-HCT in CR1. This indicates that some patients who achieve early molecular remission may be able to survive for a long time without allo-HCT in CR1. Our results suggest that relatively favorable outcomes may be obtained when allo-HCT is performed for patients with MRD− in CR2, even if recurrence will have occurred in such patients who do not undergo allo-HCT in CR1. Further improvement of chemotherapy will be expected with recent advances in ALL, which may change the status of allo-HCT as standard treatment.
This study included data for patients undergoing allo-HCT between 2002 and 2017. Before allo-HCT, a few patients were administered ponatinib, a third-generation TKI showing promising data in Ph+ ALL treatment, including in those with T315I mutation36,37 ; however, ponatinib was only approved for relapsed/refractory Ph+ ALL in September 2016. In addition, because Ino and blinatumomab were approved in 2018 and tisagenlecleucel was approved in 2019 for treatment of relapsed/refractory ALL in Japan, no patients received these drugs before allo-HCT in clinical practice. These new drugs have been reported to achieve high CR rates when used to treat relapsed/refractory ALL (Ino, 57% to 92%; blinatumomab, 36% to 44%; and tisagenlecleucel, 81%), with high probability of obtaining MRD− with achievement of CR (Ino, 63% to 93%; blinatumomab, 71% to 88%; and tisagenlecleucel, 100%).38-45 Treatment strategy may be more important when allo-HCT is performed during CR2 instead of during CR1, particularly when CR2 can be achieved with high probability. Low relapse rates associated with patients with CR2 MRD− support this strategy, which may help inform the decision of when to perform allo-HCT. More patients undergoing allo-HCT in CR2 received pretransplantation dasatinib than those undergoing allo-HCT in CR1 in this study. Although type of TKI before allo-HCT was not identified as a significant prognostic factor in multivariate analyses, different activity of pretransplantation TKIs could affect transplantation outcome, considering dasatinib is a more potent multitargeted inhibitor compared with imatinib.46,47 This study demonstrates the importance of MRD at time of allo-HCT, even during CR2.
Several limitations exist because of the retrospective nature of this study, which was performed using database entries. Unfortunately, induction/consolidation treatment data were not available, except for pretransplantation TKI information, in this study. This is a major limitation of transplantation registry–based studies.48-52 In addition to the small number of patients undergoing allo-HCT during CR2, backgrounds of these patients were considered heterogeneous because eligibility criteria for allo-HCT depended on individual transplantation centers. Although data on pre- and posttransplantation TKIs were available in our database, treatment was not controlled as in prospective trials. Therefore, examination of effects of posttransplantation treatment was difficult to perform, even though posttransplantation TKI administration could represent an important option for posttransplantation therapeutic intervention.53 NRM might have been underestimated in patients who received posttransplantation TKI because of technical issues in competing risk analyses; of 367 patients receiving posttransplantation TKI, NRM was observed in only 14 patients (3.8%), whereas relapse was observed in 257 (70%). Given that the decision to prescribe a TKI after allo-HCT was made by each institution in this study, the TKI might have been administered to patients who were potentially at high risk of relapse.9 Although posttransplantation TKI administration cannot prevent relapse, achieving comparable survival in such patients may be beneficial with the possibility that innovative new technologies will be developed in the near future.
In summary, the importance of MRD at time of allo-HCT was demonstrated in patients undergoing transplantation during both CR1 and CR2. The present study provides helpful data when considering appropriate timing of allo-HCT in the era of emerging useful new drugs, which may expand therapeutic options for ALL beyond allo-HCT. In patients who must undergo allo-HCT for Ph+ ALL, a therapeutic strategy that can achieve MRD− before allo-HCT is desirable, regardless of disease stage.
For data sharing requests, e-mail the corresponding author, Satoshi Nishiwaki (n-3104@tf7.so-net.ne.jp).
Acknowledgments
This study was supported, in part, by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI) grant JP 20K08730, and a research grant from the Hori Sciences and Arts Foundation.
Authorship
Contribution: S.N., Y. Akahoshi, S.M., A.S., S.H., and S.K. designed the research, performed the statistical analyses, interpreted the data, and wrote the manuscript; Y.N., T.F., N.U., M.T., M.O., Y. Ozawa, S.O., S.S., Y. Onishi, Y.K., and M.S. provided patient data; J.T., T.F. and Y. Atsuta collected patient data; and all authors reviewed and approved the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
All group members of the Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation who contributed to this study were included as authors of this article.
Correspondence: Satoshi Nishiwaki, Department of Advanced Medicine, Nagoya University Hospital, 65 Tsurumai-cho, Showa-ku, Nagoya 4668560, Japan; e-mail: n-3104@tf7.so-net.ne.jp.