Abstract
Although the majority of adult patients with both acute lymphoblastic leukemia and acute myelogenous leukemia achieve remission with upfront chemotherapy, many patients still suffer relapse. Often, the strategy is proposed of treating patients with relapsed leukemia into a second remission (CR2) and then proceeding to allogeneic transplantation as the definitive curative approach. However, the long-term outcomes of such a strategy are poor: the 5-year overall survival from first relapse for patients with acute leukemia is only approximately 10%. This Perspective highlights the fact that most patients do not achieve CR2 and therefore never really have an opportunity for a potential curative therapy. Although patients who undergo transplantation after relapse may be cured, those who do not achieve CR2 are rarely candidates for transplantation; therefore, the overall outcome for patients who relapse is dismal. There is therefore an urgent need not only for more effective upfront therapy to prevent relapse, but also for the development of therapies that can serve as effective bridging treatments between relapse and transplantation. We suggest that more optimal use of minimal residual disease detection during first remission may also improve the chances for successful transplantation therapy via earlier reinduction therapy, allowing transplantation before overt relapse.
Introduction
When adults are diagnosed with acute leukemia, it is very common for the physician to discuss with them the potential role, timing, risks, and impact of hematopoietic cell transplantation in the management of their disease. This often leads to recommending early transplantation for patients with high-risk disease in first remission (CR1) and delaying transplantation for those with a lower risk of relapse until there is evidence of recurrent disease. The assumption is that after relapse or progression, patients will undergo transplantation after successful reinduction therapy to achieve CR2.
In general, for patients with acute myeloid leukemia (AML), allogeneic related or unrelated transplantation is currently pursued for those with intermediate- or high-risk disease, specifically those with FLT3 mutations, intermediate- or high-risk cytogenetics, complex cytogenetics, monosomy karyotypes, or who did not achieve remission with the initial induction therapy. For those patients who have favorable-risk AML, transplantations have not been shown to be beneficial and are not generally performed in CR1.1 These include normal cytogenetics if the leukemia cells display molecular features such as NPM1 mutations because these patients often have comparable outcomes with standard chemotherapy. This also applies to patients with good-risk cytogenetics such as inv(16) or t(8;21) for whom, in the absence of other concomitant risk factors such as genes associated with a stem cell phenotype, transplantation is generally delayed in the hopes that they will be cured with repeated courses of upfront cytarabine-based consolidation therapy.2-4 For such good-risk patients, transplantation is pursued in CR2 after successful reinduction therapy. However, Schoch et al found age to be an independent poor prognostic variable for de novo AML patients and that even within the good-risk cytogenetic groups inv(16) or t(8;21), age older than 60 years significantly reduces overall survival (OS).5 Many hematologists also do not recommend allogeneic transplantation in CR1 for patients with a normal karyotype in the absence of adverse molecular phenotype, such as FLT3 mutation, once again with the hope of curing such patients if they relapse.
For patients with acute lymphoblastic leukemia (ALL), the strategy of early transplantation is still used predominantly for patients who have significant high-risk features such as the presence of the Philadelphia (Ph) chromosome, a slow response to induction therapy, or a high WBC count at the time of presentation.6 In 2008, the large international ALL Medical Research Council (MRC) UKALL12/Eastern Cooperative Oncology Group (ECOG) 2993 trial demonstrated that OS is significantly improved by transplantation in CR1 for patients with a sibling donor compared with those with no donor (53% vs 45%, P = .01).7 Improved OS is significantly better after allogeneic transplantation in standard-risk patients (Ph−, age ≤ 35 years, or high WBC count at presentation), but not in high-risk patients. The relapse rate is improved in both standard- and high-risk patients; however, TRM is increased in the high-risk patient group, likely because of the impact of older age. In practice, the idea of transplantation for standard-risk ALL in CR1 has not been uniformly accepted and there is a lack of consensus on the indications for transplantation in ALL. The LALA-87 and LALA-94 trials found allogeneic transplantation in CR1 to be beneficial for high-risk patients (standard-risk patients were not studied in the larger LALA-94 trial), whereas the MRC/ECOG trial found it to be beneficial only for standard-risk patients.7-9 Many hematologists and oncologists do not recommend an allogeneic transplantation in CR1 because the superior survival is tempered by the risk of transplantation-related complications, particularly in older patients, and the risk of late effects, particularly in younger patients. The result is that many patients will continue on with consolidation and maintenance chemotherapy regimens and pursue transplantation only at the time of relapse after successful reinduction treatment. Although data show decreases in relapse rate and modest increases in OS for specific risk groups of AML and ALL if transplanted in CR1, transplantation is frequently offered only after relapse.
In these discussions between patients and physicians regarding how to proceed, it is reasonable to discuss the available data and then encourage patients to enroll in a clinical trial if one is available. However, many physicians convey the idea that transplantation should appropriately be delayed and that if relapse does occur, then the patient “can be transplanted in CR2.” This approach seems a wise and practical decision to the patient. So what are the results of this strategy; namely, in adults, how successful is the treatment of relapsed acute leukemia followed by transplantation in CR2?
Transplantation for AML and ALL in CR2
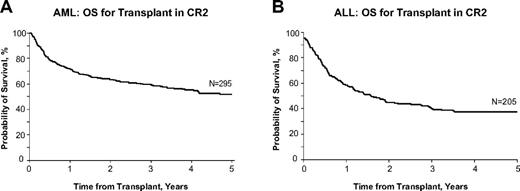
Most studies analyze the results of transplantation in patients with varying disease status (CR1, CR2, and subsequent remission, early relapse, refractory relapse, and induction failure). It is generally agreed that relapsed leukemia in the adult is a fatal condition that cannot be cured by therapy other than allogeneic transplantation. Single-institution trials of full-intensity transplantation suggest that patients with AML or ALL who are transplanted after achieving CR2 have a disease-free survival rate of approximately 40%-50%, a number that is often used in the discussion with the patient, implying that the possibility of cure remains even after the failure of upfront therapy to cure the patient. Figure 1 shows data from the Center for International Blood and Marrow Transplant Research (CIBMTR) for patients with AML or ALL who underwent transplantation in CR2 from related or unrelated donors after an ablative conditioning regimen. These data show that approximately 40%-50% of patients are cured of leukemia with this approach (Dr Mary Horowitz, personal communication, May 2012). These data imply the potential curability of patients if they are not cured by the initial upfront chemotherapy and then undergo transplantation in CR2. What is not always considered when discussing this theoretical 40%-50% cure rate is the inherent bias in evaluating the success of CR2 transplantation only as it applies to those select patients who are able to attain CR2 and who are suitable candidates for allogeneic transplantation based on age, medical, financial, and psychological criteria.
Acute leukemia OS after CR2 transplantation. Probability of survival after allogeneic hematopoietic stem cell transplantation with myeloablative conditioning for AML (A) or ALL (B) in CR2 in adults 18-50 years of age in the United States, 2005-2007. Used with permission from the CIBMTR.
Acute leukemia OS after CR2 transplantation. Probability of survival after allogeneic hematopoietic stem cell transplantation with myeloablative conditioning for AML (A) or ALL (B) in CR2 in adults 18-50 years of age in the United States, 2005-2007. Used with permission from the CIBMTR.
What is the likelihood of achieving CR2 after relapse in adult patients with acute leukemia?
Most induction regimens use intensive chemotherapy to achieve remission, followed by consolidation treatments and, in the case of ALL, maintenance therapy. For AML, induction is still built around an anthracycline/cytarabine treatment regimen. Multiple regimens have been suggested for treatment of relapse.10-14 Depending on the duration of remission, these same drugs may still be effective in achieving CR2, particularly if CR1 lasted more than 1 or 2 years. For example, in patients undergoing reinduction therapy, one of the most important factors predictive of reinduction success is the length of the CR1; the 1-year OS for the relapse-free interval from first CR of ≤ 6 months is 14%, 7-18 months is 36%, and > 18 months is 57%.15 For patients with ALL, often the same induction chemotherapy drug regimen that achieved CR1 is used to attempt CR2 and the same prognostic features, namely duration of the CR1, have an impact on the success of this strategy (Table 1).16 For patients whose remission has lasted longer than a year, CR2 can be achieved in approximately 50%,17 whereas those patients whose duration of remission was shorter have a lower likelihood of achieving remission with the same drugs and should be considered for alternative investigational therapy. Therefore, the ability to achieve CR2 is contingent on several clinical and biologic factors, including the duration of response to the first treatment, the nature of that treatment, and the overall condition of the patient at the time of relapse. Although patients who relapse typically have a reduction in their leukemic burden with additional treatment, it is the very rare patient who is cured by this approach. Among 547 patients who relapsed in the German ALL study, no patient without a transplantation survived more than 1 year after relapse.16 Therefore, these salvage therapies in transplantation-eligible patients are best considered as bridges to transplantation performed with curative intent.18 For those patients who achieve CR2 without any limiting organ toxicities that would preclude proceeding to transplantation and who have an identified HLA-matched sibling, an unrelated donor, or potentially a cord blood donor, the cure rate can approach 50%. It is these patients who have had a successful reinduction therapy and who can proceed to transplantation before another relapse that represent the best-case scenario for outcome after relapsed ALL (Figure 1B).
Response to first salvage therapy in patients with relapse during/after chemotherapy
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 224 . | CR = 95 (42%)* . | P . | n = 159 . | CR = 73 (46%)* . | P . | n = 65 . | CR = 22 (34%)* . | P . | |
| Early relapse | 160 | 58 (36%) | > .05 | 114 | 44 (39%) | > .05 | 46 | 14 (30%) | > .05 |
| Consolidation I | 47 | 13 (28%) | 38 | 11 (29%) | 9 | 2 | |||
| FLAG-IDA | 39 | 16 (41%) | 38 | 16 (42%) | 1 | 0 | |||
| CLAEG | 16 | 3 (19%) | 0 | 0 | 16 | 3 (19%) | |||
| Standard induction | 9 | 3 | 8 | 2 | 1 | 1 | |||
| HDAC ± Mitox | 9 | 4 | 5 | 2 | 4 | 2 | |||
| HDMTX | 7 | 3 | 3 | 1 | 4 | 2 | |||
| Other chemotherapy | 15 | 6 (40%) | 8 | 4 | 7 | 2 | |||
| SCT in relapse† | 18 | 10 (56%) | 14 | 8 (57%) | 4 | 2 | |||
| Late relapse | 64 | 37 (58%) | < .0001 | 45 | 29 (64%) | .0003 | 19 | 8 (42%) | > .05 |
| CLAEG | 9 | 2 | 9 | 2 | |||||
| Standard induction | 30 | 27 (90%) | 27 | 24 (88%) | 3 | 3 | |||
| SCT in relapse† | 1 | 1 | 1 | 1 | 0 | 0 | |||
| FLAG-IDA | 15 | 4 (27%) | 14 | 4 (29%) | 1 | 0 | |||
| HDAC ± Mitox | 1 | 0 | 1 | 0 | 0 | 0 | |||
| Other | 8 | 3 | 2 | 0 | 6 | 3 | |||
| . | Total . | B-lineage . | T-lineage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 224 . | CR = 95 (42%)* . | P . | n = 159 . | CR = 73 (46%)* . | P . | n = 65 . | CR = 22 (34%)* . | P . | |
| Early relapse | 160 | 58 (36%) | > .05 | 114 | 44 (39%) | > .05 | 46 | 14 (30%) | > .05 |
| Consolidation I | 47 | 13 (28%) | 38 | 11 (29%) | 9 | 2 | |||
| FLAG-IDA | 39 | 16 (41%) | 38 | 16 (42%) | 1 | 0 | |||
| CLAEG | 16 | 3 (19%) | 0 | 0 | 16 | 3 (19%) | |||
| Standard induction | 9 | 3 | 8 | 2 | 1 | 1 | |||
| HDAC ± Mitox | 9 | 4 | 5 | 2 | 4 | 2 | |||
| HDMTX | 7 | 3 | 3 | 1 | 4 | 2 | |||
| Other chemotherapy | 15 | 6 (40%) | 8 | 4 | 7 | 2 | |||
| SCT in relapse† | 18 | 10 (56%) | 14 | 8 (57%) | 4 | 2 | |||
| Late relapse | 64 | 37 (58%) | < .0001 | 45 | 29 (64%) | .0003 | 19 | 8 (42%) | > .05 |
| CLAEG | 9 | 2 | 9 | 2 | |||||
| Standard induction | 30 | 27 (90%) | 27 | 24 (88%) | 3 | 3 | |||
| SCT in relapse† | 1 | 1 | 1 | 1 | 0 | 0 | |||
| FLAG-IDA | 15 | 4 (27%) | 14 | 4 (29%) | 1 | 0 | |||
| HDAC ± Mitox | 1 | 0 | 1 | 0 | 0 | 0 | |||
| Other | 8 | 3 | 2 | 0 | 6 | 3 | |||
Responses are shown for patients with evaluable information on the type of salvage therapy without CNS involvement and with Ph/ BCR-ABL− ALL. Reproduced with permission from Gökbuget et al.16
HDAC indicates high-dose cytarabine; HDMTX, high-dose methotrexate; and Mitox, mitoxantrone.
No percentage was calculated in subgroups with total number of cases < 10.
Patients received SCT as their salvage treatment and CR rate indicates the remission rate after SCT.
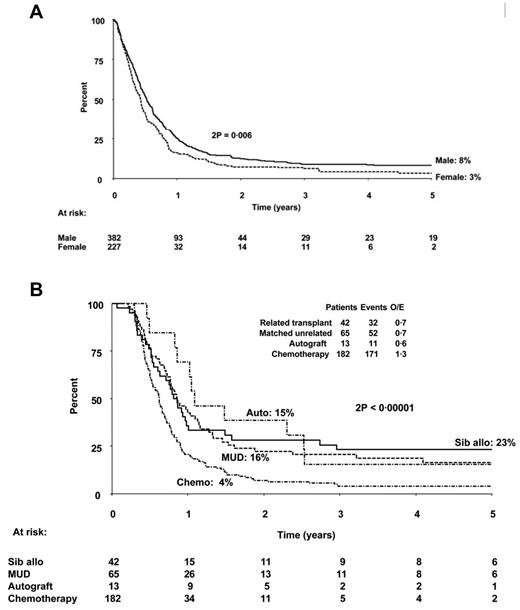
However, most patients do not achieve CR2 and proceed to a curative therapy, thus raising the question of what is the overall effectiveness of a strategy that reserves transplantation until after achievement of CR2. Recent studies suggest that the overall outcome of such an approach is really not so optimistic. The investigators who conducted the MRC/ECOG trial to determine the role of transplantation in the management of ALL7 followed not only those patients who underwent transplantation in CR1, but also those who did not, to determine the outcome of treatment. In a follow-up study of 609 patients who relapsed on the MRC UKALL12/ECOG 2993 study,19 the OS at 5 years after first relapse was only 7%, 8% for males and 3% for females (Figure 2A). Figure 2B shows the outcomes for patients in this MRC/ECOG relapse study who did not receive a sibling donor transplantation in CR1 and later relapsed and survived at least 100 days postrelapse based on the therapy they received after relapse. In fact, the percentage of patients who proceeded to allogeneic transplantation after relapse was relatively small, less than 20% of study population. The study design is partially responsible for this low rate of CR2 transplantations, because most patients with sibling donors had already been transplanted in CR1.
Probability of survival from first relapse. (A) OS in years from date of first relapse based on sex. (B) Survival postrelapse stratified according to therapy given in relapse. Patients who died within 100 days of relapse and those who were transplanted in CR1 were excluded from this analysis for better comparison of the different therapeutic modalities. MUD indicates matched unrelated donor. Used with permission from Fielding et al.19
Probability of survival from first relapse. (A) OS in years from date of first relapse based on sex. (B) Survival postrelapse stratified according to therapy given in relapse. Patients who died within 100 days of relapse and those who were transplanted in CR1 were excluded from this analysis for better comparison of the different therapeutic modalities. MUD indicates matched unrelated donor. Used with permission from Fielding et al.19
Similarly, the 5-year OS of 263 relapsed ALL patients reported by the PETHEMA study group was only 10%,17 and among 314 relapsed and refractory patients reported by the M.D. Anderson Cancer Center, it was only 3%.20 An exception to these data were recently reported by the German ALL Group, who described a 3-year OS of 24% in a group of patients who relapsed, among whom, remarkably, 75% actually proceeded to allogeneic transplantation.16 Contributing to the poor OS rates typically seen after first relapse is the inability to attain CR2, the inability to maintain sufficient performance status to undergo transplantation, and the inability to find an acceptable donor before disease progression.
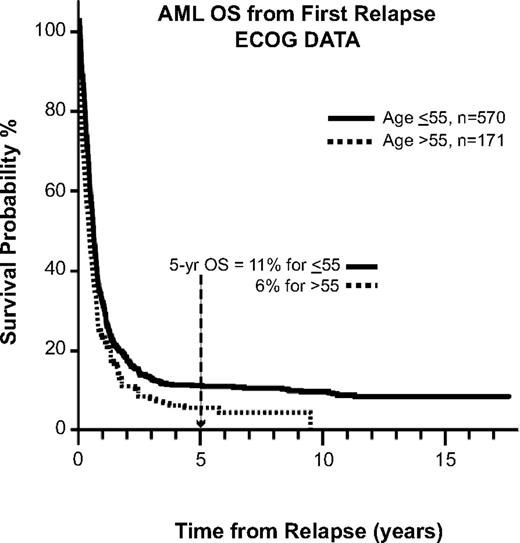
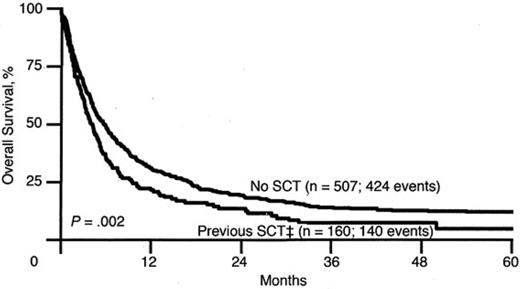
A similar trend is seen for patients with AML, as reported by ECOG.21 The 5-year OS for patients after first relapse is approximately 10%, echoing the problem facing the patient with relapsed leukemia, whose disease in general is less chemosensitive and thus less likely to achieve remission (Figure 3). Another study of relapsed AML by the Dutch-Belgian Hematology-Oncology Cooperative Group (HOVON) and the Swiss Clinical Cancer Research Collaborative Group (SAKK) reported that the survival rate after first relapse for patients who have not been transplanted previously is 12% (Figure 4). Of all of the patients treated with allogeneic transplantation postrelapse, the CR2 rate is only 18%.22 Although a few patients may still be able to proceed to transplantation after having failed to achieve remission or proceed to transplantation without a reinduction attempt, the overall cure rate remains quite low.
OS for AML after first relapse. Data are from 8 consecutive ECOG studies for newly diagnosed AML patients. Used with permission from Rowe et al.21
OS for AML after first relapse. Data are from 8 consecutive ECOG studies for newly diagnosed AML patients. Used with permission from Rowe et al.21
OS for Patients with AML after first relapse based on previous transplantation. SCT refers to both autologous and allogeneic hematopoietic stem cell transplantation. OS for the No SCT group is 12% and for the previous SCT group is 5%. Used with permission from Breems et al.22
OS for Patients with AML after first relapse based on previous transplantation. SCT refers to both autologous and allogeneic hematopoietic stem cell transplantation. OS for the No SCT group is 12% and for the previous SCT group is 5%. Used with permission from Breems et al.22
Transplantation regimens in the management of relapsed leukemia
In addition to the limitations in the efficacy of reinduction therapies to achieve remission for patients with relapsed leukemia, the problem of effective treatment is not restricted to nontransplantation therapeutics. There has been little progress in the development of transplantation regimens that could be more efficacious in treating patients with relapsed disease (ie, those not in remission at the time of transplantation). The biggest growth in transplantation therapy has been in the use of the reduced-intensity regimen to treat older patients with leukemia and other hematologic disorders. In general, these studies have shown that the leukemia patients who can be cured are those who are transplanted in CR1 when they have a low disease burden. This strategy is generally not effective for the patient with advanced disease (ie, those in relapse or induction failure). Most programs do not perform transplantation in patients who are not in remission and others are exploring new treatment approaches that might improve outcome, even for patients with active disease. These nonremission transplantation approaches include regimens incorporating radioimmunotherapy to leukemia-related antigens (ie, anti-CD45 and anti-CD33)23,24 ; helical tomotherapy,25,26 which seeks to increase the radiation dose to the diseased BM without increasing the toxicity to surrounding organs; novel-agent-based regimens that replace fludarabine with clofarabine; posttransplantation hypomethylating agents or histone deacetylase inhibitors to reduce the chances of relapse; and genetically modified leukemia-specific T cells. These are all important avenues of study designed to reduce relapse after transplantation, especially for patients at high risk because they were not in remission at the time of transplantation.
What are some new approaches that could provide a more effective bridge to transplantation?
Most reinduction regimens for AML use the same medications that were used to achieve CR1, although some use an alternative regimen, including agents such as etoposide and mitoxantrone that are not generally part of primary induction or consolidation therapies. In some patients, particularly those with an early relapse or in whom the disease kinetics are relatively slow, the use of hypomethylating-based therapy combined with histone deacetylase inhibitors can achieve remission with less toxicity to the patient, thus facilitating a transition to transplantation. There is ongoing interest in the use of FLT3 inhibitors to achieve remission, although these agents have not yet demonstrated efficacy consistently enough to rely on them as bridges to transplantation. Recent progress in the sequencing of an individual patient's leukemia cells may also provide a means of more intelligently determining the most appropriate and effective therapy to achieve CR2.27,28 A promising bispecific CD33/CD3 T-cell engager is currently in preclinical development for an AML bispecific T-cell engager (BiTE) therapy,29 which is analogous to the CD19/CD3 BiTE therapy currently in human trials for ALL.30
In ALL, the focus has been on the development of both new drugs and immunologic therapies that might be effective in achieving CR2. These include nelarabine for the treatment of T-cell ALL as an alternative to reinduction therapy with the same drugs31 and clofarabine-based reinduction regimens for the treatment of relapsed ALL have also proven effective.32 Despite the use of novel drugs for reinduction, the achievement of CR2 is still only approximately 30%-50%.15,33 Recent data on a novel therapeutic, BiTE, has shown an 80% response rate in patients with relapsed ALL. This approach uses an Ab fragment recognizing the CD19 antigen on pre-B-ALL fused to an anti-CD3 Ab fragment that “engages” a local T-cell response to enlist the patient's immune system in tumor cell killing and to increase the remission rate. These studies have shown an impressive rate of response and, importantly, acted as a bridge to transplantation in patients with relapsed ALL; 100% of the 8 patients who proceeded to transplantation were alive and in hematologic remission at analysis (Figure 5).30 These data, if confirmed, also offer the possibility that, in the future, cure may be achieved for a much higher proportion of patients with relapsed disease without transplantation, thus providing not only an effective bridge to transplantation, but also hope for those who are transplantation ineligible. The use of CD19-specific chimeric antigen-transduced T cells is also showing strong antileukemic activity in patients with CD-19+ CLL and is now being explored in patients with CD19+ ALL.27,28 Ab-targeted drug delivery is also being explored with CD19 Ab-drug conjugates.34
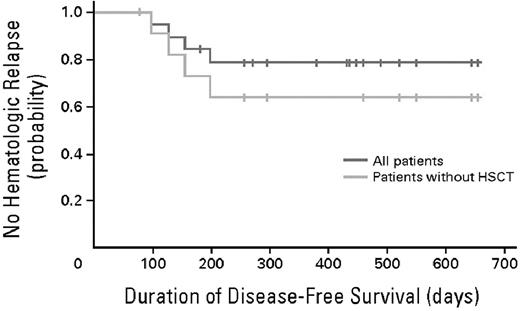
Time to clinical relapse. The probability of relapse-free survival after initiation of blinatumomab treatment in all 20 evaluable patients is shown in blue. Median follow-up for relapse-free survival is 405 days (range, 78-655). The probability of relapse-free survival after initiation of blinatumomab treatment in all 12 evaluable patients who have not undergone allogeneic transplantation after completion of blinatumomab treatment is shown in yellow. Median follow-up for relapse-free survival is 276 days (range, 78-655). HSCT indicates hematopoietic stem cell transplantation. Used with permission from Topp et al.30
Time to clinical relapse. The probability of relapse-free survival after initiation of blinatumomab treatment in all 20 evaluable patients is shown in blue. Median follow-up for relapse-free survival is 405 days (range, 78-655). The probability of relapse-free survival after initiation of blinatumomab treatment in all 12 evaluable patients who have not undergone allogeneic transplantation after completion of blinatumomab treatment is shown in yellow. Median follow-up for relapse-free survival is 276 days (range, 78-655). HSCT indicates hematopoietic stem cell transplantation. Used with permission from Topp et al.30
In addition to the development of novel agents that can be used to bridge a patient from relapse to transplantation and improved conditioning regimens that reduce the chances of relapse after transplantation, there are additional measures that can be taken to optimize the chances that a patient will actually be able to receive a transplantation after detection of relapse. The first, and simplest, is to identify a donor early on in the course of treatment of the disease. We believe that family typing should be done in all patients with acute leukemia, not so much to plan transplantations for everyone, but to know what the options are. After that, if necessary, plans can be made in an expeditious way, including performing searches through the National Marrow Donor Program and cord blood registries to know what the possibilities might be for that patient. Furthermore, with the development of haploidentical transplantations, there are increased donor options that improve the chances of moving quickly to transplantation without a reinduction attempt in patients with a low disease burden (ie, detectable by MRD or morphologically as < 10% blasts).35
The United States lags far behind Europe in performing appropriate molecular disease assessments that would enable determination of the depth of the remission and the likelihood of relapse in patients who achieve remission. Many practitioners still rely on morphology and cytogenetics, including FISH, to document remission and follow blood counts and BM results over time. For AML, the predictive value of minimal residual disease (MRD) detection is less well supported than for ALL, and MRD assessment is not routinely used for clinical decision making.36-38 Multiple studies in ALL have shown that the detection by flow cytometry or PCR of MRD after completion of induction therapy predicts relapse-free survival, and that the detection of an increasing leukemia signal is a harbinger of relapse, often by a period of months.39-43 In fact, MRD monitoring in select centers appears to have superseded other prognostic factors in adult ALL.44 Therefore, the incorporation of MRD assessment strategies into the overall treatment of ALL improves the interpretation of depth of the remission. Patients with AML could also benefit from the clinical application of reliable, highly predictive MRD assays. Regular assessments of the BM in patients in CR1 could either confirm that the remission is stable or alert physicians and patients to the need to consider alternative therapies or transplantation, because an MRD signal is an indication of incurability and chemoresistance of the leukemia. Moreover, pretransplantation MRD may also predict for increased risk of relapse after allogeneic transplantation, indicating a possible need for posttransplantation therapies.45
This commentary does not mean to imply that the difficulty of achieving CR2 and getting a patient to transplantation is justification, in and of itself, for exposing all patients to the risks of transplantation during CR1. It is simply intended to encourage transplantation in CR1 for all patients with acute leukemia in whom this is deemed appropriate, such as those with poor-prognosis AML or ALL, and to point out the many flaws and inadequacies in our therapeutic strategy related to bridging therapy from relapse to transplantation and the impact of such clinical situations on overall outcomes. The development of more effective drugs and immune approaches to achieve CR2, more effective transplantation regimens for patients who do not achieve CR2, implementation of rigorous assessment of MRD, and preemptive planning of transplantation will hopefully change the outcome of these therapies, bringing the probability of cure for patients with relapsed acute leukemia from myth to reality.
Acknowledgments
The authors thank Dr Mary Horowitz for sharing CIBMTR data, including figures, and Dr Sandra Thomas and Sarah Farkash for help in the preparation of this manuscript.
This work was made possible by funding from the National Institutes of Health (grants P30 CA33572 and P01 CA 30206).
National Institutes of Health
Authorship
Contribution: S.J.F. and J.M.R. conceptualized and wrote this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen J. Forman, MD, Department of Hematology/HCT, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: sforman@coh.org.