Key Points
Lenalidomide CHOP did not improve the complete metabolic response rate compared with historical controls in previously untreated AITL patients aged 60 to 80 years.
DNMT3A mutations were present in 32% of patients and appeared to confer shorter PFS.
Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is a frequent T-cell lymphoma in the elderly population that has a poor prognosis when treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) therapy. Lenalidomide, which has been safely combined with CHOP to treat B-cell lymphoma, has shown efficacy as a single agent in AITL treatment. We performed a multicentric phase 2 trial combining 25 mg lenalidomide daily for 14 days per cycle with 8 cycles of CHOP21 in previously untreated AITL patients aged 60 to 80 years. The primary objective was the complete metabolic response (CMR) rate at the end of treatment. Seventy-eight of the 80 patients enrolled were included in the efficacy and safety analysis. CMR was achieved in 32 (41%; 95% confidence interval [CI], 30%-52.7%) patients, which was below the prespecified CMR rate of 55% defined as success in the study. The 2-year progression-free survival (PFS) was 42.1% (95% CI, 30.9%-52.8%), and the 2-year overall survival was 59.2% (95% CI, 47.3%-69.3%). The most common toxicities were hematologic and led to treatment discontinuation in 15% of patients. This large prospective and uniform series of AITL treatment data was used to perform an integrative analysis of clinical, pathologic, biologic, and molecular data. TET2, RHOA, DNMT3A, and IDH2 mutations were present in 78%, 54%, 32%, and 22% of patients, respectively. IDH2 mutations were associated with distinct pathologic and clinical features and DNMT3A was associated with shorter PFS. In conclusion, the combination of lenalidomide and CHOP did not improve the CMR in AITL patients. This trial clarified the clinical impact of recurrent mutations in AITL. This trial was registered at www.clincialtrials.gov as #NCT01553786.
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) and related nodal peripheral T cells lymphoma with a T follicular helper (TFH) phenotype (PTCL-TFH) are highly prevalent peripheral T-cell lymphomas (PTCLs) in Western countries,1-3 preferentially affect older patients, and are associated with autoimmunity, high-stage disease, and poor outcome.4 AITL is characterized by a prominent tumor microenvironment associated with vascular hyperplasia, expansion of follicular dendritic cells, and the presence of plasma cells, lymphocytes, eosinophils, and large B cells that are frequently infected by Epstein-Barr virus (EBV).4 Neoplastic cells with a TFH phenotype5 are present in varying proportions and sometimes have an abundant clear cytoplasm. The molecular mechanisms sustaining the transformation of normal TFH cells in AITL and PTCL-TFH include disruption of the regulation of DNA methylation and hydroxymethylation by mutations in TET2,6,7 DNMT3A,8,9 and IDH210 and activation of signaling pathways by RHOA mutation encoding p.G17V11-13 or mutations affecting T-cell receptor (TCR) or costimulatory pathways.14 TET2 and DNMT3A mutations occur frequently in hematopoietic progenitor cells6,8 and are present in different cell subsets,15,16 suggesting that they may not only have an effect on neoplastic cells but also on bystander cells. However, these mutations have been mainly studied in retrospective cohorts, and whether they affect clinical presentation and outcome is unclear.
Despite an improvement in our understanding of AITL oncogenesis, there has been little therapeutic progress, and patients with AITL still have a poor prognosis when treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) therapy, making the development of new treatments an unmet need.
Lenalidomide has a complex mechanism of action, combining antiproliferative effects on tumor cells, which are mediated by the inhibition of the protein CEREBLON, antiangiogenic properties, and an increased immune response.17 The combination of lenalidomide with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy in diffuse large B-cell lymphoma18-20 and follicular lymphoma21 has shown an acceptable safety profile but variable efficacy. Furthermore, lenalidomide has been demonstrated to have anti-lymphoma efficacy in relapsed-refractory (R/R) PTCL as a single agent,22-25 especially in AITL.22
Here, we present the results of a phase 2 trial combining lenalidomide and CHOP in previously untreated AITL patients aged 60 to 80 years. Taking advantage of this prospective and homogeneous cohort, we also studied the clinical, pathologic, biologic, and molecular features of AITL and correlated them to outcome, aiming to identify predictive factors.
Methods
Study design and participants
The REVlimid in AngioImmunoblastic-T cell Lymphoma (REVAIL) study was an open-label, single-arm, multicenter, phase 2 clinical trial. Because the indication of autologous stem cell transplantation is highly debated in young patients as an upfront AITL treatment, we focused our trial on elderly patients aged 60 to 80 years who were eligible to participate in the trial if they had untreated, histologically proven AITL and a good performance status with an Eastern Cooperative Oncology Group (ECOG) score 0-2. Patients were excluded if they had central nervous system involvement; a contraindication or hypersensitivity to any of the trial drugs; active bacterial, viral, or fungal infection, particularly active hepatitis B or C or HIV-positive serologic tests; impaired renal function (creatinine clearance < 50 mL/min); impaired liver function (total bilirubin level > 30 μmol/L, transaminases > 2.5 times the upper normal limits); poor bone marrow (BM) reserves (as defined by <1.0 × 109/L neutrophils or <100 × 109/L platelets, unless related to the lymphoma); or any history of other malignancy during the past 5 years.
This study complied with all provisions of the Declaration of Helsinki and its current amendments and was conducted in accordance with Good Clinical Practice guidelines. The protocol and consent forms were approved by an independent research ethics committee (CPP Ile de France IV). Patients were required to provide written informed consent before enrollment. The protocol is available online, and the study has been registered as NCT01553786.
Procedures
Patients received 8 cycles of lenalidomide-CHOP (Len-CHOP) every 3 weeks. Each cycle of Len-CHOP consisted of 25 mg of oral lenalidomide on days 1 to 14 in combination with 50 mg/m2 intravenous doxorubicin, 750 mg/m2 intravenous cyclophosphamide, and 1.4 mg/m2 intravenous vincristine (up to a maximum dose of 2 mg) on day 1 and 40 mg/m2 oral prednisone on days 1 to 5. A reduction in the dose of lenalidomide was mandatory in case of toxicity, as described in the protocol (supplemental Appendix).
A total of 4 intrathecal injections of methotrexate were administered during the first 4 cycles for all patients, which was the standard used to treat aggressive lymphoma when the trial was designed. The administration of granulocyte colony-stimulating factor was mandatory. Patients received prophylaxis against thrombosis by treatment with low-molecular-weight heparin throughout the study. Antiemetics and supportive care were the standard of care and provided at the discretion of the treating physician. Adverse events were evaluated throughout the treatment period and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. All grade 3 and 4 events and grade 2 infections were recorded in detail. The response was evaluated by positron emission tomography-computed tomography (PET-CT) after 8 cycles, which was planned 30 days (maximum 60 days) after the last intake of lenalidomide treatment using the Lugano criteria26 and was centrally reviewed by 3 investigators (A.S.C., S.B., and M.M.). In cases of premature withdrawal during the treatment period, including for progression, the end-of-treatment evaluation was performed 30 days after the last drug administration or before the introduction of any new treatment. After treatment completion, follow-up consisted of clinical examination every 3 months for 2 years, then every 6 months for 3 years, and thereafter once a year. CT scans were performed every 6 months for 2 years and every year thereafter. The minimal expected follow-up was 18 months after the last patient had completed the treatment. The maximum standardized uptake value (SUVmax) and total metabolic tumor volume were retrospectively determined at baseline using previously described methods.27
Outcomes
The primary end point of the trial was the complete metabolic response (CMR) rate at the end of treatment according to the Lugano Classification.26 Secondary end points were the complete response (CR) rate at the end of treatment according to the International Harmonization Project,28 as assessed by the site investigator. Progression-free survival (PFS) at 2 years (2y-PFS) was defined as the time from registration to relapse for complete responders or disease progression or death from any cause, and overall survival (OS) was defined as the time from the date of registration until the date of death as a result of any cause.
Pathologic review and biologic analysis
Formalin-fixed paraffin-embedded tumor samples were centrally collected, reviewed, and classified as AITL or PTCL-TFH according to World Health Organization 2017 classification.29 Extensive immunochemistry, including the search for TFH markers and follicular dendritic cell (FDC) antigens, as well as in situ hybridization with EBV RNA (EBER) probes, were performed and scored as described in Dobay et al.30 Bone marrow trephine biopsies were examined locally. Fresh blood samples were centrally collected for T-lymphocyte immunophenotyping by flow cytometry with anti-CD2, -CD3, -CD4, -CD5, -CD8, -CD7, -CD10, and -CD26 antibodies, and T-clonality assessment was performed by polymerase chain reaction (PCR) denaturing gradient-gel electrophoresis analyses.31 Circulating neoplastic T cells were characterized by an aberrant immunophenotype (CD4 and CD10 coexpression, CD3 loss or downregulation of CD3, and/or loss of CD7) or a monoclonal TCR rearrangement. EBV viral load in plasma was centrally measured by quantitative PCR using the Qiasymphony extraction platform (Quiagen) and artus EBV RG PCR Kit. The results are expressed in international units per mL (IU/mL). DNA was extracted from formalin-fixed paraffin-embedded tumor samples, and 9 genes (RHOA, IDH2, STAT3, STAT5B, CD28, TET2, DNMT3A, SETD2,and PLCG1) were sequenced on a Ion Personal Genome Machine (ion PGM; ThermoFisher Scientific) at an expected depth of 1000×, as previously reported.32
Statistical analysis
We anticipated that 60% of patients would achieve a CR with Len-CHOP and computed the sample size to provide 80% power at an overall 5% (1-sided) significance level to detect a CMR improvement from 45% to 60% (null hypothesis, 45%; alternative hypothesis, 60%), with 45% being an early estimate based on the results of the previous LYmphoma Study Association (LYSA) trial Rituximab in AngioImmunoblastic T-cell Lymphoma (RAIL).33 Success of the study was defined as an observed number of responses statistically significantly higher than the null hypothesis, meaning that the lower limit of the 90% confidence interval (CI) is higher than 45%. If 78 patients were analyzed, 43 responding patients would correspond to 90% CI (0.453-0.648), which is the lower number of responding patients with 90% CI, excluding 0.45. This means that 43 of 78 (55%) patients are the prespecified minimum responses that would be considered a success for this trial. The sample size calculation was based on Simon’s randomized phase 2 design. The first-stage analysis was performed after 37 evaluable patients had been included. Because 17 patients achieved a CR, the trial proceeded to the second stage, and we planned to recruit 70 evaluable patients for an estimated total of 80 included participants, given the expected dropout rate. Censored data are presented as Kaplan-Meier plots of time to first event and summary tables of Kaplan-Meier estimates for criterion rates at fixed timepoints, with 95% CIs. Patients who received at least 1 dose of the study treatment were included in the safety analysis, and those who received at least 1 cycle of treatment were included in the efficacy analysis.
Correlations between the presence of a mutation and clinical or pathologic features were studied using logistic regression.
Results
Patients and treatment
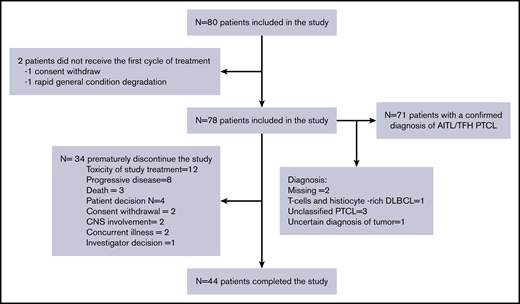
In total, 80 patients were enrolled between November 2011 and March 2017 in 23 centers in France and 2 in Belgium. Two patients did not receive at least 1 dose of treatment (1 because of withdrawal of consent and 1 because of a rapid change in general condition) and were excluded from the safety and efficacy analyses, meaning that 78 patients were included in the efficacy and safety analysis. Seventy-one patients who received at least 1 dose of lenalidomide and 1 cycle of CHOP had a confirmed diagnosis of AITL (N = 67) or nodal PTCL with a TFH phenotype (N = 4). These patients were included in the sensitivity analysis and ancillary studies and are subsequently referred to as the AITL/TFH set (Figure 1).
The baseline characteristics of the patients are listed in Table 1. Briefly, the median age was 70 years (interquartile range, 67-73), and 37.7% of patients had an IPI of 4 to 5, 49.3% had bone marrow infiltration (BMI), and 42.9% had hypergammaglobulinemia, which is a typical feature of AITL. Forty-four (55%) patients completed the study, whereas 34 discontinued the treatment prematurely because of toxicity (N = 12), progressive disease (N = 8), death (N = 3), withdrawal of consent (N = 2), major protocol violation (N = 2), concurrent illness (N = 2), patient decision (N = 4), or investigator decision (N = 1). The median dose intensity received by the patients was 2275/2800 mg (81%) for lenalidomide, 98% for doxorubicin, and 97% for cyclophosphamide. The median interval between cycles was 21 days.
Clinical activity
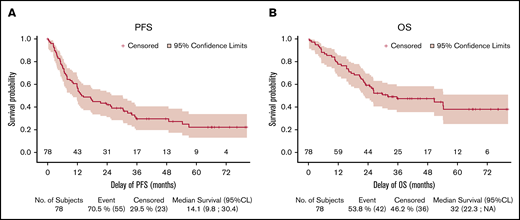
Forty-five patients received 8 cycles of Len-CHOP. A patient died of myocardial infarction after the eighth cycle but before the evaluation by PET-CT, resulting in 44 evaluated patients. Among the evaluated patients, 43 of 44 patients were evaluated by PET-CT, and CMR was achieved in 32 (41%; 95% CI: 30%-52.7%) patients and a partial response in 12 (15.4%), representing an overall response rate of 56.4%. In addition, 1 patient evaluated by CT presented a CR on CT, had a normal BM trephine, and was therefore considered to be in CMR. Thirteen patients (16.7%) had stable or progressive disease, 3 patients died (4%), and 18 (23%) were not evaluated, meaning that they were not assessed by PET-CT at the end of treatment or after a premature withdrawal (Table 2; Figure 1). The CMR rate was lower than the prespecified CMR of 55% to meet the primary end point of this study. With a median follow-up of 45 (95% CI: 36-65.9) months, the 2-year PFS was 42.1% (95% CI: 30.9-52.8) and the 2-year OS was 59.2% (95% CI: 47.3-69.3; Figure 2). Fifteen patients in complete response relapsed during the follow-up, and the median duration of response was 42.6 (95% CI: 24-not estimable) months. Similar results were observed for the 71 patients with a confirmed diagnosis of AITL/TFH (supplemental Figure 1).
At the time of the analysis, 41 patients had experienced relapsed or refractory disease. Thirty-three patients received second-line chemotherapy, the most frequent being bendamustine in 9 patients and a combination of gemcitabine and oxaliplatin in 7 patients, resulting in a second complete response in only 5 patients. Only 1 patient received second-line autologous stem cell transplantation.
Forty-two patients died during the follow-up. The cause of death was lymphoma in 29 (69%) patients, toxicity of the study treatment in 4 (10%), concurrent illness in 3 (7%), and unknown in 4 (9.5%).
Safety
Neutropenia was the most frequent adverse event, with grade 4 neutropenia occurring in 55 (71%) patients (Table 3). Eighteen patients (23%) experienced grade 3 or 4 infections. A grade 5 adverse event occurred in 4 patients: 3 patients developed sepsis, including 2 during the neutropenic phase, and 1 patient experienced fatal myocardial infarction occurring after the eighth cycle. Eight patients presented a rash, which was grade 3 in 2 patients. Ten episodes of venous thrombosis were detected in 8 patients. Constipation was reported in 10 (13%) patients, including 9 grade 2 and 1 grade 3 constipation, and peripheral neuropathy was reported in 14 (18%) patients, 2 of whom were affected by grade 3 neuropathy. Four secondary primary malignancies were reported, 1 myelodysplastic syndrome, 1 acute myeloid leukemia, 1 marginal zone B-cell lymphoma, and 1 neuroendocrine tumor, which were diagnosed 17, 23, 36, and 16 months after inclusion, respectively.
Pathologic and molecular features
The pathologic features of the 71 confirmed AITL/TFH biopsies are shown in Figure 3. BMI was diagnosed on the pretherapeutic staging BM biopsy in 32 of 66 (48%) patients. A significant circulating clonal T-cell population (ie, representing ≥10% of the T-cell compartment, subsequently called a clonal population) was detected in 28 of 55 (51%) patients, and a phenotypically aberrant population was detected by flow cytometry in 30 of 53 patients (57%). This aberrant population, presumed to be circulating tumor cells, was less than 100 cells/mm3 in 16 of 53 (30%) patients. There was no correlation between the presence of BMI and the detection of an abnormal population in the blood (supplemental Figure 2), suggesting that BM evaluation using BM trephine is still required in this disease. EBV was detected in the blood in 48 of 59 (81%) patients, with a median of 5812 (interquartile range, 1413-48 480) copies. The detection of the EBV DNA in the plasma did not correlate with the detection of EBER in the biopsy (P = .78; supplemental Figure 3). The SUVmax was not associated with a higher percentage of tumor cells estimated in the tumor biopsy by immunochemistry, but samples with a higher number of EBER-positive cells (>5 EBER-positive cells by high-power field) showed a trend toward a higher SUVmax (P = .06), suggesting that B cells from the tumor microenvironment could, at least in part, participate in the glucose avidity of the AITL lymph nodes (supplemental Figure 2).
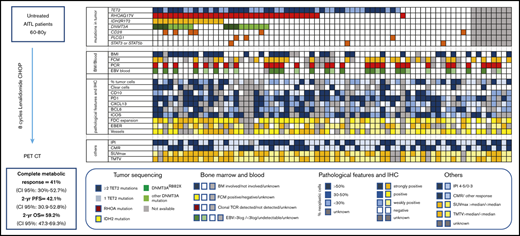
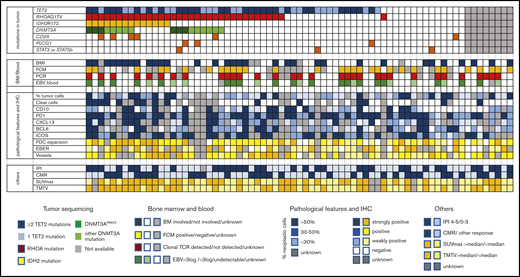
Description of molecular, pathologic, biologic, and imaging features of 71 AITL/TFH patients in the REVAIL trial. The section “mutations in tumor” describes distribution of mutations in TET2, RHOA, DNMT3A IDH2, CD28, PLCG1, STAT3, and STAT5B. Patients represented in dark blue bore at least 2 TET2 mutations, whereas only 1 TET2 mutation was detected in light blue samples. DNMT3AR882X variants are represented in dark green, whereas samples in light green harbored DNMT3A mutations altering another residue than R882. Sequencing failure, in gray, was because of the absence of enough material for DNA extraction or the presence of degraded DNA. In the section “BM/Blood,” BMI represents the presence of bone marrow involvement, as determined locally by the examination of BM trephine. Flow cytometry positivity means that cells with an aberrant phenotype were detected in blood by flow cytometry before treatment. PCR positive means that a significant (ie, representing at least 10% of the T-cell population) TCR clonal population was detected by denaturing gradient-gel electrophoresis PCR. EBV blood shows patients with a high level of EBV > 3 log, a low level of EBV (<3 log), or the absence of detectable EBV replication in blood. “pathological features and IHC” section describes the pathologic features of AITL biopsy. Blue is used to describe neoplastic cells, whereas the tumor microenvironment is scored in yellow. The percentage of neoplastic T cells was estimated by pathologists after IHC interpretation: >50% in darker blue cases, 30% to 50% in blue cases, and <30% in lighter blue cases. Clear cells with a large cytoplasm were detected in cases in dark blue, absent in cases in white, or unknown in gray. Immunohistochemistry assays for TFH markers (CD10, PD1, CXCL13, BCL6, ICOS) are described in the legend, with score 3 indicating >50% positive tumor cells, score 2 indicating 30% to 50% positive tumor cells, score 1 indicating <30% positive cells, and score 0 indicating no staining on tumor cells. FDC distribution was evaluated by CD21 and/or CD23 immunostaining and was assigned a score of 0 when the signal was restricted to germinal centers; 1 in case of perifollicular expansion; 2 in case of perifollicular and perivascular expansion; or 3 for diffuse expansion. The EBV status in large lymphoid cells was based on counting EBER-positive large cells and scored as follows: negative, absence of large EBV-positive cells; positive, up to 5 large EBV-positive cells per high power field (hpf); strongly positive, more than 5 per hpf or sheets or aggregates of large EBV-positive cells. As indicated in the legend, the sections “others” represent IPI at diagnosis (IPI 4-5 vs 1-3), CMR (CMR vs other response or nonevaluable), and SUVmax and TMTV, both being dichotomized at the median of the cohort.
Description of molecular, pathologic, biologic, and imaging features of 71 AITL/TFH patients in the REVAIL trial. The section “mutations in tumor” describes distribution of mutations in TET2, RHOA, DNMT3A IDH2, CD28, PLCG1, STAT3, and STAT5B. Patients represented in dark blue bore at least 2 TET2 mutations, whereas only 1 TET2 mutation was detected in light blue samples. DNMT3AR882X variants are represented in dark green, whereas samples in light green harbored DNMT3A mutations altering another residue than R882. Sequencing failure, in gray, was because of the absence of enough material for DNA extraction or the presence of degraded DNA. In the section “BM/Blood,” BMI represents the presence of bone marrow involvement, as determined locally by the examination of BM trephine. Flow cytometry positivity means that cells with an aberrant phenotype were detected in blood by flow cytometry before treatment. PCR positive means that a significant (ie, representing at least 10% of the T-cell population) TCR clonal population was detected by denaturing gradient-gel electrophoresis PCR. EBV blood shows patients with a high level of EBV > 3 log, a low level of EBV (<3 log), or the absence of detectable EBV replication in blood. “pathological features and IHC” section describes the pathologic features of AITL biopsy. Blue is used to describe neoplastic cells, whereas the tumor microenvironment is scored in yellow. The percentage of neoplastic T cells was estimated by pathologists after IHC interpretation: >50% in darker blue cases, 30% to 50% in blue cases, and <30% in lighter blue cases. Clear cells with a large cytoplasm were detected in cases in dark blue, absent in cases in white, or unknown in gray. Immunohistochemistry assays for TFH markers (CD10, PD1, CXCL13, BCL6, ICOS) are described in the legend, with score 3 indicating >50% positive tumor cells, score 2 indicating 30% to 50% positive tumor cells, score 1 indicating <30% positive cells, and score 0 indicating no staining on tumor cells. FDC distribution was evaluated by CD21 and/or CD23 immunostaining and was assigned a score of 0 when the signal was restricted to germinal centers; 1 in case of perifollicular expansion; 2 in case of perifollicular and perivascular expansion; or 3 for diffuse expansion. The EBV status in large lymphoid cells was based on counting EBER-positive large cells and scored as follows: negative, absence of large EBV-positive cells; positive, up to 5 large EBV-positive cells per high power field (hpf); strongly positive, more than 5 per hpf or sheets or aggregates of large EBV-positive cells. As indicated in the legend, the sections “others” represent IPI at diagnosis (IPI 4-5 vs 1-3), CMR (CMR vs other response or nonevaluable), and SUVmax and TMTV, both being dichotomized at the median of the cohort.
Sixty-three samples were successfully sequenced. As previously described, TET2 mutations were the most common alteration and were found in 49 of 63 (78%) patients; multiple mutations were often detected (28 of 49, 57%). The RHOA mutation encoding p.G17V was detected in 34 (54%) patients. DNMT3A mutations were present in 20 (32%) patients, including 6 (9.5%) with a mutation affecting the R882 residue (DNMT3AR882X). IDH2 mutations, all affecting residue R172, were present in 14 (22%) patients, and mutations in CD28, PLCG1, STAT3, and STAT5B were detected in 6, 3, 2, and 1 patients, respectively (Figure 3). As previously reported, the variant allele frequencies of TET2 and DNMT3A were higher than those of RHOA or IDH2, which was consistent with TET2 and DNMT3 being ancestral mutations in this disease (supplemental Figure 4).
TET2 mutations were associated with an age > 65 years and higher IPI (supplemental Table 1). TET2 and RHOA mutations, known to be associated with PTCL with a TFH phenotype, were associated with strong Inducible T-cell costimulator (ICOS) expression (>30% positive cells). RHOA mutations were associated with significant FDC expansion (defined as a score ≥ 2, meaning they had at least perivascular or perifollicular expansion30 ). IDH2 mutations, also associated with FDC expansion, were associated with the presence of large clear cells with an abundant cytoplasm, which are a characteristic of a subset of AITL, and these findings were consistent with a previous report.34 Unexpectedly, IDH2 and RHOA mutations, which are known to be restricted to neoplastic T cells,35 were significantly associated with BMI (supplemental Table 1).
Although most of the mutations did not affect the response rate or PFS, DNMT3A mutations showed a trend toward a lower rate of CMR and correlated with significantly shorter PFS (supplemental Table 2). Notably, none of the patients bearing the frequent mutation DNMT3AR882X responded to Len-CHOP therapy. Overall, no single mutation predicted OS, but the 8 patients harboring TET2, DNMT3A, and IDH2 mutations showed shorter survival than the others (supplemental Table 1; supplemental Figure 5).
Predictive factors of outcome and prognosis markers
We took advantage of this well-annotated series to identify clinical, biologic, metabolic imaging, and molecular factors that may predict the response to treatment or prognosis in AITL (Figure 4).
Forest plot representing the impact of clinical, biologic, and molecular features on CMR, PFS, and OS. BMI, bone marrow involvement; TMTV, total metabolic tumor volume; FCM, flow cytometry; PB EBV, EBV viral load measured in peripheral blood > median.
Forest plot representing the impact of clinical, biologic, and molecular features on CMR, PFS, and OS. BMI, bone marrow involvement; TMTV, total metabolic tumor volume; FCM, flow cytometry; PB EBV, EBV viral load measured in peripheral blood > median.
An IPI score of 3 to 5 was associated with a lower CMR rate and a shorter OS, confirming the value of this prognostic index in this disease. Apart from the IPI, a detectable phenotypically aberrant population in the blood was associated with a lower CMR rate but did not affect PFS or OS. More than 1 extranodal site, BMI, albumin levels inferior to normal, β2 microglobulin > 3.5 g/L, and DNMT3A mutation were associated with shorter PFS, whereas male sex, low albumin levels, and β2 microglobulin > 3.5 g/L were associated with shorter OS. Remarkably, BMI was associated with shorter PFS and OS, but the detection of an abnormal circulating lymphoid cell population, which was presumed to be neoplastic cells, by flow cytometry or PCR, did not highlight the difference between BM and blood dissemination in TFH-derived lymphoma.
Total metabolic tumor volume (TMTV) was not associated with PFS or OS in dichotomizing patients, either by the median (433 mm3) or the previously determined36 threshold of 230 mm3 (supplemental Figure 6). Given the limited statistical power, multivariate analyses were not performed in this cohort.
Discussion
Our results do not support the addition of lenalidomide to CHOP in untreated elderly AITL patients. The primary end point of an increase in the CMR rate from 45% to 60% was not reached, and the CMR was 41% (95% CI: 30%-52.7%). Another trial assessing the combination of lenalidomide with cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (CHOEP) in nodal PTCL showed a CR rate of 48% and a high rate of early discontinuation,37 consistent with our results. Taken together, these data indicate the lack of benefit of the addition of lenalidomide to chemotherapy in the upfront treatment of AITL.
This trial is one of the largest studies dedicated to AITL and TFH lymphomas and allowed us to prospectively study the mutational landscape of AITL and its association with clinical features and outcomes. Patients bearing both TET2 and RHOA mutations appeared to exhibit high ICOS expression more frequently than those without these mutations. The expression of RHOAG17V in the T-cell lineage was recently shown to result in ICOS overexpression in a mouse model.38 Our observation reinforces the relationship between ICOS, which may be a critical player in the oncogenesis of AITL, and the RHOA mutation. DNMT3A mutation was associated with a trend toward a lower CMR rate and significantly shorter PFS. Furthermore, no patient carrying the DNMT3AR882X variant achieved CR. This finding suggests that previous results observed in acute myeloid leukemia, showing that DNMT3AR882X confers resistance to anthracyclins,39 by impairing nucleosome eviction and chromatin remodeling in response to anthracycline treatment, could translate to AITL. Non–anthracycline-based frontline chemotherapy might be given to such patients or participation in clinical trials exploring other novel combinations that could potentially overcome this resistance.
Predictive factors for survival have been previously investigated. In our study, TMTV was not predictive of PFS or OS, in contradiction to previous reports from our group and another.36,40 However, these 2 previous publications included a large proportion of PTCL that was not otherwise specified and anaplastic large-cell lymphomas, for which the disease presentation and metabolic volume frequently might differ from those of AITL, possibly explaining the observed discrepancy.
AITL remains a difficult-to-treat lymphoma, and no significant therapeutic progress has been made in years. No positive signal of efficacy was seen in the previous LYSA phase 2 trial RAIL, in which 27 previously untreated patients with AITL, aged 60 to 80 years, were treated with a combination of rituximab and CHOP between 2005 and 2008.33 The CR rate was 44%, median PFS was 16 months, and median OS was 28 months, similar to the results of the REVAIL trial, for which the CMR rate was 43.6%, median PFS was 14 months, and median OS was 32 months, confirming the lack of benefit from the addition of rituximab or lenalidomide to CHOP in elderly patients with AITL. These results also emphasize the absence of the improvement in AITL patient survival during the last few decades and stress the need to develop alternative therapies to increase the response rate and the duration of response of AITL patients, including new strategies, such as maintenance to increase the duration of response of AITL patients.
In this trial, patients with mutations in the 3 epigenetic modifiers TET2, DNMT3A, and IDH2 had a poor prognosis, with a median OS of 14 months when treated with CHOP-based therapy. Targeting the modification of the epigenome is an attractive option in this disease, which shows a high frequency of mutations that disrupt epigenetic regulator genes. Indeed, we and others have observed sustained responses with the hypomethylating agent 5-azacytidine in refractory relapsed AITL patients.32 The oral form of 5-azacytidine, CC-486, has been combined with romidepsin in a phase 1 study,41 with promising results and is currently being tested as a single agent in R/R AITL in an international phase 3 trial (NCT03593018) or in combination with CHOP therapy in previously untreated patients (NCT03542266). Interestingly, although our results suggest the absence of the efficacy of lenalidomide in combination with chemotherapy, lenalidomide showed promising activity when combined with 5-azacytidine in high-risk myelodysplastic syndrome,42 suggesting that combining lenalidomide and epigenetic-targeting drugs might be considered for treating AITL.
Send data sharing requests via e-mail to the corresponding author.
Acknowledgments
The authors thank the Lymphoma Academic Research Organization (LYSARC) teams for their contribution to the management of various aspects of the trial; the patients, families, caregivers, and investigators of the LYSA who participated in the REVAIL clinical trial; and AJE for English editing.
This work was supported by the Celgene Corporation, the Leukemia Lymphoma Society (SCOR grant LLS SCOR 7013-17), the ARTGIL association, and the Federation pour la Recherche Médicale (N. DEQ20160334875). This trial was sponsored by LYSARC with financial support from Celgene Corporation. The study was designed by LYSA investigators, and all logistical aspects were managed by the LYSARC. All authors had full access to the data, and Celgene reviewed the manuscript before submission.
Authorship
Contribution: F.L., V.S., A.B.-F., A.-S.C., M.-H.D.-L., M.M., L.d.L., P.G., and C.H. designed the study; V.S., E.B., G.C., M.-P.M.-M., A.D., R.B., L.V., O.C., V. Cacheux, C. Régny, and V. Camus recruited patients for the trial; F.L., V.F., C. Robe, L.P., A.L., and E.M. performed the molecular analysis; A.B.-F. and M.-H.D.-L. performed flow cytometry analysis and clonality analysis; A.-S.C., S.B., and M.M. performed PET CT analysis; S.F. performed EBV viral load analysis; and P.G., L.d.L., M.P., and C.B. performed the pathologic review.
Conflict-of-interest disclosure: G.C. has consulted for Roche and Celgene and received honoraria from Sanofi, Gilead, Jansen, AbbVie, Roche, and Celgene. C.H. reports personal fees from Celgene during the conduct of the study and personal fees from Roche, Amgen, Janssen, Gilead, Novartis, and Takeda outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Corinne Haioun, Unité Hémopathies Lymphoïdes, Hôpitaux Universitaires Henri Mondor, 51 Av de Lattre de Tassigny, 94000 Créteil, France; e-mail: corinne.haioun@ aphp.fr.
References
Author notes
F.L. and V.S. contributed equally to this work.
The full-text version of this article contains a data supplement.