Key Points
We obsrved excellent outcome of PMBL patients treated with first-line R-CHOP14 and R-ACVBP without radiotherapy.
We confirmed the prognostic importance of baseline TMTV in this population.
Abstract
Primary mediastinal B-cell lymphoma (PMBL) is a rare type of aggressive lymphoma typically affecting young female patients. The first-line standard of care remains debated. We performed a large multicenter retrospective study in 25 centers in France and Belgium to describe PMBL patient outcomes after first-line treatment in real-life settings. A total of 313 patients were enrolled and received rituximab (R) plus ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) (n = 180) or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) delivered every 14 days (R-CHOP14, n = 76) or 21 days (R-CHOP21, n = 57) and consolidation strategies in modalities that varied according to time and institution, mainly guided by positron emission tomography. Consolidation autologous stem cell transplantation was performed for 46 (25.6%), 24 (31.6%), and 1 (1.8%) patient in the R-ACVBP, R-CHOP14, and R-CHOP21 groups, respectively (P < .001); only 17 (5.4%) patients received mediastinal radiotherapy. The end-of-treatment complete metabolic response rates were 86.3%, 86.8%, and 76.6% (P = .23) in the R-ACVBP, R-CHOP14, and R-CHOP21 groups. The median follow-up was 44 months, and the R-ACVBP, R-CHOP14, and R-CHOP21 three-year progression-free survival probabilities were 89.4% (95% confidence interval [CI], 84.8-94.2), 89.4% (95% CI, 82.7-96.6), and 74.7% (95% CI, 64-87.1) (P = .018). A baseline total metabolic tumor volume (TMTV) ≥360 cm3 was associated with a lower progression-free survival (hazard ratio, 2.18; 95% CI, 1.05-4.53). Excess febrile neutropenia (24.4% vs 5.3% vs 5.3%; P < .001) and mucositis (22.8% vs 3.9% vs 1.8%; P < .001) were observed with R-ACVBP compared with the R-CHOP regimens. Patients with PMBL treated with dose-dense immunochemotherapy without radiotherapy have excellent outcomes. R-ACVBP acute toxicity was higher than that of R-CHOP14. Our data confirmed the prognostic importance of baseline TMTV.
Introduction
Primary mediastinal B-cell lymphoma (PMBL) is a rare type of aggressive B-cell lymphoma that is clinically and biologically distinct from diffuse large B-cell lymphoma (DLBCL). Accounting for ∼2% to 4% of newly diagnosed non-Hodgkin lymphomas,1 it typically affects young female patients.2 Most studies have reported a very favorable prognosis for PMBL, with a survival rate exceeding 80% at 5 years,3-5 and this excellent prognosis is a particular feature of PMBL.
However, therapeutic management of PMBL differs across countries, with no fully established standard of care. Therapeutic options include standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone),5,6 dose-dense immunochemotherapy with rituximab (R) plus ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone)3,7 or DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab),4,8 and mediastinal consolidation radiation therapy (CRT).9,10 The use of CRT remains controversial among hematology centers worldwide; some centers choose to apply it systematically9,11 or optionally (for metabolic partial responders),6,12,13 and other centers no longer use this approach.14-16 Finally, the place of autologous stem cell transplant (ASCT) in first-line consolidation,17,18 mainly guided by fluorodeoxyglucose positron emission tomography (PET) response assessment,19 also remains a cause of debate due to the paucity of available evidence in PMBL. Indeed, because PMBL is uncommon, few prospective data exist, resulting in the present situation of no clearly established standard of care. Patients with PMBL are poorly represented in clinical trials, and treatment practices are heterogeneous. This prompted us to conduct a real-life, large, multicenter retrospective study in Lymphoma Study Association (LYSA) centers.
Methods
Patients and data collection
All adult patients with newly diagnosed PMBL treated in France and Belgium by participating LYSA centers from 1 January 2007 to 31 December 2017, were identified from local databases. The inclusion criteria were as follows: first-line ACVBP or CHOP plus anti-CD20, available pretreatment (baseline) PET, and patients’ nonopposition statement. PMBL diagnosis was made locally in each center and based on typical clinical presentation and histologic criteria described in the 2008 and 2016 World Health Organization classification,1,20 with the support of the French Lymphopath21 network after 2009. Data were retrospectively collected from medical records in all centers. “Bulky” was defined as disease ≥10 cm in axial diameter. Primary refractory disease was defined as lymphoma progression during treatment or within 3 months of treatment completion.
The primary end point was progression-free survival (PFS). Secondary end points were: overall survival (OS), baseline total metabolic tumor volume (TMTV) prognostic impact, OS according to uptake levels based on the Deauville Score (DS) at the end of treatment (EoT), complete metabolic response (CMR) rates, and maximum standardized uptake value (SUVmax) reduction between baseline (PET0) and PET4 (ΔSUVmax PET0-4). The study was approved by Centre Henri Becquerel’s institutional review board (CHB-1801B) and by the LYSA scientific committee (01/2018). The study was conducted according to the criteria set by the Declaration of Helsinki.
Treatments and assessment of PET response
The “induction” phase was defined as 4 cycles of immunochemotherapy. Granulocyte colony-stimulating factor use and central nervous system (CNS) prophylaxis were left up to the discretion of the investigators. PET was routinely performed before treatment (baseline), after 2, 3, or 4 cycles of chemotherapy (PET2, PET3, and PET4), and at EoT, depending on the center’s usage. Centers mainly used interim PET (iPET) results to guide “postinduction” treatments in modalities that varied according to the time and institutions, mainly managed according to the design of the major LYSA clinical trials.19,22 Patients received one or more of the following options, according to their physician’s decision (details in supplemental Table 1): (1) continuation of R-CHOP to achieve 6 to 8 cycles; (2) consolidation ASCT (BEAM [BiCNU (carmustine), etoposide, cytarabine, and melphalan]23 conditioning regimen or equivalent)19 ; or (3) LYSA sequential consolidation chemotherapy (SCC)19,22 (mainly after ACVBP induction) and/or CRT. Notably, patients could receive ASCT at the discretion of the investigators in each of the treatment groups. EoT PET assessment was performed after consolidation ASCT or at the end of standard chemotherapy if patients did not receive an ASCT.
Response evaluation was performed in each institution and was mainly based on the DS24 or the International Harmonization Project (IHP) criteria,25 depending on the time and the centers’ habits. CMR was defined as follows: DS1-3 (5-point scale, for PET examinations performed in 2010 and after) or negative PET (IHP criteria, for PET examinations performed between 2007 and 2009). TMTV using the 41% SUVmax threshold method and ΔSUVmax PET0-4 were centrally retrospectively assessed by 4 independent senior nuclear medicine physicians.
Statistical analysis
Comparisons of characteristics according to treatments were conducted with χ2 tests (or Fisher’s exact tests) for qualitative variables and nonparametric Wilcoxon Mann-Whitney tests for quantitative variables. The best cutoff value for baseline TMTV was determined with receiver-operating curve analysis.
OS was calculated from the date of diagnosis to the date of death from any cause or the date of last follow-up while alive. PFS was calculated from the date of diagnosis until disease progression, relapse, or death from any cause or the last patient follow-up. Patients free of disease progression and relapse were censored on the date of the last follow-up visit or contact. Second PFS (PFS2) was calculated from the date of first disease relapse until subsequent disease progression, relapse, or death from any cause or the last patient follow-up. Survival outcomes were estimated by using the Kaplan-Meier method, with significant differences evaluated with the log-rank test. Multivariable analysis (MVA) was conducted by using a Cox proportional hazards model. As soon as a patient has missing data in one of the MVA variables, the patient was no longer part of the model. The level of significance retained for each test was 5%. Statistics were performed with R software version 4.0.2 (R Foundation for Statistical Computing).
Results
Patients’ characteristics
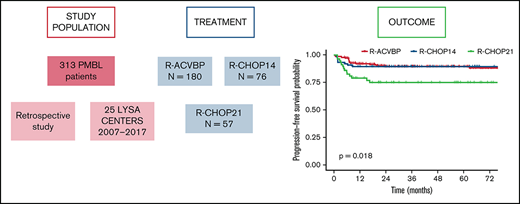
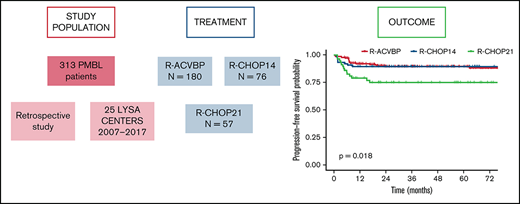
A total of 313 patients were enrolled from 25 LYSA centers. The patients’ characteristics are listed in Table 1. The median age at diagnosis was 32 (18-88) years; the majority of patients were female (n = 190 [60.7%]) and presented at diagnosis with a good performance status (Eastern Cooperative Oncology Group performance status 0-1, 81.8%), Ann Arbor stage I to II (57.5%), elevated lactate dehydrogenase (81.8%), bulky disease (58.5%), international prognostic index (IPI) 1 to 2 (60.7%), and CNS IPI 2 to 3 (36.4%). No patient had CNS involvement at diagnosis. Sixteen (5.1%) patients presented with thrombotic events related to tumor mass before treatment. Mediastinal masses were reported in 278 (96%, data not available = 23) patients and were the main biopsy site for diagnosis. Tumors typically displayed a CD20+/CD30+/CD23+/MUM1+/CD15– profile (supplemental Table 1). Extranodal involvement was reported in 151 (48%) patients (supplemental Table 2).
Treatments received
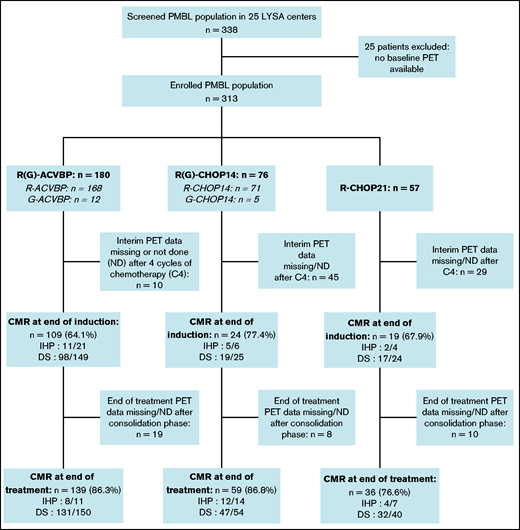
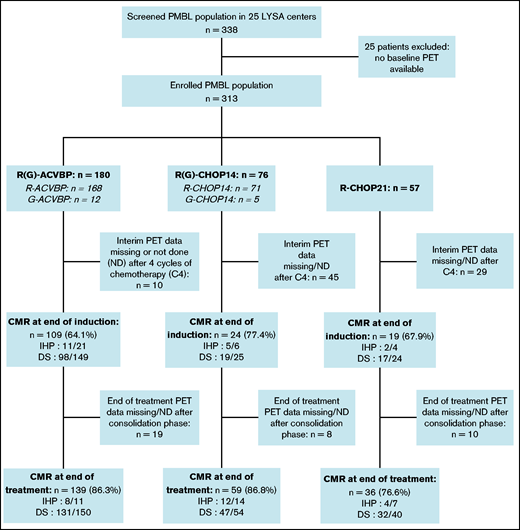
Three induction chemotherapy regimens were used: ACVBP7 delivered every 14 days (n = 180) and CHOP administered every 14 days (CHOP14,26,27 n = 76) or every 21 days (CHOP21,5 n = 57) (Figure 1). Anti-CD20 combined with chemotherapy was rituximab (R; n = 296 [94.6%]) or obinutuzumab (G; n = 17 [5.4%]). CNS prophylaxis was performed in 261 (83.4%) patients (Table 2). Patients treated with R-CHOP21 were enrolled homogeneously over the entire study period, similar to the other patients. Forty-seven (15%) patients were treated in an LYSA trial open at the time of diagnosis7,19,22,28,29 (supplemental Table 3). The total median number of R-CHOP cycles received by patients was 7.5 (1-8) vs 6 (1-8) in the R-CHOP14 and R-CHOP21 groups, respectively.
Study flowchart and treatment CMR rate. CMR was defined as follows: DS 1 to 3 (5-point scale, for PET performed in 2010 and after), or negative PET (IHP criteria, for PET examinations performed between 2007 and 2009).
Study flowchart and treatment CMR rate. CMR was defined as follows: DS 1 to 3 (5-point scale, for PET performed in 2010 and after), or negative PET (IHP criteria, for PET examinations performed between 2007 and 2009).
Consolidation ASCT was performed for 46 (25.6%), 24 (31.6%), and 1 (1.8%) patient (P < .001), and CRT was delivered to 4 (2.2%), 11 (14.5%), and 2 (3.5%) patients in the R-ACVBP, R-CHOP14, and R-CHOP21 groups, respectively (P < .001) (Table 2). By regrouping the R-CHOP groups, ASCT was performed for 25 (R-CHOP) vs 46 (R-ACVBP) patients (P = .2) and CRT was delivered to 13 (R-CHOP) vs 4 (R-ACVBP) patients (P = .0047).
Interim PET results
A total of 308 (98.4%) patients had at least an iPET after 2 (n = 178 [56.9%]), 3 (n = 13 [4.2%]), and/or 4 (n = 241 [77%]) cycles of chemotherapy (supplemental Table 4). Regarding patients in the R-CHOP groups, we only had PET results after 2, 3, or 4 cycles of chemotherapy, but no consecutive PET2/PET4 results (data missing or PET examination not performed), in contrast to the R-ACVBP group.
The CMR rates after induction for the 229 (73.2%) evaluable patients with available PET4 were similar across the R-ACVBP, R-CHOP14, and R-CHOP21 groups: 64.1%, 77.4%, and 67.9% (P = .35), respectively (Figure 1). We then selected 72 patients who had both an interpretable PET0 and PET4, and we observed that ΔSUVmax PET0-4 ≤70% was associated with unfavorable outcomes (supplemental Figure 1).
Patient outcomes
EoT CMR rates were fairly similar across the R-ACVBP, R-CHOP14, and R-CHOP21 groups: 86.3%, 86.8%, and 76.6% (P = .23), respectively (Figure 1). Thirty-seven (11.8%) patients progressed, including 32 (10.2%) who had a primary refractory disease. Among those 37 events, 15 (40.6%) patients had an extra-mediastinal relapse, and 6 (16.2%) progressed after first-line consolidation ASCT in a median time of 3 (2-58) months. A single late relapse was observed beyond 2 years’ postdiagnosis (Figure 2). CNS relapse occurred in 9 (2.9%) patients, of whom 8 had received CNS intrathecal methotrexate prophylaxis (supplemental Table 5).
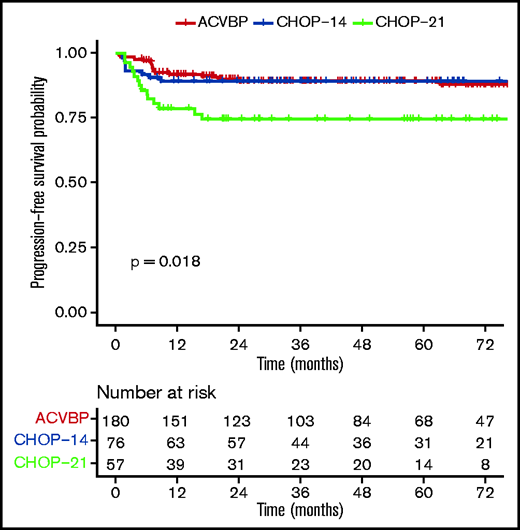
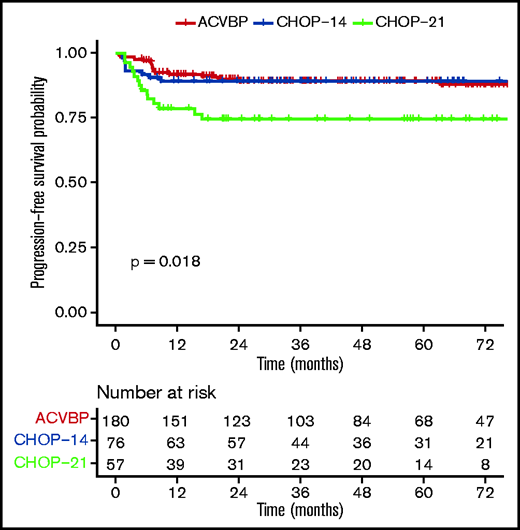
PFS according to ACVBP, CHOP14, and CHOP21 plus anti-CD20 treatment groups.
PFS and OS by treatment groups
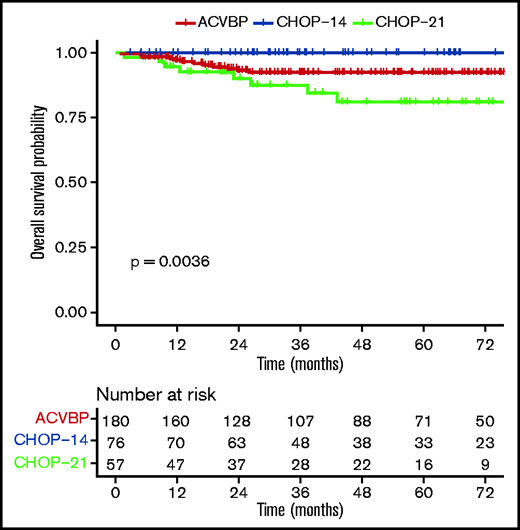
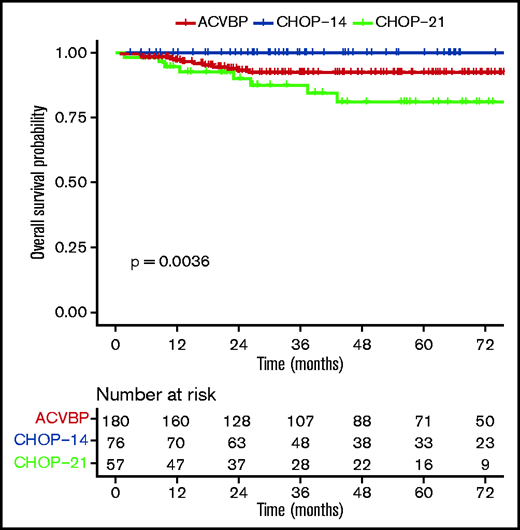
The median follow-up was 44 (1-153) months, and the R-ACVBP, R-CHOP14, and R-CHOP21 3-year PFS and OS were 89.4% (95% confidence interval [CI], 84.8-94.2), 89.4% (95% CI, 82.7-96.6), and 74.7% (95% CI, 64-87.1), respectively (P = .018) (Figure 2) and 92.4% (95% CI, 88.4-96.7), 100% (95% CI, 100-100), and 87.5% (95% CI, 78.6-97.6) (P = .0036) (Figure 3). Twenty-two patients died (R-CHOP21, n = 8; R-ACVBP, n = 14), mainly due to lymphoma progression (n = 15) and toxicity (n = 2) (supplemental Table 6).
OS according to ACVBP, CHOP14, and CHOP21 plus anti-CD20 treatment groups.
By univariate analysis, R-CHOP21 treatment (hazard ratio [HR], 2.37; 95% CI, 1.20-4.67) (Table 3), presence of B symptoms (HR, 1.91; 95% CI, 1.05-3.47), and baseline TMTV ≥360 cm3 (HR, 2.18; 95% CI, 1.05-4.53) (supplemental Figure 2) were associated with a shorter PFS. The type of anti-CD20 antibody (R or G) had no impact on outcome (2-year PFS, 86.4% vs 93.3%, P = .35; data not shown). In the TMTV ≥360 cm3 subgroup, the numbers of events were too low to test the impact of the treatment modalities on PFS. The outcome was similar between patients receiving R-ACVBP plus ASCT, R-ACVBP plus SCC, R-CHOP14 alone, and R-CHOP14 plus ASCT, but R-CHOP21 still performed worse in this subgroup analysis (3-year PFS, 74.7%; P = .001) (supplemental Figure 3). In an MVA including treatment group (Table 3), IPI 3 to 5, bulky disease, TMTV, pericardial or pleural effusion, and B symptoms, TMTV ≥360 cm3 was associated with inferior OS (HR, 5.68; 95% CI, 1.61-20.06; P = .007), independent of treatment modality. CRT was not included in the model due to the low number of patients (5.4%). Treatment group was not associated with outcome in the MVA.
EoT PET results for prognostic assessment
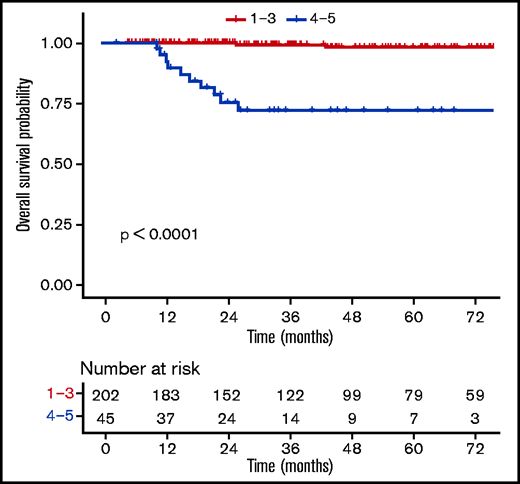
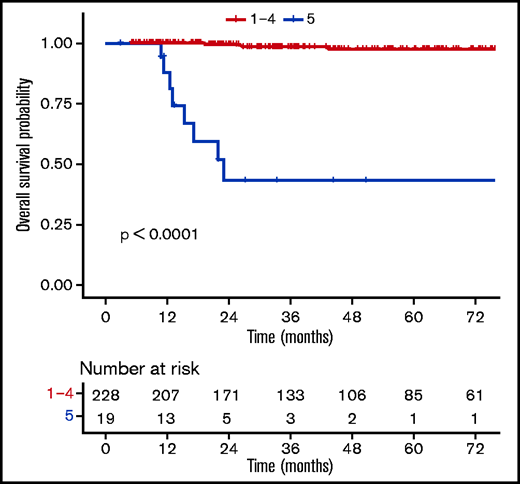
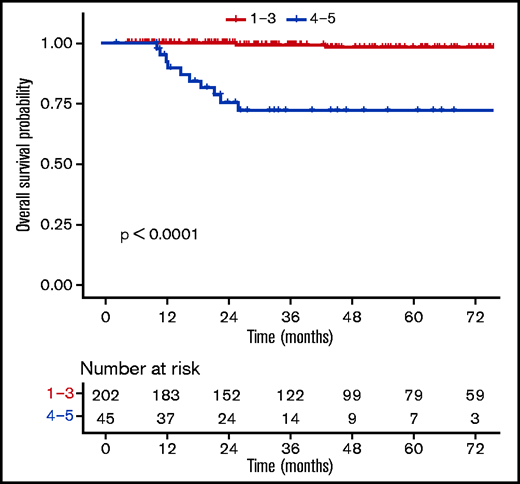
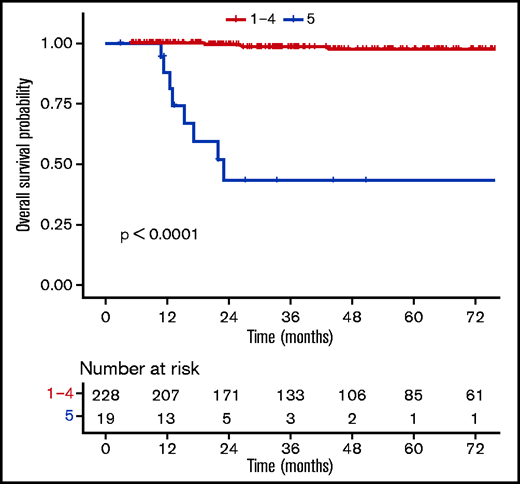
Patients with a negative PET (DS1-3) at EoT (n = 202) had favorable outcomes compared with those with a positive PET (DS4-5, n = 45) (supplemental Table 7), with a 3-year OS rate of 99.3% (98% to 100%) vs 72% (58.6%-88.6%) (P ≤ .0001) (Figure 4). When using a different threshold, patients with DS1-4 at the EoT (n = 228) also had excellent outcomes compared with DS5 (n = 19), with a 3-year OS rate of 98.3% (96.3% to 100%) vs 43.3% (23.4% to 80.2%) (P ≤ .0001) (Figure 5). No statistically significant prognostic difference was observed between DS3 (n = 71) and DS4 (n = 26) at EoT, with a 3-year OS rate of 98% (94.1% to 100%) for DS3 vs 89.8% (77.3% to 100%) for DS4, with only 1 event in each subgroup (supplemental Figure 4). The outcome (ie, PFS, OS) of patients who obtained a negative PET at EoT is excellent and identical between the 3 treatment arms, whether using the IHP or DS criteria (supplemental Figure 5).
OS according to levels (DS1-3 vs DS4-5) at the end of the first-line treatment.
OS according to levels (DS1-3 vs DS4-5) at the end of the first-line treatment.
OS according to uptake levels (DS1-4 vs DS5) at the end of the first-line treatment.
OS according to uptake levels (DS1-4 vs DS5) at the end of the first-line treatment.
Salvage treatments and PFS2
Among the 37 progression events, salvage treatments administered at first progression were high-dose chemotherapy (R-ICE [rituximab, ifosfamide, carboplatin, and etoposide] or R-DHAOX-like regimens) (n = 30) followed by second-line consolidation ASCT (n = 11/30) or ASCT + CRT (n = 5/11); CRT alone (n = 1); other regimens (ie, R-CHOP, R-GEMOX [rituximab, gemcitabine, and oxaliplatin]) (n = 3); and none (n = 3). Among the 6 patients who received second-line CRT, 5 progressed, and 1 was still alive in a CR (the patient who received salvage ASCT + CRT).
The 2-year PFS2 and overall survival after the first progression 2 were 29% and 59.7%, respectively (supplemental Figure 6).
Toxicity
Safety data were retrospectively collected for 313 (100%) patients. All-grade treatment-related adverse events were similar among the groups, except for an excess of febrile neutropenia (5.3% vs 5.3% vs 24.4%; P < .001) and mucositis (1.8% vs 3.9% vs 22.8%; P < .001) (supplemental Table 8) in the R-ACVBP group. Two toxic deaths were observed (R-CHOP21, n = 1; R-ACVBP, n = 1). A very low rate (n = 7 [2.2%]) of cardiac events was reported (including 2 episodes of chest pain and 1 of atrial fibrillation), with only 3 events (1%) related to acute anthracycline toxicity (cardiomyopathy with the development of heart failure).
Secondary malignancies (SM) appeared in 7 (2.2%) patients (R-CHOP21, n = 2; R-ACVBP, n = 5 [2.8%]), including 3 cases of acute myeloid leukemia (R-ACVBP group), 1 Ewing sarcoma, 1 thyroid papillary microcarcinoma, 1 carcinoma in situ of the uterine cervix, and 1 squamous cell carcinoma. Only 2 of 7 patients who developed SM had received an ASCT, and no one developed acute myeloid leukemia. Median time between first-line treatment and SM appearance was 3.1 (1.1-6.2) years. One patient died of an SM (R-ACVBP group) (supplemental Table 9).
Discussion
We conducted a large retrospective study describing the outcome of newly diagnosed PMBL patients treated in 25 LYSA centers in real-life settings. In this study, patients who received R-CHOP14 or R-ACVBP had excellent outcomes with limited acute toxicity. The majority of failures occurred in patients with primary refractory disease. These results are consistent with other published data confirming the excellent outcomes of patients with PMBL receiving first-line immunochemotherapy.3,4,6,15,30
Regarding the factors explaining these favorable results, we noted that dose intensity seems to play a role in the outcome of PMBL. Patients treated with standard R-CHOP21 had a trend toward inferior results compared with R-ACVBP and R-CHOP14. However, the MVA shows that treatment group was not associated with outcome in our study. However, the inferior results of R-CHOP21 in our study can be explained partly by a higher proportion of patients >60 years of age in this subgroup, who are usually not eligible for R-ACVBP and for whom the prognosis is generally less favorable. In total, 4.5% of patients were aged >60 years. This small proportion of elderly patients with PMBL is consistent with other data in the literature.12,31-33 The higher median age of the R-CHOP21 group (40 years and almost 20% aged >60 years) probably influenced the decision to not treat these patients with R-CHOP14 or R-ACVBP. These dose-dense regimens are associated with higher toxicity in patients aged >60 years.34,35 This is also the likely reason why patients in the R-CHOP21 group did not receive consolidation ASCT for the most part.
In the pre-rituximab era, Massoud et al3 included 67 patients treated with ACVBP and 38 treated with CHOP21 and CRT, and found a better OS and PFS with ACVBP. In a small subgroup analysis of UK National Cancer Research Institute R-CHOP21 vs R-CHOP14 plus CRT at the physician’s discretion, Gleeson et al5 also established that R-CHOP14 performed better than R-CHOP21. In addition, a recent randomized study that included 96 Ukrainian patients with PMBL confirmed that DA-EPOCH-R is more effective than standard R-CHOP; however, DA-EPOCH-R had a higher rate of grade 3 to 4 neutropenia and the use of CRT.8
A recent study report in the form of an abstract by Held et al,36 however, revealed no outcome differences between PMBL treated with R-CHOP21 and R-CHOP14 in a cohort of 131 patients. In this subgroup analysis of the UNFOLDER 21/14 trial, only patients with age-adjusted IPI = 0 plus bulky disease or age-adjusted IPI = 1 were included and received either CRT or observation at EoT, which could partially explain the results. In our study, the R-ACVBP and R-CHOP14 groups were comparable based on clinical characteristics, but the groups were unbalanced in size, with more patients who received R-ACVBP. R-CHOP14 was associated with a PFS comparable to that of R-ACVBP but led to slightly more frequent use of consolidation ASCT and CRT. Variations in the criteria for the interpretation of PET over time, as well as the habits of the centers, may have partially contributed to this greater number of ASCT and CRT in the R-CHOP14 group, whereas the tumor control at the end of induction seems similar. The centers mainly chose between 2 treatment strategies: either R-ACVBP or R-CHOP14 with consolidation ASCT if PET2+/PET4–, or a classic strategy with no ASCT consisting of 6 to 8 cycles of R-CHOP21. As a result, almost all of the ASCT occurred in the R-ACVBP and R-CHOP14 groups. Because the postinduction treatments were adapted to iPET results, the outcomes were similar between R-ACVBP plus ASCT, R-ACVBP plus SCC, R-CHOP14 plus ASCT, and R-CHOP14 alone. In addition, the CNS relapse rate was low (2.9%) and consistent with the literature.33,37,38 No consensus exists to recommend CNS prophylaxis in PMBL, and thus CNS-IPI is commonly used, as in DLBCL.
Regarding safety, the treatments were generally well tolerated in our study, and the reported cardiac event rate while on anthracycline therapy was low (2.2%), far from the 7% to 18% of cardiac event rates commonly described in the literature39 at cumulative doses of 150 to 350 mg/m2. This finding is possibly due to the young age of the population and the insufficient follow-up, as well as the missing reports linked to retrospective data collection. Nevertheless, R-ACVBP was more toxic than R-CHOP14, with a higher rate of febrile neutropenia and mucositis, without taking into account the number of rehospitalizations, which is not possible to specify in this retrospective work. Furthermore, the incidence of SM in the R-ACVBP arm at 2.8% is notable with 3 cases of acute myeloid leukemia. This finding was previously described in the literature.40 Considering retrospective data collection with limited follow-up (44 months) and time between chemotherapy and SM appearance in our cohort, we may expect additional SM onset in the future. A watchful follow-up of these young patients is recommended.
PMBL is a radiosensitive disease, and various procedures historically combined chemotherapy with CRT, but there are many concerns about the long-term toxicity of CRT in this young, mostly female population.41,42 However, real-life data on large cohorts of patients treated with chemotherapy with or without CRT are lacking, and thus no consensus exists on omitting CRT. In our study, the number of patients who received CRT was very low (n = 17), and none received CRT after 2014, probably due to an evolution of practices in LYSA centers. This means that from that date, in the event of a positive iPET, physicians preferred consolidation ASCT to CRT. The study reported by Dunleavy et al4 previously showed that treatment with an intensive chemotherapy regimen (DA-EPOCH-R) obviated the need for CRT. In a large series of patients with PMBL treated with R-CHOP, Hayden et al also reported that a PET-guided approach may reduce CRT use in the majority of patients. In our experience, CRT can be safely omitted in this population of predominantly young female patients treated with dose-dense immunochemotherapy to avoid CRT long-term side effects.43-46 In addition, the frequent extra-mediastinal relapses observed in our study also argue against CRT. The results of the IELSG-37 (#NCT01599559) study are awaited to provide results based on a randomized study and may conclusively settle the debate.47
Relapses occurred in 12% of patients in our study, consistent with previously published data.48-50 In this situation, retreatment with salvage high-dose chemotherapy followed by ASCT is the standard of care.19,51 Nevertheless, relapsed PMBL is often chemoresistant, and the patients will either not actually undergo ASCT or will relapse early after ASCT with a poor prognosis (2-year PFS2, 30%). PMBL is associated with genetic aberrations at 9p24 and overexpression of programmed cell death-1 ligands; therefore, pembrolizumab, an anti–programmed cell death-1 checkpoint antibody, was tested as a single agent52 in patients with PMBL relapsing after ASCT, with good results and a manageable safety profile. In addition, a combination of checkpoint inhibitors and brentuximab vedotin53 has been reported as promising in third-line or more therapy.
Our results confirm favorable outcomes of DS4 at EoT, as previously reported in the literature but mainly after CRT. Indeed, the prospective IELSG-26 study54 evaluated PET after first-line R-CHOP–like plus CRT in 115 patients with PMBL, and the positive predictive value was only 32% for DS4. Filippi et al55 also reported in a series of 51 patients with PMBL that all 17 patients with EoT DS4 had excellent outcomes. We believe EoT PET is useful to establish an overall prognosis. Similar to other teams, we think DS4 at the EoT should lead to serial PET surveillance56 and not to CRT.41
We also showed that a baseline TMTV ≥360 cm3 was associated with an unfavorable prognosis independent of treatment. This threshold is different from the cutoff values reported in the literature57 for DLBCL (65 cm3 to 600 cm3) and classical Hodgkin lymphoma (147 cm3 to 313 cm3), mainly determined by receiver-operating curve analyses; all studies agree, however, that a higher baseline TMTV predicts a significantly worse PFS and OS in patients with various types of lymphoma. Three methods exist for PET volume autosegmentation, which complicates TMTV use as a prognostic factor in real life. Nevertheless, expert nuclear medicine physicians independently re-evaluated all available images, reinforcing the identified threshold’s value. There is probably an interaction in the MVA between TMTV and R-ACVBP, which is not significant due to a lack of power.
Regarding iPET, Lazarovici et al58 previously reported the low predictive value of positive iPET scored by IHP or DS, which pinpoints the need for an additional tool to help physicians more precisely assess therapeutic response. As a consequence, we suggest using the semi-quantitative tool ΔSUVmax PET0-4 (cutoff ≤70% or >70%), as previously reported in patients with DLBCL,19,59 to help guide consolidation decisions, as previously described by Casasnovas et al,60 who showed that the prognostic impact of iPET results could be increased by using ΔSUVmax compared with visual analysis.
Our study has several limitations that should be highlighted. First, it was a retrospective study that inevitably involved missing data. More centers from the former Groupe d’Etude des Lymphomes de l’Adulte (GELA), who are used to R-ACVBP, participated in this study, in contrast to the former Groupe Ouest Est des Leucémies et Autres Maladies du Sang (GOELAMS) centers, who are used to R-CHOP and who participated less; this may represent a selection bias. We have no information on the factors that motivated the centers to choose R-CHOP over R-ACVBP. The decision was left to each investigator and mainly corresponded to a center’s habit. Patients’ comorbidities were taken into account in addition to patients’ age, but comorbidity data were not collected. Regarding other limitations, we do not have sufficient data on physicians’ motivations for their choice of consolidative options (eg, ASCT, CRT, SCC) according to iPET results, for several reasons: (1) iPET was performed at different times during the treatment course in a heterogeneous way; (2) serial PET2/PET4 data were missing in the R-CHOP groups; (3) variable methods were used for interpreting PET responses; (4) local treatment guidelines have evolved over time; and (5) no data are available on whether biopsies were performed by the centers based on DS4 at EoT. Our cohort may not be representative of the overall PMBL population because baseline PET was mandatory for inclusion. Because PMBL is sometimes diagnosed in an emergency context, and patients may receive treatment with no baseline PET, we may have possibly missed the sickest patients. Furthermore, no dedicated PMBL registry exists in LYSA centers. The identification of PMBL patients in this study has called on local databases, and selection biases are inevitable.
In addition, our study does not have enough power to explore the results of the 3 regimens in the IPI 0 subgroup. We cannot directly compare our results vs those published with DA-EPOCH-R,4,61,62 but the outcomes seem globally similar. We did not review biopsy results to confirm the local PMBL diagnosis, but since 2009, LYSA centers routinely address all lymphoma biopsy samples to the French Lymphopath Network21 and thus we can hypothesize that there are very few samples that would not have been diagnosed by experts in our cohort. In addition, the patients’ clinical characteristics and outcomes presented here correspond to a typical PMBL population. Finally, regarding the features correlated with an excellent R-CHOP14 outcome, we cannot conclude whether RCHOP14 is the important “key” factor or if it is the consolidation phase. However, the results of our study suggest that next PMBL patients may be treated with R-ACVBP or R-CHOP14 without CRT according to the “GAINED” trial19 design with an iPET-driven consolidation strategy. A prospective trial in the PMBL population evaluating this treatment design and the role of circulating tumor DNA as a biomarker of molecular response as a complement to PET is ongoing (#NCT04824950).
Finally, these results confirm the remarkable outcomes of patients with PMBL treated with dose-dense immunochemotherapy without CRT. Our data also support the prognostic importance of baseline TMTV and the favorable outcomes of DS4 at EoT.
Acknowledgments
The authors thank the patients and their families; the reviewers at the LYSA, notably Thierry Lamy and Hervé Ghesquières; the LYSARC; and all of the investigators in the LYSA centers. They also thank Julie Libraire, clinical data manager at the Clinical Research Unit, Centre Henri Becquerel, and Doriane Richard, CRA manager, for support in this study. The authors thank Arthur Dumouchel and Pierrick Gouel for imaging data management in this study.
This work was supported by grants from the Centre Henri Becquerel and the Ligue Contre le Cancer (Comité de la Manche).
Authorship
Contribution: V.C. designed and supervised the study, analyzed and interpreted the data, and wrote the manuscript; C.R., P.S., C.H., E.D., A.W., M.G., M.-P.M.-M., C.A., J. Lazarovici, H.M., S. Bernard, M.T., C. Besson, L.L., S.C., K.L.D., C. Bonnet, S. Bailly, G.D., K.L., H.M., R.H., A.C., F.J.., and A.T.-G. collected the data; D.T., P.D., S. Becker, and A.B.-R. collected the data and reviewed all available PET images; J. Lequesne performed the statistical analysis; and H.T. designed and supervised the study, analyzed and interpreted the data, and edited the paper.
Conflict-of-interest disclosure: V.C. reports honoraria from Roche, Amgen, Gilead-Kite, BMS, and Sanofi; and travel grants from Pfizer and Roche. H.T. reports honoraria from Celgene, Roche, Karyopharm, AstraZeneca, and Bristol Myers Squibb; and grants from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Vincent Camus, Centre Henri Becquerel, Département d’Hématologie, 1 rue d’amiens, 76038 Rouen Cedex, France; e-mail: vincent.camus@chb.unicancer.fr.
References
Author notes
Presented in abstract form at the 62nd American Society of Hematology virtual annual meeting, 5-8 December 2020; and as an oral communication at the International Conference on Malignant Lymphoma, 18-21 June 2021, Lugano (abstract 50).
Requests for data sharing may be submitted to the corresponding author (Vincent Camus; e-mail: vincent.camus@chb.unicancer.fr).
The full-text version of this article contains a data supplement.