Key Points
The baseline EASIX score combined with ferritin is associated with onset of CRS in patients with LBCL treated with axi-cel.
Baseline EASIX score combined with ferritin and CRP is associated with the onset of ICANS in patients with LBCL treated with axi-cel.
Abstract
The Endothelial Activation and Stress Index (EASIX) score, defined as [(creatinine × lactate dehydrogenase [LDH])/platelets], is a marker of endothelial activation that has been validated in the allogeneic hematopoietic stem cell transplant setting. Endothelial activation is one of the mechanisms driving immune-mediated toxicities in patients treated with chimeric antigen receptor-T (CAR-T)-cell therapy. This study’s objective was to evaluate the association between EASIX and other laboratory parameters collected before lymphodepletion and the subsequent onset of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) those patients. Toxicity data were collected prospectively on 171 patients treated with axicabtagene ciloleucel (axi-cel) for large B-cell lymphoma (LBCL). CRS grades 2 to 4 were diagnosed in 81 (47%) patients and ICANS grades 2 to 4 in 84 (49%). EASIX combined with ferritin (EASIX-F) identified 3 risk groups with CRS grades 2 to 4 cumulative incidence of 74% (hazards ratio [HR], 4.8; 95% confidence interval [CI], 2.1-11; P < .001), 49% (HR, 2.3; 95% CI, 1.02-5; P = .04), and 23% (reference), respectively. EASIX combined with CRP and ferritin (EASIX-FC) identified 3 risk groups with an ICANS grade 2 to 4 cumulative incidence of 74% (HR, 3.6; 95% CI, 1.9-6.9; P < .001), 51% (HR, 2.1; 95% CI, 1.1-3.9; P = .025), and 29% (reference). Our results indicate that common laboratory parameters before lymphodepletion correlate with CAR-T–related toxicities and can help support clinical decisions, such as preemptive toxicity management, hospitalization length, and proper setting for CAR-T administration.
Introduction
Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) are common immune-related toxicities associated with chimeric antigen receptor (CAR)–T-cell therapy. Their clinical manifestations can be severe and potentially life threatening. In clinical trials of autologous anti-CD19–directed CAR T cells, CRS was observed in 42% to 92% of recipients, and ICANS was observed in 21% to 67%.1-3 However, predicting which patients are at increased risk for CRS and ICANS is difficult, limiting the consistent development of preemptive toxicity management strategies and decision-making tools for determining hospitalization length and the appropriate setting for administration. Different studies have reported on several biomarkers, including lactate dehydrogenase (LDH), as the surrogate marker of tumor burden, and C-reactive protein (CRP) and ferritin as general inflammatory markers, all 3 of whichare associated with severe CRS and/or ICANS.2,4-8 However, their individual predictive power is limited, and novel biomarkers are strongly needed.
Upon recognition of their target antigen, CAR T cells are activated and expand through a cascade of immune stimuli, not limited to the CAR T cells themselves.4 Interferon-γ (IFN-γ) secreted by CAR T cells leads to stimulation of macrophages, which may in turn cause vascular endothelial activation and coagulopathy, the latter correlating with the severity of CRS.4 ICANS often occurs simultaneously or immediately after a CRS episode, and similar to CRS, its severity correlates with higher cytokine levels and elevated inflammatory markers, such as ferritin, CRP, interleukin-6 (IL-6), IFN-γ, and tumor necrosis factor-α,9 Severe neurotoxicity has also been associated with elevated prothrombin time, activated partial thromboplastin time, D-dimer, and other markers of endothelial activation.9 The endothelial activation and stress index (EASIX) score, defined as [(creatinine × LDH)/platelets], has been developed as a surrogate marker of endothelial activation in the setting of allogeneic hematopoietic stem cell transplantation, and it correlates strongly with other biomarkers of endothelial dysfunction and complement activation.10 Furthermore, it is predictive of transplant-related toxicities, such as fluid overload and sinusoidal obstruction syndrome, both caused by endothelial dysfunction and associated coagulopathy.11,12 Finally, higher EASIX scores are associated with higher nonrelapse mortality and decreased overall survival in the allogeneic transplant setting.13,14
We therefore sought to determine the association between the EASIX score before lymphodepletion and the subsequent onset of CRS and ICANS. In addition, we examined whether other inflammatory biomarkers could add to the predictive power of EASIX and propose an enhanced risk-stratification algorithm to guide clinical decisions before the start of lymphodepletion.
Methods
Patient population
All adult patients with relapsed or refractory large B-cell lymphoma (LBCL), who received standard of care commercial axicabtagene ciloleucel at the University of Texas MD Anderson Cancer Center from January 2018 through April 2020 were consecutively included in the study. Lymphodepleting chemotherapy (LDC) consisted of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) administered IV on days −5, −4, and −3, followed by CAR T cells (2 × 106 cells/kg) infused on day 0. Patients who received other CAR-T products or who had received CAR-T therapy as part of a clinical trial were excluded from the study. Patient data were prospectively collected through the institutional lymphoma CAR T-cell therapy database. The study was approved by the Institutional Review Board of MD Anderson Cancer Center and was conducted in accordance with the principles of the Declaration of Helsinki. Baseline laboratory values were defined as the latest values before the initiation of chemotherapy on day −5, but no earlier than day −15. Because of a change in the range of normality over time, LDH levels were corrected to the upper limit of normal of the newer scale used. No prophylactic corticosteroids and/or tocilizumab were used. Toxicity was prospectively graded according to the CARTOX15 and ASTCT consensus grading systems,16 and treatment was administered according to the CARTOX treatment protocol.15 Grades 2 to 4 CRS and ICANS were selected, with the intention of having an adequate number of events to allow for statistical power and of identifying a clinically meaningful toxicity severity level. Performance status was graded according to the Eastern Cooperative Oncology Group (ECOG) guidelines,17 and the International Prognostic Index (IPI) was calculated based on the revised International Prognostic Index (R-IPI).18
Statistical methods
The cumulative incidence of CRS and ICANS was estimated starting on the date of infusion, considering death before toxicity as a competing risk. Factors associated with toxicity after infusion were evaluated using the Fine and Gray regression analysis on univariate and multivariate analyses to account for death as a competing event. In addition, recursive partitioning analysis was performed using classification and regression tree analysis, to account for the strong correlation among baseline characteristics and to develop a risk-stratification algorithm for development of CRS and ICANS. Internal validation of the multivariate models was evaluated by using bootstrapping analysis based on 3000 resampled data sets. The estimated bias-corrected 95% confidence intervals around the relative risk measures were consistent with minimal bias. Statistical significance was set at P < .05. Analyses were primarily performed with STATA, 14.0 (College Station, TX).
Results
Patient characteristics
A total of 171 patients were included in the study, with a median follow-up among those surviving of 8.5 months (range, 0.8-26). The median age was 59 years (range, 18-85), and 120 of the patients (70%) were men (Table 1). The diagnosis was diffuse LBCL (DLBCL) in 133 patients (78%), transformed follicular lymphoma in 28 (16%), and primary mediastinal B-cell lymphoma in 10 (6%). At the time of CAR-T infusion, 140 patients (82%) had stage III or IV disease, and 96 patients (56%) had an IPI score of 3 to 5. Data for baseline EASIX parameters (LDH, platelets, and creatinine; supplemental Table 1) were available for all patients. During the first 30 days, CRS of any grade was diagnosed in 160 patients (94%), of whom 81 patients (47%) had grade 2 to 4 CRS, and 14 (8%) had grade 3 to 4 CRS. During the same time frame, ICANS of any grade was diagnosed in 109 patients (64%), of whom 84 (49%) had grade 2 to 4 ICANS and 64 (37%) had grade 3 to 4 ICANS. CRS grades 2 to 4 and ICANS grades 2 to 4 were diagnosed at a median of 6 days (range, 1-27) and 7 days (range, 1-26), respectively. Fewer than 5 patients in our cohort had CAR-T–related macrophage–activating syndrome, precluding biomarker analysis for this subgroup.
Factors associated with increased risk of CRS
On univariate analysis, among baseline (pre-LDC) laboratory parameters (supplemental Table 1), the EASIX score shows the strongest association with CRS grades 2 to 4 (hazard ratio [HR], for upper quartile, 2.4; 95% confidence interval [CI], 1.5-3.7; P < .001). Additional parameters associated with CRS grades 2 to 4 included ferritin (HR for levels above the lowest quartile, 2.3; 95% CI, 1.1-4.6; P = .02), LDH (HR for levels above the median, 1.6; 95% CI, 1.05-2.5; P = .03), and low platelets (HR for lowest quartile, 1.7; 95% CI, 1.1-2.7; P = .02). Notably, the EASIX score showed a stronger association with CRS than its individual components; therefore, all subsequent analyses were based on the EASIX score. Other parameters, such as absolute lymphocyte count, absolute neutrophil count, albumin, and CRP, were not significantly associated with CRS grades 2 to 4 on univariate analysis. In a subgroup analysis looking at grade 2 vs grades 3 to 4, EASIX values within the upper quartile were associated with grade 2 (HR, 2.1; 95% CI, 1.3-3.4; P = .003) and showed a trend for an association with grades 3 to 4 (HR, 2.4; 95% CI, 0.8-6.9; P = .1) that did not reach statistical significance.
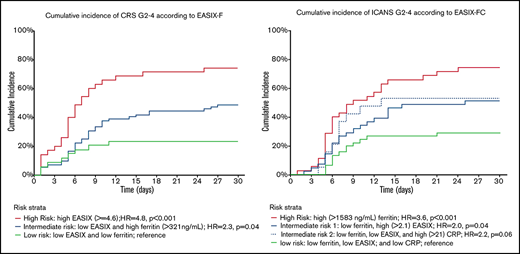
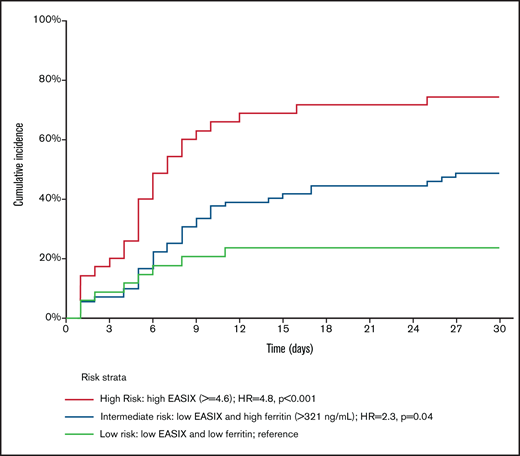
Evaluation of the independent effects (Table 2) of EASIX and ferritin showed a strong correlation between high EASIX (EASIX >4.6, in the upper quartile) and high ferritin levels (ferritin >321 ng/mL, greater than the first quartile), as 33 of 35 patients (94%) with high EASIX scores also had high ferritin levels. This strong correlation precluded the evaluation of the independent effect of ferritin in patients with high EASIX scores; thus, patients with high scores were considered to fall into an independent risk stratum. Among patients with EASIX scores lower than the upper quartile, we identified 2 risk strata, as high ferritin level was significantly associated with an increased rate of CRS grades 2 to 4 (HR, 2.3; 95% CI, 1.02-5.0; P = .04). Thus, based on EASIX score and ferritin level, a modified “EASIX-F” identified 3 risk groups with a cumulative incidence of CRS grades 2 to 4 of 74%, 49%, and 23% for the high (HR, 4.8; 95% CI, 2.1-11; P < .001), intermediate (HR, 2.3; 95% CI, 1.02-5; P = .04), and low (reference) risk groups, respectively (Table 2; Figure 1). Of note, LDH above the upper limit of normal as a separate variable was not significantly associated with CRS grades 2 to 4 when evaluated in the low-, intermediate-, and high-risk groups defined based on EASIX-F.
Cumulative incidence of CRS grades 2 to 4, according to EASIX-F. High- vs intermediate-risk groups: HR, 2.1 (95% CI, 1.3-3.5); P = .003. Only patients with available ferritin levels were eligible for this analysis.
Cumulative incidence of CRS grades 2 to 4, according to EASIX-F. High- vs intermediate-risk groups: HR, 2.1 (95% CI, 1.3-3.5); P = .003. Only patients with available ferritin levels were eligible for this analysis.
Among baseline clinical variables (supplemental Table 2), an IPI score of 3 to 5 (HR, 1.7; 95% CI, 1.1-2.6; P = .03) and prior autologous transplantation (HR, 0.5; 95% CI, 0.3-0.9; P = .02) were the only 2 factors that were significantly associated with CRS grades 2 to 4 on univariate analysis. Multivariate analysis incorporating the EASIX-F risk groups, IPI, and prior autologous transplantation showed that the EASIX-F risk groups were independently associated with CRS grades 2 to 4 (supplemental Table 3). A subgroup analysis of the EASIX-F for the different histology groups showed a similar stratification for DLBCL and transformed follicular lymphoma, with the primary mediastinal B-cell lymphoma group being too small to analyze.
Factors associated with increased risk of ICANS
On univariate analysis, significant laboratory factors that were associated with increased rates (supplemental Table 1) of ICANS grades 2 to 4 included the EASIX score (HR for levels above the median, 2.2; 95% CI, 1.4-3.4; P < .001), ferritin (HR for the upper quartile, 2.3; 95% CI, 1.4-3.6; P < .001), albumin (HR for levels below the median, 1.9; 95% CI, 1.2-2.9; P = .006), CRP (HR for levels above the median, 1.7; 95% CI, 1.1-2.7; P = .02), LDH (HR for levels above the median, 2; 95% CI, 1.4-3.3; P = .001), and platelets (HR for the lower 3 quartiles, 2.1; 95% CI, 1.2-3.8; P = .01). In a subgroup analysis looking at grade 2 vs grades 3 to 4, EASIX scores above the median were associated with grade 2 (HR, 2.4; 95% CI, 0.9-6.4; P = .07) and grade 3 to 4 (HR, 1.9; 95% CI, 1.2-3.1;), P = .01) ICANS. Similar to the observation for CRS, the EASIX score demonstrated a stronger association with ICANS than its individual components. Therefore, all subsequent analyses were based on the EASIX score.
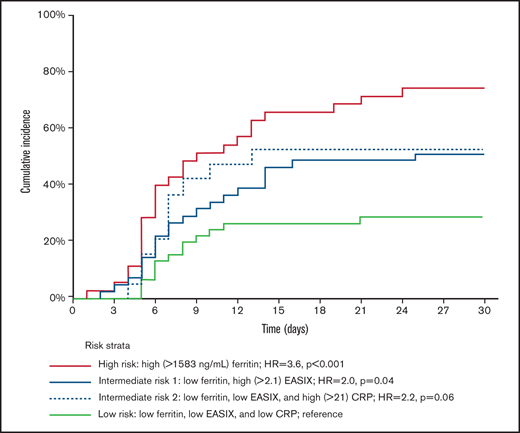
Classification and regression tree analysis was used to evaluate the independent effect of the laboratory markers found to be significant on univariate analysis (supplemental Figure 1). This analysis revealed 3 risk groups based on ferritin (>1583 ng/mL, the upper quartile), EASIX (>2.1, above the median), and CRP (>21 mg\L, above the median) levels (EASIX-FC) with a cumulative incidence of ICANS grades 2 to 4 of 74%, 51% (high EASIX) or 53% (high CRP), and 29% for the high (HR, 3.6; 95% CI, 1.9-6.9; P < .001), intermediate (HR, 2.1; 95% CI, 1.1-3.9; P = .025), and low (reference) risk groups respectively (Table 3; Figure 2).
Cumulative incidence of ICANS grades 2 to 4, according to EASIX-FC. Both intermediate-risk groups vs reference: HR, 2.1 (95% CI, 1.1-3.9); P = .025. High- vs intermediate-risk groups: HR, 1.8 (95% CI 1.1-2.9); P = .03. Only patients with available ferritin levels were eligible for this analysis.
Cumulative incidence of ICANS grades 2 to 4, according to EASIX-FC. Both intermediate-risk groups vs reference: HR, 2.1 (95% CI, 1.1-3.9); P = .025. High- vs intermediate-risk groups: HR, 1.8 (95% CI 1.1-2.9); P = .03. Only patients with available ferritin levels were eligible for this analysis.
Stratified analysis evaluating the effect of LDH within each of these risk groups showed no significant impact of above-normal LDH level.
Of the clinical factors evaluated, IPI was the only factor significantly associated with ICANS grades 2 to 4 on univariate analysis (supplemental Table 2). A multivariate analysis adjusted for IPI score and the 3 risk groups defined based on EASIX-FC demonstrated that EASIX-FC was the only factor significantly associated with ICANS grades 2 to 4. Results were unchanged when above-normal LDH levels were forced into the multivariate model (supplemental Table 4). A subgroup analysis of the EASIX-FC for the different histology groups showed a similar stratification for DLBCL and transformed follicular lymphoma, with the primary mediastinal B-cell lymphoma group being too small to analyze.
When the EASIX-FC risk groups were applied to ICANS grades 3 to 4, there was a trend for an association with ICANS grades 3 to 4, yet it reached significance only for the highest risk group, with a cumulative incidence of ICANS grades 3 to 4 of 60% for the high-risk group (HR, 3.8; 95% CI, 1.8-8; P < .001; supplemental Table 5).
Discussion
In this study, we demonstrated for the first time that the EASIX, a clinical surrogate for endothelial dysfunction, measured before lymphodepletion and combined with the inflammatory markers ferritin and CRP, is associated with the incidence and severity of CRS and ICANS in patients with LBCL treated with axi-cel. If validated in larger trials, the modified EASIX-scores: EASIX-F (for CRS) and EASIX-FC (for ICANS) may be used as an enhanced risk-stratification algorithm for patient evaluation before starting their treatment.
Pre-LDC factors shown to associate with increased risk of CRS in previously published results of clinical trials and retrospective studies include elevated serum CRP, LDH, IL-6, ferritin, monocyte chemoattractant protein-1 (MCP-1) and bilirubin levels, as well as low platelet count, low absolute neutrophil count, and low serum fibrinogen and total protein levels.2,4,7,8 Similar pre-LDC factors were shown to associate with increased the risk of ICANS, including elevated serum CRP, LDH, IL-6, ferritin, and MCP-1 levels, as well as low albumin levels and platelet counts.2,5,7,8 Likewise, CRP and ferritin levels measured on the day of CAR-T-cell infusion or shortly thereafter were also associated with increased risk of severe CRS and ICANS.1,5,6
In preclinical models, the development of CRS has been shown to be secondary to both CAR T-cell expansion and to subsequent activation of the immune microenvironment, leading to a cytokine storm, including peak levels of in IL-1, IL-6, and other proinflammatory molecules.19,20 Of interest, in patient-derived samples,4 these chemokine levels associated not only with higher CAR-T-cell expansion, but also with endothelial activation. In this regard, IL-6 levels can also be elevated in capillary leak syndromes, such as acute respiratory distress syndrome, severe burns, or sepsis, along with IL-8, IL-10, MCP-1, and plasminogen activator inhibitor-1, all of which are surrogate markers of endothelial injury.21 These findings emphasize the link between inflammation and endothelial dysfunction, likely explaining the association between the modified EASIX score and rate of CRS reported in this study.
Endothelial dysfunction has also been recognized as a potential mechanism of ICANS. A primate model demonstrated an association between elevated levels of serum IL-6, IL-8, IL-1RA, and other cytokines and elevated levels of IL-6, IL-2, granulocyte-macrophage colony-stimulating factor, and vascular endothelial growth factor in the cerebrospinal fluid, the latter associating with more severe neurological symptoms.22 A mouse model showed that in the setting of CRS, IL-1 secretion by monocytes preceded IL-6 production, and although blocking of IL-6 with tocilizumab ameliorated CRS, the mice had delayed neurotoxicity, accompanied by meningeal macrophage infiltration.20 In a study of 133 patients with B-cell malignancies who received a CD19 CAR T-cell product, high-grade ICANS was associated with low blood pressure, hypoxia, low serum protein, and albumin levels, and weight gain, suggesting a systemic capillary leak syndrome, potentially related to endothelial dysfunction.9 In the same study, high-grade ICANS was associated with high peak levels of IL-6, angiopoietin, and other markers of disseminated intravascular coagulation. Interestingly, higher pre-LDC levels of biomarkers of endothelial activation correlated with subsequent development of severe neurotoxicity.4 These mechanisms support our findings of a correlation of ICANS with EASIX, an endothelial activation marker, in combination with CRP, an inflammatory marker, and ferritin, a biomarker of monocyte and macrophages activation.
Of interest, in our study the modified EASIX score showed a stronger association with CRS and ICANS than its individual components, including LDH. The latter is commonly used as a surrogate marker of tumor burden, a known predictive factor of CAR-T–related toxicity.4,7,8,23 In addition, the laboratory values needed to calculate the EASIX score, combined with ferritin and CRP, are widely available as part of the current recommended pretreatment workup,24 thus providing an easily accessible tool for clinicians to estimate the probability of severe toxicity, even before starting the treatment. Two recent studies, published in abstract form, showed results similar to ours, with correlations of the EASIX score to outcomes after CAR-T-cell therapy.25,26 Interestingly, in one of these, when creatinine was replaced with CRP, the predictive power of the model was similar, and in our analysis, the role of creatinine was most likely minor as well. Larger trials are needed to assess the precise role of kidney function in predicting CAR-T toxicity.
We acknowledge the multiple limitations of our study. Only patients who received axi-cel were included in this analysis, limiting its application to other CAR-T products. The population sample is limited, and a validation cohort is missing. Finally, baseline tumor metabolic volume, a more robust surrogate of tumor burden than serum LDH levels, which were recently shown to be associated with CAR T outcomes,8,27 was not available, and, as such, it could not be included in the multivariate analysis. Further studies are needed to assess the added prognostic value of tumor metabolic volume to biomarkers in predicting CAR-T–related toxicity.
In conclusion, we demonstrated that a modified EASIX score is associated with increased rates of CRS and ICANS in patients with LBCL treated with axi-cel. This novel and inexpensive tool could support clinical decision making, including the setting for infusions (outpatient vs inpatient) and the duration of monitoring. It may also assist in the selection of high-risk patients for clinical trials and prophylactic strategies aimed at preventing toxicities.28 Larger studies aimed at validating these findings and the large-scale applicability of this tool are warranted.
Acknowledgments
U.G. is a recipient of a fellowship grant from the American Physicians’ Fellowship (APF) for Medicine in Israel. P.S. is supported by the Lymphoma Research Foundation Career Development Award.
Authorship
Contribution: U.G., P.S., R.M.S., P.K., and S.A. designed the study and wrote the initial draft of the manuscript; R.M.S. performed the statistical analysis and contributed to interpretation of the results; J.T., G.R., C.C.P., J.L.R., F.F.Y., and M.H., contributed to data collection and analysis; Y.N., C.H., S.A.S., J.W., L.E.F., H.J.L., S.P.I., R.N., L.J.N., S.P., M.A.R., F.S., R.E.S., M.W., C.R.F., S.T., K.R., R.E.C., E.J.S., and S.S.N. contributed to data interpretation and critical review; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: Y.N. has received grant support from Secura Bio, Novartis, and AstraZeneca and grant support and consultancy fees from Affimed. C.H. has received consultancy fees from NKARTA Inc. J.W. has received research funding and consultancy fees from Amgen, Morphosys. Janssen, BMS, Kite, Curis, Novartis, and AstraZenaca and consultancy fees from Genentech Inc. H.J.L. has received research funding from Celgene, Oncternal Therapeutics, Seattle Genetics, and Takeda; consultancy fees and research funding from Bristol-Myers Squibb; and consultancy fees from Guidepoint Blogal and has served on the speakers bureau of Aptitude Health. L.J.N. has received honoraria and research funding from Celgene, Genentech Inc, and Novartis; research funding from Karus Therapeutics, LAM Therapeutics Jansson, and Pfizer; honoraria from Bayer and Gamida Cell; and consultancy fees and research funding from Bristol-Myers Squib. S.P. is a current equity holder, is a member of the board of directors or advisory committees, holds patents and receives royalties, and has received research funding from Cellenkos Inc. M.W. has received consultancy fees from Guidepoint Global, Nobel Insights, MoreHealth, InnoCare, and Pulse Biosciences; honoraria from Dava Oncology, OncLive, and Targeted Oncology; research funding from Verastem, BioInvent, VelosBio, Acerta Pharma, and Molecular Templates; honoraria from Lu Daopei Medical Group and Beijing Medical Award Foundation; consultancy fees and research funding from Loxo Oncology and Juno; consultancy fees, research funding, and travel, accommodation, and expense reimbursements from Celgene and Kite Pharma; honoraria and travel, accommodation, and expense reimbursements from OMI; and consultancy fees, honoraria, research funding, and travel, accommodation, and expense reimbursements from Pharmacyclics, AstraZeneca, and Janssen. C.R.F: has received consultancy fees from Spectrum, Beigene, Denovo Biopharma, Pharmacyclics/Janssen, Karyopharm, and OptumRx; consultancy fees and research funding from AbbVie, Genentech, Inc/F Hoffmann-LaRoche Ltd, Millennium/Takeda, Gilead, and Celgene; and research funding from Kite, V Foundation National Cancer Institute, Eastern Cooperative Oncology Group, Burroughs Welcome Fund, and Acerta. M.H. has been a member of the board of directors or advisory committee of Kite. K.R. has been a member of the board of directors or advisory committee of GemoAb, Adicet Bio, Takeda and Formula Pharma; has received educational grants from Affimed, Pharmacyclics, and Virogen; and holds a licensing agreement with Takeda. R.E.C: holds patents and receives royalties from Takeda; has received consultancy fees from Actinium, Johnson and Johnson, Omeros, and Cytonus: has been a member of the board of directors or advisory committee of DKMS America; and serves on the speakers bureau for Genzyme. E.J.S: holds a licensing agreement with Takeda and has been a member of the board of directors or advisory committee of Magenta, Novartis, Celgene, Zelluna, and Adaptimmune. S.S.N: has received personal fees from Novartis, Cell Medica/Kuur, Incyte, and Pfizer; research funding and personal fees from Bristol-Myers Squibb, Merck, Kite/Gilead, Celgene, Allogene Therapeutics, and Precision Biosciences; research funding from Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics, Poseida, Cellectis, Karus Therapeutics, and Acerta; consultancy fees from Kebriaei and Jazz; and research support from Ziopharm and Amgen; has served on the advisory board of Novartis, Kite, and Pfizer; and has served on the board of directors or advisory committee of Ahmed and Tessa Therapeutics, and holds patents and receives royalties from Takeda.
Correspondence: Sairah Ahmed, Department of Lymphoma and Myeloma, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: sahmed3@mdanderson.org; and Partow Kebriaei, Department of Stem Cell Transplantation and Cellular Therapy, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: pkebriae@mdanderson.org.
References
Author notes
U.G. and P.S. contributed equally to this study.
P.K. and S.A. contributed equally to this study.
For original data, please contact the corresponding authors at sahmed3@mdanderson.org and pkebriae@mdanderson.org.
The full-text version of this article contains a data supplement.