Key Points
G3139 can be safely added to conventional chemotherapy for older patients with AML.
Adding G3139 did not improve complete remission rates, but patients with secondary AML had improved DFS.
Abstract
Overexpression of B-cell leukemia/lymphoma 2 (BCL2) renders acute myeloid leukemia (AML) cells resistant to chemotherapy and has been associated with unfavorable outcomes. Oblimersen (G3139) is a phosphorothioate 18-mer antisense oligonucleotide directed against the first 6 BCL2 codons. In a phase 1 study of AML patients treated with G3139, cytarabine, and daunorubicin induction with cytarabine consolidation, no antisense-related toxicity was reported, and BCL2 downregulation occurred in patients achieving complete remission. In this phase 3 trial, untreated older AML patients were randomized to cytarabine (100 mg/m2 per day on days 4-10) and daunorubicin (60 mg/m2 per day on days 4-6) followed by cytarabine consolidation (2000 mg/m2 per day on days 4-8) with (arm A) or without (arm B) G3139 (7 mg/m2 per day on days 1-10 [induction] or days 1-8 [consolidation]). A total of 506 patients were enrolled. No differences in toxicity were observed between arms. Estimated overall survival (OS) at 1 year was 43% for arm A and 40% for arm B (1-sided log rank P = .13), with no differences in disease-free (DFS; P = .26) or event-free survival (P = .80). Subgroup analyses showed patients age <70 years in arm A had improved OS by 1 month vs those in arm B (P = .04), and patients with secondary AML in arm A had better DFS vs those in arm B (P = .04). We conclude that addition of G3139 to chemotherapy failed to improve outcomes of older AML patients. However, more effective means of inhibiting BCL2 are showing promising results in combination with chemotherapy in AML. This trial was registered at www.clinicaltrials.gov as #NCT00085124.
Introduction
The identification of mechanisms of leukemogenesis that lead to maturation arrest, myeloid blast proliferation, and resistance to chemotherapy has helped guide the development of targeted therapies for the treatment of patients with acute myeloid leukemia (AML). While applicable to all patients, those age ≥60 years are in need of novel induction strategies, because they often have adverse cytogenetic and/or molecular abnormalities that confer an inferior response to standard cytarabine and anthracycline chemotherapy.1,2 Current treatment approaches attempt to improve complete remission (CR) rates by adding an agent with a unique mechanism of action that will not increase the toxicity of standard chemotherapy and will possibly prolong disease-free (DFS) and overall survival (OS).3,4
Mitochondrial processes involved in cell survival and death signaling are critically important in cancer biology, because defects within the intrinsic apoptotic pathway confer a survival advantage and contribute to chemotherapy resistance.5 The proteins that seem to exert the most control over this pathway are those within the B-cell leukemia/lymphoma 2 (BCL2) family.6 By binding to proapoptotic moieties, which disrupt mitochondrial integrity and allow the release of cytochrome c, BCL2 prevents the initiation of apoptosis and thus prolongs cell survival.7 Because the percentage of BCL2-expressing cells increases in patients with AML, the CR rates after intensive treatment decrease, and patients with BCL2-expressing leukemia (≥20% BCL2+ cells) have shorter survival than those with BCL2− leukemia (<20% BCL2+ cells).8 Additionally, upregulation of BCL2 within leukemic stem cells may promote leukemic stem cell survival in response to stressful stimuli such as chemotherapy or radiotherapy.9 The small-molecule oral BCL2 inhibitor venetoclax was recently approved for the treatment of newly diagnosed older and/or unfit AML patients in combination with hypomethylating agents or low-dose cytarabine based on high rates of durable remissions.10 On the basis of these observations, downregulation of the BCL2 gene itself as a therapeutic strategy could be used as a way to lower the apoptotic threshold and restore chemosensitivity.
Oblimersen sodium (G3139) is an 18-mer phosphorothioate oligodeoxynucleotide antisense molecule designed to bind the first 6 codons of human BCL2 messenger RNA (mRNA). In a phase 1 trial of G3139 in combination with escalated doses of fludarabine, cytarabine, and granulocyte colony-stimulating factor in patients with refractory or relapsed acute leukemia, downregulation of BCL2 mRNA after G3139 administration was observed. The agent was well tolerated, with 45% of patients achieving CR or CR with incomplete count recovery.11 In a larger phase 1 trial of 29 patients age ≥60 years with untreated AML, G3139 in combination with standard cytarabine and anthracycline induction and high-dose cytarabine consolidation was deemed safe and tolerable, and BCL2 downregulation was observed to occur preferentially in the 14 patients who achieved CR.12 Indeed, those who achieved CR had a decrease in median BCL2 mRNA copies compared with baseline (P = .03), whereas nonresponders had an increase in median BCL2 mRNA copies (P = .05).12
The primary objective of the Cancer and Leukemia Group B (CALGB) 10201 study was to determine whether the addition of G3139 to cytarabine/daunorubicin induction chemotherapy and high-dose cytarabine consolidation therapy improves outcomes with regard to CR rate, DFS, event-free survival (EFS), and OS in previously untreated AML patients age ≥60 years.
Patients and methods
Eligibility criteria and study design
Patients age ≥60 years with an unequivocal diagnosis of AML based on World Health Organization and/or French-American-British classification, excluding acute promyelocytic leukemia, were enrolled at CALGB centers and through the Cancer Trials Support Unit. CALGB is now part of the Alliance for Clinical Trials in Oncology (Alliance). Patients with a history of antecedent myelodysplasia (MDS) who had not received prior cytotoxic chemotherapy and those with therapy-related AML were eligible. Signed informed consent was obtained before study entry. G3139 was provided to the National Cancer Institute under a Clinical Research and Development Agreement between Genta, Inc., and the National Cancer Institute, whereby the drug was shipped directly to the institutions where the patients were to be treated.
For remission induction, patients were randomized 1:1 to 1 of the 2 treatment arms, as shown in Table 1. Patients in arm A received 7 mg/kg of G3139 per day as a continuous IV infusion (CIVI) through a central line on days 1 to 10; 100 mg/m2 of cytarabine per day was administered as a CIVI through a central line on days 4 to 10, and 60 mg/m2 of daunorubicin per day was administered by IV push through a central line on days 4 to 6. G3139 was administered before the initiation of chemotherapy based on the hypothesis that downregulation of BCL2 would lower the apoptotic threshold and restore chemosensitivity in otherwise resistant leukemic cells. A bone marrow (BM) aspirate and biopsy were performed on day 17 for patients enrolled in arm A to determine the need for a second induction cycle. Patients enrolled in arm B received cytarabine and daunorubicin alone via the same doses, schedules, and methods of administration as patients enrolled in arm A; however, a BM aspirate and biopsy were performed on day 14 to assess the need for a second induction cycle. The dose of daunorubicin was reduced if the bilirubin levels were elevated (daunorubicin was reduced by 25% for total bilirubin between 2 and 3 mg/dL and by 50% for total bilirubin >3 mg/dL). Patients with residual leukemia received a second induction course according to the arm to which they had been randomized. Patients in arm A received 7 mg/kg of G3139 per day by CIVI on days 1 to 8, 100 mg/m2 of cytarabine per day on days 4 to 8, and 60 mg/m2 of daunorubicin per day on days 4 to 5. Patients enrolled in arm B received 100 mg/m2 of cytarabine per day by CIVI on days 1 to 5 and 60 mg/m2 of daunorubicin per day by IV push on days 1 to 2. Regardless of treatment arm, BM aspiration and biopsy were performed within 1 week after recovery of absolute neutrophil count ≥1500/μL and platelets ≥100 × 109/L, but no later than on day 42 of the second induction. Patients who were documented to have residual leukemia at this time were removed from protocol therapy and followed for survival.
Patients who achieved CR were eligible to receive consolidation according to their initial randomization. Patients were required to have resolution of all significant induction toxicities to grade <2. Patients who had experienced a decline in left ventricular ejection fraction to <35% and had been randomized to receive G3139 had it omitted from consolidation therapy. Patients enrolled in arm A received 7 mg/kg of G3139 per day by CIVI on days 1 to 8 and 2000 mg/m2 of cytarabine per day IV over 3 hours on days 4 to 8. Patients enrolled in arm B received 2000 mg/m2 of cytarabine per day IV over 3 hours on days 1 to 5. In both arms, serial neurologic evaluation was performed before and after each infusion of cytarabine, and corticosteroid ophthalmic drops to each eye were administered 4 times daily and continued for at least 24 hours after the final dose of cytarabine. Patients who completed the first consolidation were eligible to proceed to a second cycle after confirmation of continued remission on a BM aspirate and biopsy.
Patients who experienced grade 3 or 4 nonhematologic toxicity during G3139 infusion during remission induction or consolidation that was considered to be probably or possibly related to G3139 were managed according to dose-reduction and discontinuation guidelines set forth in the protocol.
Cytogenetic analyses
Pretreatment cytogenetic analyses of BM and/or blood samples were performed by CALGB-approved institutional cytogenetic laboratories, and the results were confirmed by central karyotype review.13,14 For the karyotype to be designated as normal, at least 20 metaphase cells from BM specimens that were subjected to short-term (24- and/or 48-hour) culture must have been analyzed and no clonal abnormality found.13,14
Molecular analyses
The mutational status of NPM1, ASXL1, RUNX1, and TP53 genes was determined centrally at The Ohio State University by targeted amplicon sequencing using the MiSeq platform (Illumina, San Diego, CA) as described previously.15 Determination of CEBPA mutational status and testing for internal tandem duplication of the FLT3 gene were performed as described by Marcucci et al16 and Whitman et al,17 respectively. Patients for whom cytogenetic and molecular data were available were categorized according to the 2017 European LeukemiaNet (ELN) genetic risk classification.18
Statistical analyses
The primary objective was to determine whether the addition of G3139 to cytarabine/daunorubicin induction chemotherapy and high-dose cytarabine consolidation therapy improves outcomes with regard to OS, DFS, EFS, and CR rates in previously untreated AML patients age ≥60 years. There were no stratification factors used in the randomization. The primary end point was OS, which was defined as the time from randomization to death resulting from any cause. This study was designed to have a power of 0.77 to detect a 33% increase in median OS (9 vs 12 months) with a type 1 error of 0.025 (1 sided); this required a target sample size of 500 patients to be evenly randomized to 1 of the 2 treatments to achieve the target number of events of 350 deaths. DFS was defined as the time from remission to relapse or death resulting from any cause. EFS was defined as the time from randomization to relapse, death resulting from any cause, or failure to achieve CR by the end of the induction courses. Interim efficacy analyses were planned for OS, DFS, EFS, and CR rates to coincide with semiannual CALGB/Alliance Data and Safety Monitoring B meetings, beginning after 25% of the expected events for each end point (OS, DFS, EFS, and CR rate) had been observed. Each analysis was to use a Lan-DeMets spending function analog of a 1-sided O’Brien-Fleming stopping rule truncated at 0.001 when relevant.
χ2 tests were used to compare categorical variables,19 and 2 sample Student t tests were used to compare continuous variables.20 The distributions of time-to-event end points were estimated using the Kaplan-Meier method21 and compared between 2 treatment arms using log-rank tests.22 Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox proportional hazards model.23P value for primary analysis of OS was determined by 1-sided log-rank test per study design, and P values for all other end points and secondary analyses were 2 sided. All primary analyses were performed by intent to treatment. The secondary analyses were performed among patients who were eligible and received at least 1 cycle of treatment. Subgroup analyses based on pretreatment clinical and cytogenetic characteristics were planned. Clinical data were locked on 14 July 2016, although the molecular analyses were more recently completed.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Results
Patient characteristics
A total of 506 patients were enrolled between October 2004 and October 2006 at 75 CALGB member institutions and their affiliated hospitals; 254 patients were randomized to arm A and 252 to arm B (supplemental Figure 1). Sixty-eight patients with either therapy-related AML or AML after prior MDS were enrolled in arm A, and 59 were enrolled in arm B. Eleven patients never began protocol therapy after they were registered and randomized; they were included in the intent-to-treat analysis but excluded from the correlative analyses. A patient age 48.5 years old was included in this group of 11 patients. Baseline clinical characteristics are listed in Tables 2 and 3.
Response to induction chemotherapy
Of the 506 patients, 277 achieved remission. The CR rate was 53% in arm A vs 56% in arm B. There was no statistical difference between the 2 arms (P = .53; Table 4). Sixty-four patients (26%) in arm A and 66 (27%) in arm B (P = .84) received a second induction course. Subgroup analysis by age (<70 vs ≥70 years; supplemental Table 1) and by AML type (supplemental Table 2) showed no difference in CR rate between the 2 arms. Response rates of patients classified according to 2017 ELN genetic risk classification18 are shown in Table 5. There were no statistically significant differences in CR rate between patients in arm A and those in arm B in any of the 2017 ELN genetic risk groups. A minority of patients in arm A from select institutions had pretreatment and follow-up samples submitted for reverse transcription polymerase chain reaction analysis of BCL2 expression, limiting the interpretation of the results. Given that these findings are inconclusive, they are not included here.
Consolidation treatment
Of the 277 patients who achieved CR, 211 received at least 1 cycle of consolidation. By arm, 70 patients (68%) in arm A and 73 (68%) in arm B completed both cycles of consolidation. Five patients in arm A did not receive G3139 during consolidation. None had experienced a decline in cardiac ejection fraction as the reason for discontinuation. Patients who did not receive both cycles of chemotherapy withdrew from the study because of disease progression (n = 29), toxicity (n = 14), switching to an alternative therapy (n = 4), or death (n = 6).
Treatment toxicities during induction
During induction, toxicities that commonly follow intensive chemotherapy for AML were observed. Grade 4 neutropenia and thrombocytopenia were nearly universal, occurring in >90% of patients in both groups. The most common grade 3 or 4 nonhematologic toxicities were febrile neutropenia and infection, occurring equally between the 2 arms (febrile neutropenia: arm A, 73% vs arm B, 77%; infection: arm A, 57% vs arm B, 56%). The next most common toxicities included electrolyte abnormalities, dyspnea, diarrhea, and fatigue. Twelve patients in arm A and 8 in arm B developed left ventricular systolic dysfunction during induction; this difference was not statistically significant. One patient in arm A developed tumor lysis syndrome compared with 2 in arm B. Deaths during the first 30 days of treatment are reported in Table 4 and did not differ by treatment arm (13% vs 12%; P = .81). Table 6 lists all grade ≥3 nonhematologic adverse events that occurred in ≥10% of patients during the induction course.
Treatment toxicities during consolidation
During consolidation therapy, >90% of patients experienced grade 4 neutropenia and thrombocytopenia. Febrile neutropenia and grade 3 or 4 infection were common in both groups, but not as frequent as during induction (febrile neutropenia: arm A, 38% vs arm B, 47%; infection: arm A, 27% vs arm B, 33%). Grade 3 rash (4% vs 5%) and diarrhea (6% vs 2%) were also less common during consolidation than during induction. There were 9 patients (4%) in arm A who developed grade 3 ataxia compared with 2 in arm B (1%). There were 4 treatment-related deaths (arm A, n = 2; arm B, n = 2) during consolidation.
Survival and long-term follow-up
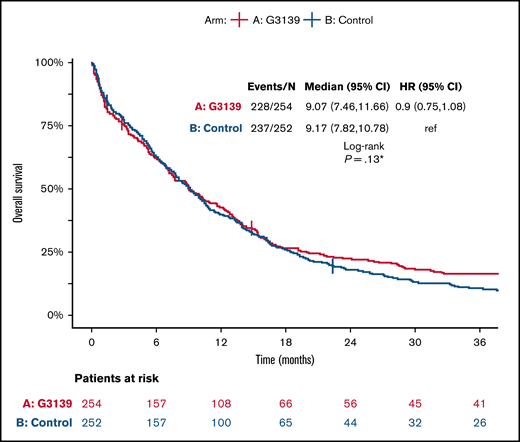
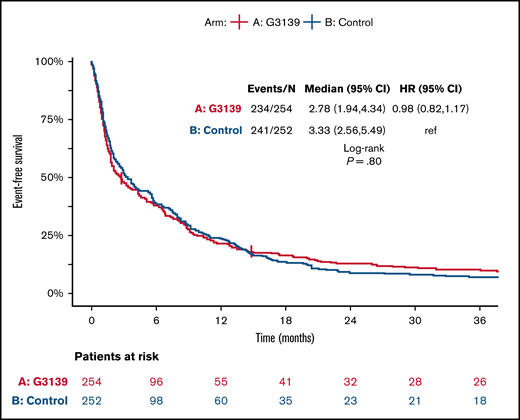
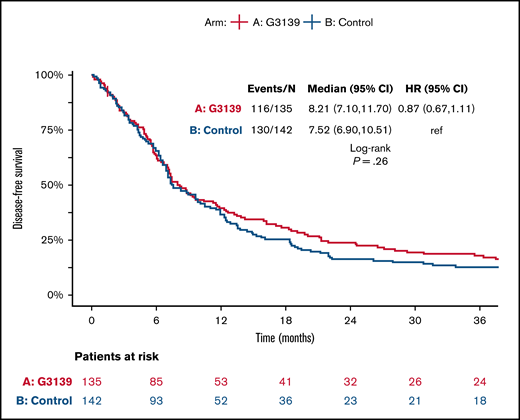
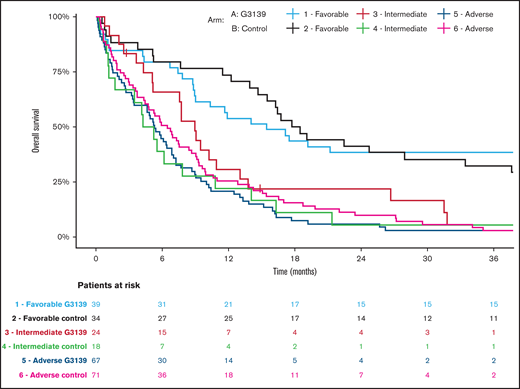
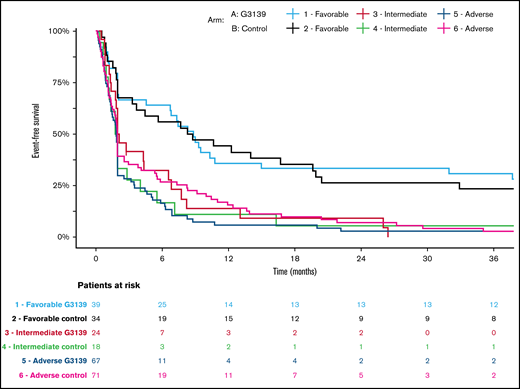
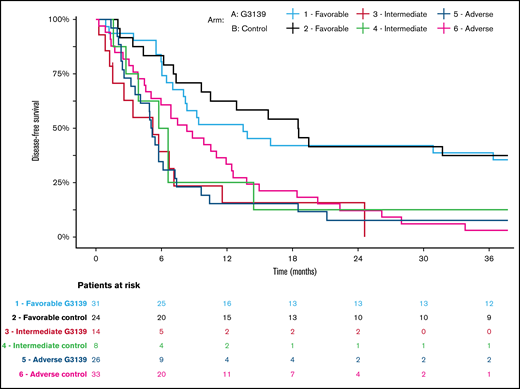
Among the 506 randomized patients, median follow-up was 100 months (range, 1-119) for the 41 surviving patients. There were no significant differences between the 2 arms in OS, EFS, or DFS (Table 4; Figures 1-3). Median OS was 9 months (95% CI, 7-12) in the G3139 group and 9 months (95% CI, 8-11) in the control group (1-sided log-rank P = .13), with an HR of 0.9 (95% CI, 0.75-1.08). The 1-year OS rate was 43% in the G3139 group and 40% in the control group. Median EFS was 3 months (95% CI, 2-4) in the G3139 group and 3 months (95% CI, 3-5) in the control group (P = .80), with an HR of 0.98 (95% CI, 0.82-1.17). Median DFS was 8 months (95% CI, 7-12) in the G3139 group and 8 months (95% CI, 7-11) in the control group (P = .26), with an HR of 0.87 (95% CI, 0.67-1.11).
OS. Kaplan-Meier curves for OS in the G3139 and control groups. Tick marks indicate censoring of data. *One-sided P value.
OS. Kaplan-Meier curves for OS in the G3139 and control groups. Tick marks indicate censoring of data. *One-sided P value.
EFS. Kaplan-Meier curves for EFS in G3139 and control groups. Tick marks indicate censoring of data.
EFS. Kaplan-Meier curves for EFS in G3139 and control groups. Tick marks indicate censoring of data.
DFS. Kaplan-Meier curves for DFS in G3139 and control groups. Tick marks indicate censoring of data.
DFS. Kaplan-Meier curves for DFS in G3139 and control groups. Tick marks indicate censoring of data.
Subgroup analysis showed no significant differences between the 2 arms in EFS or DFS based on age (supplemental Table 1). However, OS for patients age <70 years in arm A was significantly longer than OS for patients age <70 years in arm B, with a median of 10 vs 9 months (P = .04; supplemental Table 1). A subgroup analysis based on AML type showed no significant differences between the 2 arms in EFS or OS (supplemental Table 2). However, DFS for patients with secondary AML in arm A was significantly improved compared with DFS for this patient subset in arm B, with a median of 10 vs 6 months (P = .04; supplemental Table 2).
A total of 46 patients underwent stem cell transplantation. A majority received an allogeneic hematopoietic cell transplantation (alloHCT) from either a matched sibling (n = 25) or matched unrelated donor (n = 11; supplemental Table 3). Three patients received an alloHCT from an alternative donor source (ie, either umbilical cord blood or haploidentical transplant). Seven patients underwent autologous transplantation, 3 using BM and 4 using peripheral blood stem cells. There was no difference in the number of patients undergoing transplantation by arm (n = 24 vs 22; P = .99).
A total of 19 patients in arm A and 20 in arm B received an alloHCT; no significant differences in OS, EFS, or DFS were observed between the 2 arms (supplemental Figures 2-4). Because allogeneic transplantation was an important alternative therapy, we performed a sensitivity analysis of OS, EFS, and DFS, in which data were censored at the time patients received an alloHCT. In this analysis, there was an 11% lower risk of death in arm A than in arm B (HR, 0.89; 95% CI, 0.74-1.08), but the difference did not reach statistical significance (P = .24; supplemental Figure 5). DFS and EFS were also similar between the 2 arms after censoring at the time of alloHCT (EFS, P = .99; DFS, P = .20; supplemental Figures 6 and 7).
For patients who did not receive an alloHCT (n = 467), OS, DFS, and EFS were also similar between the 2 arms (OS, P = .23; EFS, P = .55; DFS, P = .17; supplemental Figures 8-10). However, for patients with secondary AML who did not undergo transplantation, G3139 was associated with improved OS and DFS as compared with control (OS: median, 10 vs 7 months; P = .03; DFS: median, 10 vs 6 months; P < .01; supplemental Figures 11 and 12).
Discussion
Despite advances in our understanding of the biology of AML and characterization of prognostic clinical and genetic factors, the overall outcome remains poor for patients age >60 years.1,24,25 Given that CR rates remain inferior to those of patients age <60 years, attempts to target mechanisms leading to primary resistance to chemotherapy with novel induction strategies are needed.1 One approach in AML, as well as in other hematologic malignancies, has been to develop agents that enhance apoptosis in leukemic cells. Several proteins within the mitochondrial apoptotic pathway have been identified as potential targets because of their antiapoptotic function, including BCL2, BCL-XL, and MCL-1.6 Aberrant BCL2 expression has been associated with lower CR rates and poor outcomes in patients with AML, and we hypothesized that downregulation of BCL2 expression may induce a lower apoptotic threshold and restore chemosensitivity.8 Oblimersen sodium (G3139) is an oligodeoxynucleotide antisense molecule designed to hybridize to BCL2 mRNA, thus becoming a substrate for degradation and creating a net decrease in BCL2 translation.26 Early-phase clinical trials of G3139 in combination with cytotoxic chemotherapy showed the agent to be well tolerated and able to downregulate BCL2 mRNA levels in select patients.11,12
In the current study, which enrolled older patients with newly diagnosed de novo and secondary AML, we assessed whether patients randomized to receive G3139 in combination with standard intensive cytarabine/daunorubicin induction chemotherapy and high-dose cytarabine consolidation would experience an improvement in CR rates and other disease-specific outcomes when compared with those randomized to arm B, who did not receive G3139. We observed no added toxicities. G3139 was well tolerated in this group of patients and did not seem to increase or prolong the adverse events normally seen during anthracycline-based induction. However, when considering all treated participants, there were no overall differences in CR rates, OS, DFS, or EFS between those who received G3139 and those who did not, regardless of the 2017 ELN genetic risk group (Table 5; Figures 4-6).18 Importantly, patients randomized to receive G3139, who thus had a 3-day delay in starting cytotoxic chemotherapy, had similar clinical outcomes and did not experience a worse early death rate compared with the control group. A subset analysis showed that patients with secondary AML, defined as therapy-related AML or AML after prior MDS, who received G3139 had improved DFS when compared with those who did not. Additionally, for patients with secondary AML who received G3139 and did not undergo transplantation, G3139 was associated with improved OS and DFS when compared with the standard therapy arm.
Kaplan-Meier estimates of OS in AML patients categorized according to 2017 ELN genetic risk classification by treatment arm. Patients receiving G3139 (arm A) and those in the control arm (arm B) were arranged into 3 genetic risk groups (favorable, intermediate, or adverse) based on their cytogenetic and molecular genetic findings at diagnosis.18
Kaplan-Meier estimates of OS in AML patients categorized according to 2017 ELN genetic risk classification by treatment arm. Patients receiving G3139 (arm A) and those in the control arm (arm B) were arranged into 3 genetic risk groups (favorable, intermediate, or adverse) based on their cytogenetic and molecular genetic findings at diagnosis.18
Kaplan-Meier estimates of EFS in AML patients categorized according to 2017 ELN genetic risk classification by treatment arm. Patients receiving G3139 (arm A) and those in the control arm (arm B) were arranged into 3 genetic risk groups (favorable, intermediate, or adverse) based on their cytogenetic and molecular genetic findings at diagnosis.18
Kaplan-Meier estimates of EFS in AML patients categorized according to 2017 ELN genetic risk classification by treatment arm. Patients receiving G3139 (arm A) and those in the control arm (arm B) were arranged into 3 genetic risk groups (favorable, intermediate, or adverse) based on their cytogenetic and molecular genetic findings at diagnosis.18
Kaplan-Meier estimates of DFS in AML patients categorized according to 2017 ELN genetic risk classification by treatment arm. Patients receiving G3139 (arm A) and those in the control arm (arm B) were arranged into 3 genetic risk groups (favorable, intermediate, or adverse) based on their cytogenetic and molecular genetic findings at diagnosis.18
Kaplan-Meier estimates of DFS in AML patients categorized according to 2017 ELN genetic risk classification by treatment arm. Patients receiving G3139 (arm A) and those in the control arm (arm B) were arranged into 3 genetic risk groups (favorable, intermediate, or adverse) based on their cytogenetic and molecular genetic findings at diagnosis.18
There are limitations to the study. Given the small number of pretreatment and follow-up samples submitted for BCL2 expression analysis, it is difficult to determine whether the lack of benefit between the 2 treatment groups was due to a lack of target inhibition or other mechanisms that prevented response to chemotherapy. Antisense oligonucleotides are known to have issues with delivery, targeting, and off-target effects,27 and it is not known whether these played a role in this trial. Nonetheless, we did see some benefit in patients with secondary AML, usually a more refractory subgroup, and this may suggest that this subtype is more sensitive to BCL2 downregulation.
Whereas G3139 attempts to inhibit BCL2 at the level of translation, newer agents that target the BCL2 protein directly have been developed. Venetoclax is an oral highly selective BCL2 inhibitor that was granted accelerated approval by the US Food and Drug Administration for chronic lymphocytic leukemia with chromosome 17p deletion in April 2016.28 Given the concern for drug delivery and target accuracy with antisense molecules, the more potent direct inhibitory effect of venetoclax would be an advantage over G3139. Preclinical studies in primary AML samples in vitro and in xenograft models demonstrated that BCL2 inhibition resulted in cell death.29 Although single-agent venetoclax was well tolerated in patients, it had limited antileukemic activity in relapsed or refractory AML.30 However, early-phase studies of venetoclax combined with low-intensity chemotherapy demonstrated high response rates and longer OS in older adults with previously untreated AML.31 The superiority of venetoclax-based combination therapy has since been confirmed in 2 separate phase 3 randomized trials. In the VIALE-A32 and VIALE-C33 trials, patients with previously untreated AML were randomized to venetoclax or placebo plus azacitidine or low-dose cytarabine, respectively. Patients randomized to venetoclax experienced a higher composite CR (CR plus CR with incomplete hematologic recovery) and longer OS compared with those in the placebo arm of either trial. These results, in addition to the tolerability of these regimens, have solidified venetoclax-based induction strategies as a standard of care for patients with untreated AML. Additional trials of venetoclax in younger patients and in combination with cytotoxic chemotherapy are under way.34
AML cells seem to be particularly dependent on BCL2 for their survival. The approval of venetoclax in combination with hypomethylating agents or low-intensity chemotherapy has already changed the therapeutic landscape for older patients with AML. Whether using an antisense molecule such as G3139 to lower the intracellular levels of BCL2 would provide any synergistic benefit beyond the use of venetoclax plus chemotherapy is uncertain. Although there has been no further development of G3139, the concept of attacking antiapoptotic proteins, as attempted in the current trial, may represent a new paradigm in AML therapy.
Acknowledgments
The authors thank patients and their families, collaborators, and staff in the CALGB and Alliance member institutions/data center and the CALGB/Alliance Leukemia Tissue Bank. The authors acknowledge C.D.B.’s key role in the design and completion of this study.
This work was supported by the National Cancer Institute, National Institutes of Health, under awards U10CA180821, U10CA180882, and U24CA196171 (Alliance for Clinical Trials in Oncology) and P30CA033572, UG1CA189848, UG1CA189850, UG1CA189858, UG1CA233180, UG1CA233191, UG1CA233327, UG1CA233331, UG1CA233338, and UG1CA233339. Support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs is listed at https://acknowledgments.alliancefound.org. This work was also supported in part by Deltec, Inc.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: G.M. and R.A.L. were responsible for study conception and design; A.R.W., G.M., W.B., W.S., A.J.C., A.-K.E., E.S.W., J.E.K., M.T., G.S., R.V., R.K.S., J.C.B., C.D.B., R.M.S., and R.A.L. acquired data; A.R.W., G.M., J.Y., J.K., K.M., S.J., C.D.B., and R.A.L. analyzed and interpreted the data; A.R.W., K.M., R.A.L., R.M.S., and J.Y. drafted the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: R.A.L. has acted as a consultant or advisor for Novartis, Amgen, Ariad/Takeda, Astellas, Celgene/Bristol-Myers Squibb, CVS/Caremark, Epizyme, and MorphoSys and has received clinical research support from Novartis, Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven, and Rafael Pharmaceuticals and royalties from UpToDate. G.M. has acted as a consultant or advisor for Novartis. R.M.S. has acted as a consultant or advisor for AbbVie, Actinium, and Agios; has received personal fees from Amgen, Argenx, AROG, Astellas, AstraZeneca, BioLineRx, Celgene, Cornerstone, Daiichi-Sankyo, Fujifilm, Jazz Pharmaceuticals, MacroGenics, Novartis, Ono/Theradex Oncology, Orsenix, Otsuka/Astex, Pfizer, Roche, Stemline Therapeutics, Takeda, and Trovagene; and has received institutional research support from AbbVie, Agios, AROG, and Novartis. A.-K.E. has received a research grant from Novartis and has ownership interest in Karyopharm Therapeutics. A.R.W. has received clinical research support from Gilead, Novartis, Geron, and Newave. The remaining authors declare no competing financial interests.
Clara D. Bloomfield died on 1 March 2020.
Correspondence: Alison R. Walker, Division of Hematology, Department of Internal Medicine, The Ohio State University, 410 West 10th Ave, Columbus, OH 43210; e-mail: alison.walker@osumc.edu.
References
Author notes
For data sharing, contact the corresponding author at alison.walker@osumc.edu.
The full-text version of this article contains a data supplement.