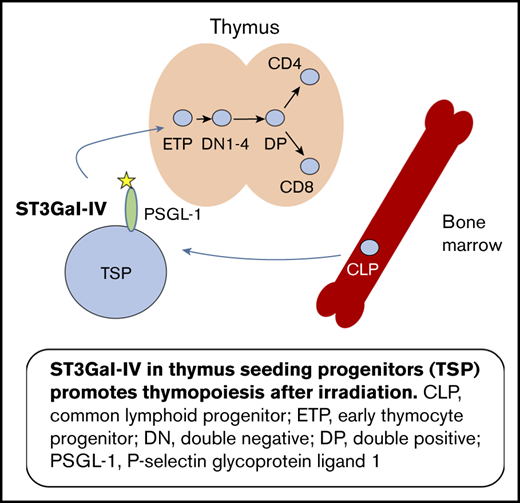
Key Points
ST3Gal-IV–deficient T-cell progenitors have a competitive disadvantage in seeding the thymus of bone marrow chimeric mice.
Retroviral reconstitution of ST3Gal-IV promotes T-cell lineage development in a competitive in vivo setting.
Abstract
T lymphocytes are important players in beneficial and detrimental immune responses. In contrast to other lymphocyte populations that develop in the bone marrow, T-cell precursors need to migrate to the thymus for further development. The interaction of P-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) is crucial for thymic entry of T-cell precursors during settings of T-cell lineage reconstitution. PSGL-1 has to be sialylated to function as a ligand for P-selectin, and the sialyltransferase ST3Gal-IV might play a critical role in this process. We therefore investigated the role of ST3Gal-IV for T-cell development using competitive mixed bone marrow chimeric mice. We found that ST3Gal-IV is dispensable for homing and engraftment of hematopoietic precursors in the bone marrow. However, ST3Gal-IV deficiency affects seeding of the thymus by early T-cell progenitors, leading to impaired restoration of the peripheral T-cell compartment. This defect could be restored by ectopic retroviral expression of ST3Gal-IV in hematopoietic stem cells derived from ST3Gal-IV–deficient donor mice. Our findings show that ST3Gal-IV plays a critical and nonredundant role for efficient T-cell lineage reconstitution after bone marrow transplantation.
Introduction
T-cell development occurs in the thymus but needs continuous import of T-cell progenitor cells from the bone marrow by mechanisms that are poorly understood. The lineage-negative, Sca1-positive, c-Kit-positive (LSK) cell population in the bone marrow contains hematopoietic stem cells (HSCs) and multipotent precursor cells.1,2 The latter differentiate into common lymphoid progenitors (CLPs) characterized by interleukin-7 (IL-7) receptor expression3 and common myeloid progenitors.4 The CLPs are supposed to include T-cell progenitors in the bone marrow, but the exact nature of the circulating T-cell progenitors in the blood remains unknown.5-9 It has been shown that multiple T-cell progenitor populations exist in physiological conditions.10 The circulating T-cell progenitors reach the thymus via the bloodstream, enter the thymus, and give rise to the early thymic progenitors (ETPs), which generate all downstream thymocytes.11
Thymus settling T-cell progenitors (TSPs) enter the thymus via a stepwise cascade of rolling, activation, adhesion, and diapedesis.12 The rolling of the TSPs depends on the interaction of P-selectin expressed on thymic endothelial cells and P-selectin glycoprotein ligand-1 (PSGL-1) expressed on the TSPs.12,13 Of note, functional PSGL-1 is not expressed on HSCs but on cells capable of thymic settling.14 PSGL-1 is a versatile molecule influencing many aspects of T-cell biology as migration of activated T helper 1 cells to sites of inflammation and immune regulation by induction of exhaustion and tolerance.15 To function as a ligand for P-selectin, PSGL-1 has to be posttranslationally modified by various enzymatic steps.16,17
One of these crucial modifications is the addition of α-2,3-linked sialic acid to the tetrasaccharide Lewis X residue of PSGL-1. There are currently 2 α-2,3-sialyltransferases that have been cloned and characterized with substrate preferences indicating they may generate P-selectin ligands, namely ST3Gal-IV and ST3Gal-VI.18,19 Both sialyltransferases were subsequently shown to contribute to selectin ligand formation and to mediate E- and P-selectin–dependent rolling of murine neutrophils in in vitro flow chamber systems, as well as under inflammatory conditions in vivo.20,21 In addition, ST3Gal-IV was shown to mediate L-selectin–dependent leukocyte–leukocyte interactions (secondary tethering) under in vivo conditions.22 Furthermore, ST3Gal-IV is upregulated in T helper 1 cells and mediates their migration into inflammatory sites,23 but the functions of ST3Gal-IV in physiological noninflammatory conditions is poorly understood. The initial characterization of ST3Gal-IV–deficient mice showed a reduction of the von Willebrand factor in plasma and a thrombocytopenia in these mice mimicking the human bleeding disorder von Willebrand disease.24 Although the expression of ST3Gal-IV in human and murine thymus was reported >2 decades ago,25,26 no data exist about its role in this organ.
Because interaction of P-selectin and PSGL-1 is crucial for T-cell progenitors to settle the thymus, and PSGL-1 needs to be sialylated to function as a ligand for P-selectin, we were interested in the role of the α-2,3-sialyl-transferase ST3Gal-IV for T-cell development. We found that in mixed bone marrow chimeric (MBMC) mice, ST3Gal-IV–deficient cells had a pronounced defect in reconstituting the thymus and the peripheral T-cell compartments. Early hematopoietic precursor cells in the bone marrow were not dependent on ST3Gal-IV, but ETPs in the thymus were generated less efficiently from ST3Gal-IV–deficient cells. The proliferation of ST3Gal-IV–deficient ETPs was not diminished, and ST3Gal-IV–deficient LSK cells had no defect in generating thymocytes in the OP9-DL1 coculture system. These data point to an important role of α-2,3-sialic acid in mediating thymic settling during T-cell lineage reconstitution.
Materials and methods
Mice
ST3Gal-IV–deficient mice on C57BL/6 background24 and C57BL/6_CD45.1 congenic mice (B6.SJL-Ptprca Pepcb/BoyJ) were maintained in the Franz-Penzoldt Center in Erlangen, Germany, under specific pathogen-free conditions. All experiments were performed in accordance with German animal protection law and European Union guidelines 86/809 and were approved by the Federal Government of Lower Franconia.
Generation of MBMC mice
Bone marrow cells were flushed with phosphate-buffered saline (Merck Millipore, Darmstadt, Germany) from the tibia and femur of ST3Gal-IV–deficient or CD45.1/CD45.2 heterozygous mice. Erythrocytes were lysed with ACK-buffer (0.15 M NH4Cl, 1 mM KHO3, 0.1 mM Na2EDTA). Recipient wild-type (WT) mice (CD45.1) were irradiated by doses of 500 rad and 400 rad 4 hours apart. A mixture of either 50% ST3Gal-IV–deficient cells and 50% WT cells (50:50) or 90% ST3Gal-IV–deficient cells and 10% WT cells (90:10) (1 × 106 total cells) was IV injected into recipient mice. Mice were kept with antibiotic-containing drinking water (2 g/L neomycin sulfate, 100 mg/L polymyxin B sulfate; MilliporeSigma, Burlington, MA) for 8 weeks after reconstitution and analyzed after 8 to 10 weeks.
Plasmids
St3gal4 coding sequence was amplified with the Pfu polymerase using complementary DNA from murine spleen as the template (Thermo Fisher Scientific, Waltham, MA) and the following primers: forward primer 5′-ggatccGCTATCTGCTGAGAAACATGAC-3′ and reverse primer 5′-gcggccgcCTCCCATCCAAGTCAGAAGTAT-3′. The polymerase chain reaction product was cloned with BamHI and NotI (New England Biolabs, Frankfurt/Main, Germany) into the retroviral vector pMXpie to generate ST3Gal-IV–expressing retroviruses.
Production of retroviral supernatants
Cells (3.5 × 106) of the packaging cell line Phoenix were seeded 1 day before transfection in 10 cm cell culture dishes in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 µg/mL streptomycin (Biochrom, Berlin, Germany). Calcium-phosphate-transfection was conducted by using 20 µg of the ST3Gal-IV-pMXpie plasmid or as control the empty pMXpie plasmid together with 5 µg of the helper plasmid pCLEco (Biocarta Europe GmbH, Hamburg, Germany). Medium was changed 1 day later, and retroviral supernatants were harvested after an additional day, aliquoted, and stored at −80°C.
Generation of retrogenic mice
Donor mice (ST3Gal-IV–deficient or CD45.1/CD45.2 heterozygous) were injected intraperitoneally with 0.15 mg/g 5-fluorouracil (5-FU) (MilliporeSigma) to enrich for HSCs 3 to 4 days before harvesting the bone marrow cells. Bone marrow cells were seeded at a density of 2 to 3 × 106 cells per well in a 24-well plate in 1 mL BM-medium (Dulbecco’s modified Eagle medium supplemented with 15% FCS, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 20 ng/mL IL-3 [ImmunoTools, Friesoythe, Germany], 50 ng/mL IL-6, and 100 ng/mL stem cell factor [PeproTech, Hamburg, Germany]). One day later, the ST3Gal-IV–deficient cells were transduced with ST3Gal-IV–expressing retroviruses or control retroviruses as follows: 1 mL retroviral supernatant containing 4 µg/mL polybrene was added per well, and cells were centrifuged for 2 hours at 1100g and 25°C. Afterward, the retroviral supernatant was exchanged with BM-medium. The transduction was repeated 1 day later. WT cells were control retrovirus transduced. Cells were harvested after an additional day. Transduction efficiencies were 30% to 60% as measured by flow cytometry for green fluorescent protein–positive cells (supplemental Figure 5). Sex-matched recipient mice (CD45.1) were irradiated by doses of 500 rad and 400 rad 4 hours apart. A mixture of 90% ST3Gal-IV–transduced ST3Gal-IV–deficient cells and 10% control-transduced WT cells or 90% control-transduced ST3Gal-IV–deficient cells and 10% control-transduced WT cells (5 × 105 total cells) were IV injected into recipient mice. Mice were kept with antibiotic-containing drinking water for 8 weeks and analyzed after 8 to 9 weeks following reconstitution. The fold increase was calculated as follows: The cells of interest were gated (eg, CD4 T cells), and the donor cells were then gated for CD45.2. This method excludes the radio-resistant recipient cells. Next, the ST3Gal-IV–deficient cells were gated for CD45.1-negative. The mean of the percentage of ST3Gal-IV–deficient cells in mice that received control-transduced ST3Gal-IV–deficient cells was calculated and set to 1. The percentage of ST3Gal-IV–deficient cells in each mouse that was given ST3Gal-IV–transduced ST3Gal-IV–deficient cells was then divided by this mean percent value.
Flow cytometry
Single-cell suspensions of spleens, thymi, and mesenteric lymph nodes (mLNs) were generated by mechanical disruption. Bone marrow cells were flushed from the tibia with phosphate-buffered saline containing 2% FCS. Blood was directly withdrawn from the heart. Erythrocytes were lysed with ACK-buffer. Cells were preincubated with anti-CD16/CD32 (clone 2.4G2) (Bio X Cell, West Lebanon, NH) to block unspecific binding and stained with respective antibodies in phosphate-buffered saline containing 2% FCS for 20 minutes at 4°C. The following antibodies were used: FITC-, PE-, or PECy7-labeled anti-CD4 (clone RM4-5), PE-labeled anti-CD8 (clone 53-6.7), Alexa Fluor 700–labeled anti-CD11b (clone M1/70), PerCP-labeled anti-CD19 (clone eBio 1D3), APCe780- or PE-labeled anti-CD25 (clone PC61.5), APCe780-labeled anti-CD45R (clone RA3-6B2), FITC-, PerCP-, or APCe700-labeled anti-CD45.1 (clone A20), PerCP- or APCe780-labeled anti-CD45.2 (clone 104), PECy7-labeled anti-CD117 (clone 2B8), PE-labeled anti-CD127 (clone A7R34), PE-labeled anti-Gr1 (clone RB6-8C5), PerCPCy5.5-labeled anti-Ly-6A/E (clone D7) (Thermo Fisher Scientific), V500-labeled anti-CD8 (clone 53-6.7), BUV395-labeled anti-CD45.1 (clone A20) (BD Bioscience, San Jose, CA), APC-labeled anti-CD44 (clone IM7.8.1) (Miltenyi BioTec, Bergisch Gladbach, Germany), and APC- or BV421-labeled anti-CD45.2 (clone 104) (BioLegend, San Diego, CA). To stain lineage-positive cells, a mixture of the following biotin-labeled antibodies was used: anti-CD3 (clone 145-2C11), anti-CD4 (clone GK1.5), anti-CD8 (clone 53-6.7), anti-CD11b (clone M1/70), anti-CD49b (clone DX5), anti-Gr1 (clone RB6-8C5), anti-Ter119 (clone Ter-119) (Thermo Fisher Scientific), and anti-CD45R (clone RA3-6B2) (BioLegend) followed by incubation with BUV395- or Pacific Blue–labeled streptavidin (Thermo Fisher Scientific).
Dead cells were excluded by staining with DAPI, fixable viability dye APC-eFluor780, or fixable viability dye APC-eFluor506 (both from eBioscience, San Diego, CA). Samples were acquired with a FACS Canto II or a BD LSRFortessa instrument (BD Bioscience), and data were analyzed by using FlowJo software (Tree Star, Ashland, OR).
Analysis of proliferation and apoptosis in ETPs of MBMC mice
Six hours before euthanasia, 90/10 MBMC mice were injected with 1 mg 5-ethynyl-2′-deoxyuridine (EdU) IV. Single-cell suspensions of thymi were prepared, and ETPs were stained for flow cytometric analysis as described earlier. Apoptotic cells were detected with the CaspGLOW Red Active Caspase-3 Staining Kit (BioVision, Milpitas, CA) according to the manufacturer’s protocol. After flow cytometric analysis of the signal for apoptotic cells, measurement of proliferation in the same sample was performed with the Click-iT Plus EdU Flow Cytometry Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol.
OP9-DL1 cocultures with LSK cells
LSK cells were sorted with a S3 Cell Sorter (Bio-Rad Laboratories, Hercules, CA) from bone marrow of ST3Gal-IV–deficient (CD45.2) or WT (CD45.1) mice. Same cell numbers of both genotypes were seeded onto 1 plate with OP9-DL1 cells, and the cocultures were performed as previously described27 except that the concentration of IL-7 was reduced to 0.1 ng/mL from day 12 of culture onward. At days 8, 12, 16, and 21, the cells were analyzed by using flow cytometry.
Short-term thymus reconstitution
ST3Gal-IV–deficient or WT mice were sublethally irradiated with 600 rad. Thymi were analyzed after 2 and 7 days.
Statistical analysis
Normally distributed values from 2 groups of data sets were analyzed by using a 2-tailed Student t test with the following cutoff for different levels of significant differences: *P < .05; **P < .01; and ***P < .001. The Mann-Whitney U test was used for data sets that were not normally distributed and marked ###P < .001.
Results
ST3Gal-IV is important for reconstituting the thymus in MBMCs
Based on previous reports that showed a critical requirement of α-2,3-sialylated PSGL-1 for binding to P-selectin and thymic entry,13 we expected severely impaired thymocyte development from precursors that lack the α-2,3-sialyl-transferase ST3Gal-IV. To address this hypothesis, we generated competitive MBMC mice by injecting a mixture of 50% ST3Gal-IV–deficient and 50% WT bone marrow cells into lethally irradiated and congenically marked WT mice. Thus, by flow cytometric analyses, we were able to distinguish between transferred WT (CD45.1/CD45.2 heterozygous) or ST3Gal-IV–deficient (CD45.2 homozygous) cells as well as radioresistant recipient cells (CD45.1 homozygous). In this 50/50 setting, almost all thymocytes and splenic T cells were of WT origin, which indicates a critical role of ST3Gal-IV in T-cell development after bone marrow reconstitution (supplemental Figure 1).
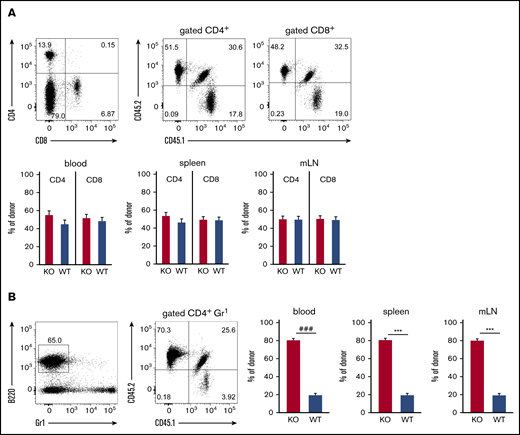
To further investigate this phenomenon, we generated 90/10 MBMC (Figure 1A) and observed in the bone marrow the expected frequency of ∼90% ST3Gal-IV–deficient and 10% WT cells among all CD45.2+ donor cells (Figure 1B). In blood, spleens, and mLNs, the frequency of ST3Gal-IV–deficient cells was reduced to 60% to 70%, and only 50% ST3Gal-IV–deficient cells were observed in the thymus. This finding indicates that ST3Gal-IV does not contribute to engraftment of the bone marrow by HSCs but clearly promotes thymocyte development in this competitive setting.
ST3Gal-IV is important for reconstituting the thymus in MBMC mice. MBMC mice generated with 90% bone marrow cells from ST3Gal-IV–deficient (KO) mice (CD45.2) and 10% bone marrow cells from WT mice (CD45.1/CD45.2 heterozygous) injected into irradiated recipient mice (CD45.1) were analyzed 9 weeks after reconstitution. (A) Schematic illustration of the experimental setup. (B) Flow cytometric analysis of indicated lymphoid organs. The dot plots show the distribution of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) from 1 representative mouse. Bars represent the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among all donor cells from 2 independent experiments with 14 mice in total per group. ***P <. 001 by 2-tailed Student t test.
ST3Gal-IV is important for reconstituting the thymus in MBMC mice. MBMC mice generated with 90% bone marrow cells from ST3Gal-IV–deficient (KO) mice (CD45.2) and 10% bone marrow cells from WT mice (CD45.1/CD45.2 heterozygous) injected into irradiated recipient mice (CD45.1) were analyzed 9 weeks after reconstitution. (A) Schematic illustration of the experimental setup. (B) Flow cytometric analysis of indicated lymphoid organs. The dot plots show the distribution of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) from 1 representative mouse. Bars represent the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among all donor cells from 2 independent experiments with 14 mice in total per group. ***P <. 001 by 2-tailed Student t test.
ST3Gal-IV is important for reconstituting the peripheral T-cell compartments in MBMCs
We further investigated the role of ST3Gal-IV for establishing T-cell and B-cell populations in peripheral lymphoid organs by using flow cytometric analysis of blood, spleens, and mLNs of MBMC mice generated with 10% WT and 90% ST3Gal-IV–deficient donor bone marrow. Both the CD4 T-cell and the CD8 T-cell compartment consisted of ∼50% WT and 50% ST3Gal-IV–deficient cells in all 3 organs (Figure 2A). In contrast, the B-cell compartment was composed of ∼20% WT cells and 80% ST3Gal-IV–deficient cells reflecting roughly the ratio of the bone marrow cell input (Figure 2B). These results indicate that ST3Gal-IV–deficient T cells are impaired in reconstituting the peripheral T-cell compartments in MBMC mice, whereas the reconstitution of the B-cell compartment is independent of ST3Gal-IV. Thus, we concluded that the observed defect of ST3Gal-IV–deficient cells in reconstituting the thymi of MBMC mice further results in defective restoration of the peripheral T-cell compartments by ST3Gal-IV–deficient cells. Interestingly, the T-cell compartments in spleens and mLNs of naive ST3Gal-IV–deficient mice were not altered, which indicates that the requirement for ST3Gal-IV is only apparent in a situation of T-cell lineage reconstitution in adult mice (supplemental Figure 2).
ST3Gal-IV is important for reconstituting the peripheral T-cell compartment in MBMC mice. Flow cytometric analysis of T cells (A) and B cells (B) in blood, spleens, and mLNs of MBMC mice generated as described in Figure 1. Dot plots show the frequencies of CD4+ and CD8+ T cells (A) or B cells (B) and the distribution of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) within the corresponding parental gate from 1 representative mouse. Bars represent the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among all donor cells from 2 independent experiments with 14 mice total per group. ***P < .001 by 2-tailed Student t test; ###P < .001 by Mann-Whitney U test.
ST3Gal-IV is important for reconstituting the peripheral T-cell compartment in MBMC mice. Flow cytometric analysis of T cells (A) and B cells (B) in blood, spleens, and mLNs of MBMC mice generated as described in Figure 1. Dot plots show the frequencies of CD4+ and CD8+ T cells (A) or B cells (B) and the distribution of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) within the corresponding parental gate from 1 representative mouse. Bars represent the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among all donor cells from 2 independent experiments with 14 mice total per group. ***P < .001 by 2-tailed Student t test; ###P < .001 by Mann-Whitney U test.
Loss of ST3Gal-IV leads to impaired thymic recruitment of T-cell progenitor
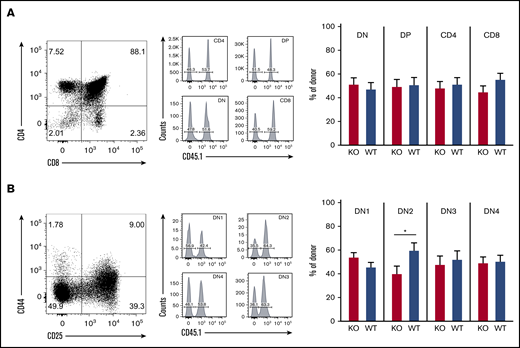
To determine the role of ST3Gal-IV in thymopoiesis, we investigated different stages of T-cell development in the thymus of MBMC mice by using flow cytometry. The CD4 single-positive, CD8 single-positive, double-positive, and double-negative (DN) thymocytes consisted of ∼50% WT cells and 50% ST3Gal-IV–deficient cells (Figure 3A). The same 50/50 ratio was observed for all stages of DN thymocytes with exception of the DN2 stage, where fewer ST3Gal-IV cells were present (Figure 3B). The exact percentages of WT and ST3Gal-IV–deficient thymocytes are depicted in supplemental Table 1. The rate of proliferation (as measured by EdU incorporation) and the rate of apoptosis (as measured by activated caspase 3 staining) were also comparable between WT and ST3Gal-IV–deficient DN thymocyte subsets (supplemental Figure 3). Taken together, these results indicate an important role for ST3Gal-IV in seeding of the thymus by bone marrow–derived T-cell progenitors. As described for the peripheral T-cell compartments, the defective thymopoiesis is also only apparent during conditions of T-cell lineage reconstitution as the different thymocyte populations were normal in untreated ST3Gal-IV–deficient mice (supplemental Figure 2). These findings are in line with the data obtained from naive PSGL-1–deficient mice, which also show normal DN1-4 frequencies but impaired thymopoiesis in MBMC mice.13
Loss of ST3Gal-IV leads to impaired T-cell development in MBMC mice. Flow cytometric analysis of thymocytes of MBMC mice generated as described in Figure 1. Dot plots show the frequency of CD4 single-positive, CD8 SP, DN, and double-positive (DP) thymocytes (A) or the frequency of DN1 to DN4 populations among total DN cells (B) of 1 representative mouse. Histograms indicate the frequency of WT (CD45.1+) and ST3Gal-IV–deficient (KO) (CD45.1−) cells among total CD45.2+ gated donor cells. Bars show the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among all donor cells in indicated gates of 2 independent experiments with 14 mice total per group. *P < .05 by 2-tailed Student t test.
Loss of ST3Gal-IV leads to impaired T-cell development in MBMC mice. Flow cytometric analysis of thymocytes of MBMC mice generated as described in Figure 1. Dot plots show the frequency of CD4 single-positive, CD8 SP, DN, and double-positive (DP) thymocytes (A) or the frequency of DN1 to DN4 populations among total DN cells (B) of 1 representative mouse. Histograms indicate the frequency of WT (CD45.1+) and ST3Gal-IV–deficient (KO) (CD45.1−) cells among total CD45.2+ gated donor cells. Bars show the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among all donor cells in indicated gates of 2 independent experiments with 14 mice total per group. *P < .05 by 2-tailed Student t test.
ST3Gal-IV promotes development of early thymic progenitors but is dispensable for the CLP and LSK populations
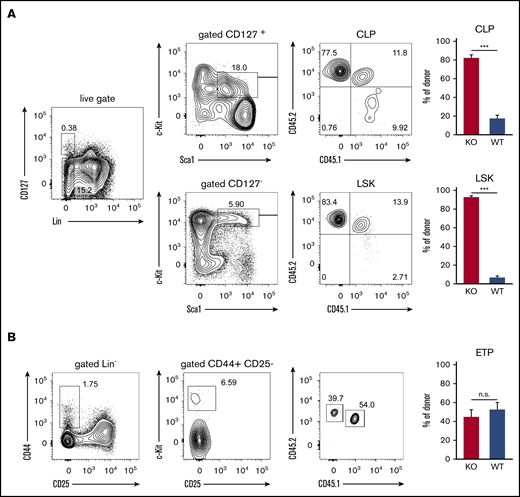
The impaired engraftment of the thymus by T-cell progenitors derived from ST3Gal-IV–deficient donors could either be due to inefficient recruitment or impaired development of precursor cells in the bone marrow. To address this point, we analyzed the hematopoietic precursor cells in the bone marrow of MBMC mice. About 92% of cells within the LSK population consisted of ST3Gal-IV–deficient donor-derived cells, and only ∼8% was derived from the WT donor, which reflects the 90/10 ratio of ST3Gal-IV–deficient/WT cells in the transferred mixture of bone marrow cells (Figure 4A). Similar results were obtained for the CLP population, indicating that ST3Gal-IV–deficient cells have no defect in entering the bone marrow and generating lymphocyte progenitor cells. In striking contrast, the ETP population in the thymi of MBMC mice consisted of ∼55% WT and 45% ST3Gal-IV–deficient donor-derived cells (Figure 4B). This reflects a significant reduction of ST3Gal-IV–deficient cells in the ETP compared with the LSK or CLP population (Mann-Whitney U test, P < .001). These data show that ETPs strongly depend on ST3Gal-IV, and we hypothesized that ST3Gal-IV is crucial for the entry of TSPs into the thymus. Due to the very low number of TSPs in the blood,7 we were unable to analyze this intermediate cell population in the MBMC mice. In addition, it was estimated that only 5 TSPs arrive in the thymus per day,28 leading to technical challenges in detecting these cells with limited mouse numbers.
ST3Gal-IV promotes development of early thymic progenitors but is dispensable for the CLP and LSK populations. Flow cytometric analysis of MBMC mice generated as described in Figure 1. (A) Contour plots from bone marrow samples show the frequency of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) from CLP (Lin−CD127+c-Kit+Sca-1+) and LSK (Lin−CD127−c-Kit+Sca-1+) populations of 1 representative mouse. (B) Contour plots show the frequency of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) from the ETP population (Lin−CD25−CD44+c-Kit+) in the thymus of 1 representative mouse. Bar graphs in panels A and B show the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among total donor cells from 2 independent experiments with 14 total mice per group. ***P < .001 by 2-tailed Student t test. n.s., not significant.
ST3Gal-IV promotes development of early thymic progenitors but is dispensable for the CLP and LSK populations. Flow cytometric analysis of MBMC mice generated as described in Figure 1. (A) Contour plots from bone marrow samples show the frequency of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) from CLP (Lin−CD127+c-Kit+Sca-1+) and LSK (Lin−CD127−c-Kit+Sca-1+) populations of 1 representative mouse. (B) Contour plots show the frequency of WT donor cells (CD45.1+CD45.2+), ST3Gal-IV–deficient (KO) donor cells (CD45.1−CD45.2+), and WT recipient cells (CD45.1+CD45.2−) from the ETP population (Lin−CD25−CD44+c-Kit+) in the thymus of 1 representative mouse. Bar graphs in panels A and B show the mean ± SEM frequency of WT (blue) and ST3Gal-IV–deficient (red) cells among total donor cells from 2 independent experiments with 14 total mice per group. ***P < .001 by 2-tailed Student t test. n.s., not significant.
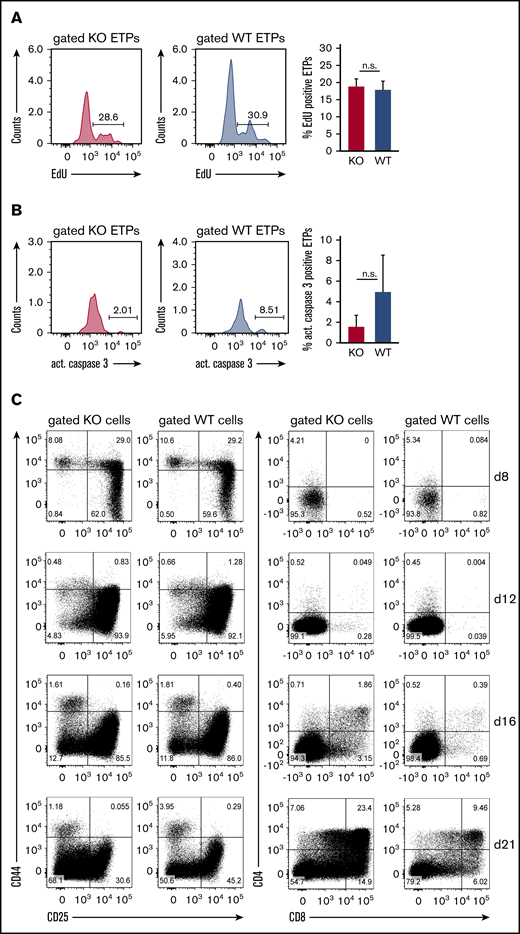
ST3Gal-IV is dispensable for the proliferation of ETPs in vivo and the in vitro development of thymocytes
The observed defect of ST3Gal-IV–deficient ETPs to reconstitute the thymus after bone marrow transplantation could also be a consequence of impaired proliferation or increased apoptosis instead of defective thymic settling. The proliferation of ETPs was therefore measured by determining the EdU incorporation rate in genomic DNA. The frequency of EdU-positive cells was similar between ST3Gal-IV–deficient and WT ETPs in the thymi of MBMC mice (Figure 5A). By staining for active caspase 3, we found no evidence for enhanced apoptosis in ETPs of ST3Gal-IV–deficient origin (Figure 5B). Thus, ST3Gal-IV seems to be dispensable for regulation of proliferation or apoptosis of ETPs strengthening our hypothesis that the enzyme is crucial for the entry of TSPs.
ST3Gal-IV is dispensable for the proliferation of ETPs in vivo and the in vitro development of thymocytes. Flow cytometric analysis of the proliferation (A) and apoptosis (B) of ST3Gal-IV–deficient and WT ETPs. MBMC mice were generated as described in Figure 1 and injected with EdU 6 hours before euthanasia. Histograms show the frequency of EdU-positive (A) or active caspase 3–positive (B) ST3Gal-IV–deficient (KO) or WT ETPs in the thymus of 1 representative mouse. Bars show the mean ± SEM frequency of EdU-positive (A) or active caspase 3–positive (B) WT (blue) or ST3Gal-IV-deficient (red) ETPs of 2 independent experiments with 8 total mice. (C) Flow cytometric analysis of thymocyte development in the OP9-DL1 coculture system. Sorted WT (CD45.1) and ST3Gal-IV–deficient (CD45.2) LSK cells were cocultured in the same well on OP9-DL1 cell layer. Dot plots show the frequencies of the various thymocyte populations at days 8, 12, 16, and 21 of culture of gated KO or WT cells. The dot plots are representative for 3 independent experiments.
ST3Gal-IV is dispensable for the proliferation of ETPs in vivo and the in vitro development of thymocytes. Flow cytometric analysis of the proliferation (A) and apoptosis (B) of ST3Gal-IV–deficient and WT ETPs. MBMC mice were generated as described in Figure 1 and injected with EdU 6 hours before euthanasia. Histograms show the frequency of EdU-positive (A) or active caspase 3–positive (B) ST3Gal-IV–deficient (KO) or WT ETPs in the thymus of 1 representative mouse. Bars show the mean ± SEM frequency of EdU-positive (A) or active caspase 3–positive (B) WT (blue) or ST3Gal-IV-deficient (red) ETPs of 2 independent experiments with 8 total mice. (C) Flow cytometric analysis of thymocyte development in the OP9-DL1 coculture system. Sorted WT (CD45.1) and ST3Gal-IV–deficient (CD45.2) LSK cells were cocultured in the same well on OP9-DL1 cell layer. Dot plots show the frequencies of the various thymocyte populations at days 8, 12, 16, and 21 of culture of gated KO or WT cells. The dot plots are representative for 3 independent experiments.
To further investigate whether ST3Gal-IV–deficient ETPs have a general developmental disadvantage compared with WT ETPs, we performed competitive in vitro cultures. Therefore, LSK from congenic WT (CD45.1) and ST3Gal-IV–deficient (CD45.2) mice were sorted and cultured in vitro for up to 3 weeks on OP9-DL1 feeder cells to support thymocyte development.27 This approach showed that ST3Gal-IV–deficient ETPs are not developmentally impaired under these conditions (Figure 5C).
ST3Gal-IV is not required for thymocyte reconstitution after low-dose irradiation
Thymocytes can also develop from intrathymic progenitors independently of recruitment of bone marrow–derived precursors.29 To determine whether ST3Gal-IV is required for recruitment-independent replenishment of thymocytes, we subjected WT and ST3Gal-IV–deficient mice to low-dose (600 rad) γ-irradiation and analyzed thymocytes by using flow cytometry. Double-positive thymocytes were efficiently depleted on day 2 after irradiation; 5 days later, this population was completely restored in both WT and ST3Gal-IV–deficient mice (supplemental Figure 4). This finding further indicates that intrathymic proliferation and differentiation of thymocytes is not affected in ST3Gal-IV–deficient mice.
Retroviral reconstitution of ST3Gal-IV–deficient cells improves T-cell development
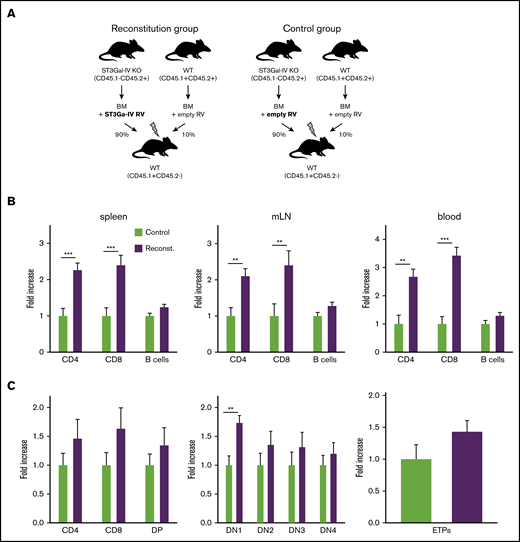
To confirm that the observed defect in T-cell development is indeed due to the deficiency of ST3Gal-IV, we generated retrogenic mice in which ST3Gal-IV–deficient cells were partially reconstituted by retroviral transduction with a ST3Gal-IV–expressing vector. Therefore, bone marrow cells were isolated from 5-FU–treated ST3Gal-IV–deficient mice to mobilize HSCs and transduced with ST3Gal-IV–expressing or empty retroviruses with a transduction efficiency of ∼30% to 60% (supplemental Figure 5). In addition, bone marrow cells from 5-FU–treated WT mice were transduced with control retroviruses. One group of irradiated recipient mice received 90% of the ST3Gal-IV–reconstituted ST3Gal-IV–deficient cells together with 10% of WT cells transduced with empty retroviral vectors (reconstitution group). Another group of irradiated recipient mice received 90% ST3Gal-IV–deficient cells together with 10% WT cells both transduced with empty vectors (control group) (Figure 6A).
Retroviral reconstitution of ST3Gal-IV KO cells partially corrects the defect in T-cell development. (A) Schematic illustration for the generation of retrogenic MBMC mice as described in the main text. Fold increase of T and B cells (B) or thymocyte subsets (C) within the reconstituted ST3Gal-IV–deficient mice-derived cell populations in the reconstitution group (purple) compared with the ST3Gal-IV–deficient mice-derived cell populations in the control group (light green) set to the value of 1. Bars represent the mean ± SEM of 3 independent experiments with 11 to 17 mice per group. **P < .01 and ***P < .001 by 2-tailed Student t test.
Retroviral reconstitution of ST3Gal-IV KO cells partially corrects the defect in T-cell development. (A) Schematic illustration for the generation of retrogenic MBMC mice as described in the main text. Fold increase of T and B cells (B) or thymocyte subsets (C) within the reconstituted ST3Gal-IV–deficient mice-derived cell populations in the reconstitution group (purple) compared with the ST3Gal-IV–deficient mice-derived cell populations in the control group (light green) set to the value of 1. Bars represent the mean ± SEM of 3 independent experiments with 11 to 17 mice per group. **P < .01 and ***P < .001 by 2-tailed Student t test.
Peripheral T-cell and B-cell compartments of the retrogenic MBMC mice were analyzed 8 and 9 weeks after transfer. Retrogenic mice that were given reconstituted ST3Gal-IV–deficient cells exhibited twofold to threefold more CD4 and CD8 T cells within the CD45.1−CD45.2+ population in spleens, mLNs, and blood compared with the control group. In contrast, reconstitution of the ST3Gal-IV–deficient cells had no effect on the B-cell compartment (Figure 6B). We next investigated thymocyte development of the retrogenic MBMC mice. The DN1 thymocyte population increased significantly, and other thymocyte populations (including the ETPs) were also increased in reconstituted mice that were given reconstituted ST3Gal-IV–deficient cells compared with the control group (Figure 6C).
These results show that the defect of ST3Gal-IV–deficient cells in thymic recruitment of T-cell progenitors and reconstitution of the peripheral T-cell compartments in MBMC mice is specific for loss of ST3Gal-IV rather than other unknown genetic factors.
Discussion
Using competitive bone marrow chimeras and retrogenic mice, we showed that recruitment of T-cell progenitors to the thymus strongly depends on the α-2,3 sialyltransferase ST3Gal-IV. Our results indicate that ST3Gal-IV mediates the homing of TSPs to the thymus after irradiation and bone marrow reconstitution so that this enzyme can be added to the multifaceted mechanism of thymic settling.
We cannot formally rule out the possibility that ST3Gal-IV is important for the TSPs to leave the bone marrow and enter the circulation. Detection and quantification of TSPs in the blood circulation are technically limited due to their extremely low frequency,7 and only ∼5 TSPs were estimated to enter the thymus every day.28 Nevertheless, previously published data on mechanisms regulating thymic settling after bone marrow reconstitution support the hypothesis that ST3Gal-IV promotes the entry of TSPs into the thymus. In an elegant study using parabiosis and competitive repopulation experiments, Rossi et al13 showed that the interaction of P-selectin and PSGL-1 is necessary for the entry of TSPs into the thymus. Because PSGL-1 can only function as a ligand for P-selectin when it is sialylated,17 we concluded that ST3Gal-IV plays a key role in sialylation of P-selectin and thereby controls thymic entry of TSPs. This finding extends the previously known role of ST3Gal-IV for neutrophil rolling and extravasation under inflammatory conditions to a further requirement for rolling of TSPs on the thymic endothelium resulting in thymic settling.20,21
Retroviral reconstitution of HSCs from ST3Gal-IV–deficient mice with a ST3Gal-IV–encoding retroviral vector improved T-cell repopulation after transfer in irradiated recipients by a factor of 2 to 3; some variability was observed, however, in the reconstitution efficiency of the thymi in the retrogenic mice. This finding might be explained by the fact that settling of the thymus is a gated process in which the gate is open for precursor entry for 1 week followed by a 3-week period of a closed gate.30 In addition, P-selectin messenger RNA is known to be expressed periodically in thymic tissue and peaks every 2 weeks.31 This leads to a complex regulation of thymic receptivity and therefore probably to the observed variable thymic reconstitution. Analysis of the study mice was performed at 2 time points 1 week apart, which could have caused some variation in the settling efficiency of the thymus.
Interestingly, untreated adult ST3Gal-IV–deficient mice have normal numbers of thymocytes and normal frequencies of thymocyte subsets.21 Similarly, PSGL-1–deficient mice were reported to have normal T-cell development.13 However, settling of the thymus by T-cell progenitors after irradiation was dependent on PSGL-1.13,32 These findings indicate that ST3Gal-IV is the critical enzyme for sialylation of PSGL-1, which promotes recruitment of T-cell progenitors to the thymus after irradiation and bone marrow reconstitution. However, this mechanism seems largely dispensable for T-cell development in untreated mice.
In addition to the results shown here, mice deficient for the polysialyltransferase ST8Sia-IV have a 30% reduction of thymocytes, including early thymocyte precursors, and this phenomenon has been attributed to impaired exit of TSPs from their bone marrow niche.33 This finding indicates that different sialyltransferases play critical and nonredundant roles in T-cell development and act at distinct developmental stages.
These study data are of clinical relevance, because it has long been known that the T-cell lineage recovers slowly after stem cell transplantation in humans,34 and this delay contributes to morbidity and mortality in these patients.35 The exact understanding of the early steps in T-cell development is crucial to develop strategies to improve T-cell counts after stem cell transplantation or T cell–destructive HIV infections. Based on our results, it could be beneficial to perform ex vivo sialylation of isolated donor cells before adoptive transfer to improve T-cell lineage reconstitution. A similar approach using ex vivo fucosylation has been successfully applied for enhanced engraftment of the bone marrow by human mesenchymal stem cells in immunocompromised mice.36,37
All original data can be obtained from the corresponding author upon reasonable request (David Voehringer; e-mail: david.voehringer@uk-erlangen.de).
Acknowledgments
The authors thank Kirstin Castiglione and Laura Handl for technical assistance, the members of the Voehringer laboratory for helpful discussions, and Andreas Krüger and Juan Carlos Zúniga-Pflücker for providing the OP9-DL1 cell line.
This work was funded by the Deutsche Forschungsgemeinschaft grant SFB643_TP_B15 to D.V. M.S. was supported by the German Research Foundation (SP621/3-1) and by the Mizutani Foundation (Project Ref. No. 090063). Contribution by J.D.M. was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK048247) and the National Heart, Lung, and Blood Institute (HL131474) of the National Institutes of Health.
Authorship
Contribution: S.S. and D.V. designed experiments; S.S. and D.D. performed experiments; M.S. provided ST3Gal-IV–deficient mice and critical advice; S.S. and D.V. wrote the manuscript; and J.D.M. and M.S. reviewed the data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Voehringer, Department of Infection Biology, University Hospital Erlangen and Friedrich-Alexander University Erlangen-Nuremberg, 91054 Erlangen, Germany; e-mail: david.voehringer@uk-erlangen.de.
References
Author notes
The full-text version of this article contains a data supplement.