Key Points
Albuminuria progresses with age in SCA, and baseline albuminuria ≥100 mg/g predicts persistent albuminuria.
Persistent albuminuria is associated with worse GFR decline and CKD development in adults with SCA.
Abstract
Sickle cell nephropathy results in chronic kidney disease (CKD), which is associated with significant morbidity and mortality in sickle cell anemia (SCA). Albuminuria is an early manifestation of sickle nephropathy; however, little is known about progression of albuminuria or its correlation with glomerular filtration rate (GFR) decline or CKD. We studied nephropathy progression in 303 SCA participants in a prospective, multicenter, longitudinal study. We collected steady-state urine and serum samples yearly and assessed albumin/creatinine ratio (ACR), estimated GFR (eGFR), and SCA and nephropathy biomarkers. Participants with albuminuria (ACR ≥30 mg/g) for ≥2 annual measurements were classified as having persistent albuminuria (PA). At baseline (mean age, 21 years; range, 2-64 years), 32% had albuminuria. In longitudinal multivariate analysis, ACR was associated with sex, anemia, older age, and higher bilirubin and kidney injury molecule-1 levels. Albuminuria increased with age by 3.5 mg/g per year (P < .0001). Of 175 participants with ≥3 annual samples, 81% with baseline albuminuria ≥100 mg/g developed PA. Decreased eGFR and adult CKD were associated with PA (P = .002 and P = .02, respectively), but not with baseline albuminuria. Rate of eGFR decline was steeper among adults (but not children) with albuminuria, compared with those without (P = .02). Participants with PA were more likely to have rapid eGFR decline compared with those without (P = .03). In this longitudinal study, albuminuria progressed with age, and adults with albuminuria had worse eGFR decline than those without. Albuminuria ≥100 mg/g predicted PA, which was associated with rapid eGFR decline and CKD development in adults with SCA. This trial was registered at www.clinicaltrials.gov as #NCT02239016.
Introduction
Sickle cell anemia (SCA) results from a point mutation in the β-globin gene that leads to the formation of sickle hemoglobin (HbS). Polymerization of HbS in the deoxygenated state leads to several SCA complications, including recurrent vasoocclusion and chronic hemolytic anemia. The hypoxic, hyperosmolar, and acidotic renal medullary environment increases the propensity for HbS polymerization and makes the kidney particularly vulnerable in SCA.1 Approximately 16% to 18% of early mortality in patients with SCA is related to renal disease, making it the most common type of chronic organ failure–related death in SCA.2,3
Kidney disease in SCA, often referred to as sickle cell nephropathy, results from glomerulopathy and tubular injury and manifests progressively as urine concentrating defect and glomerular hyperfiltration, acidification defect, hematuria, and albuminuria.1 Albuminuria, which represents an early stage of chronic kidney disease (CKD), starts in childhood and may predict early mortality in SCA.4-9 CKD occurs in ∼30% of adult SCA patients and is associated with a higher risk of mortality when resulting from SCA compared with other causes of CKD.10,11 Once end-stage renal disease (ESRD) is recognized, the 1-year mortality rate is 3 times higher among patients with SCA compared with non-SCA individuals.12 Little is known about the natural progression of albuminuria in SCA, and the association between albuminuria, glomerular filtration rate (GFR) decline, and CKD is not fully understood.
The glomerulopathy of SCA starts early in life, with increased renal blood flow and glomerular hyperfiltration. Chronic glomerular capillary hypertension predisposes to glomerular hypertrophy and sclerosis, leading to loss of permselectivity and albuminuria.6,13,14 Recently, we showed that the renin-angiotensin system is activated in SCA in the absence of hypertension, and increased angiotensin receptor-1 signaling significantly contributes to the glomerulopathy of SCA.15,16
Despite a high prevalence of albuminuria in SCA, ranging from 15% to 50%,6 the natural progression of albuminuria and sickle cell nephropathy is poorly defined, because most data are extrapolated from cross-sectional studies. Albuminuria is defined by excretion of >30 mg albumin per gram of creatinine17 ; however, measurements of urine albumin/creatinine ratio (ACR) are inherently variable, and not all ACR changes are indicative of changing kidney function.18 Transient albuminuria is common and frequently a benign finding, whereas persistent albuminuria (PA) is indicative of renal disease.19-21 The magnitude of ACR that is predictive of progressive or persistent albuminuria in SCA is unknown. In the limited longitudinal studies of individuals with SCA and albuminuria, ACR measurements have been variable; half of the patients had spontaneous resolution of albuminuria by the end of a 3-year study,22 and in another adult study, the risk of proteinuria did not change over time.23 Correctly classifying albuminuria has significant implications in clinical decision making, especially as new therapies become available for nephropathy in SCA.16,24
In this study, we sought to examine the progression of albuminuria in patients with SCA in a prospective, multicenter, longitudinal study using blood and urine samples obtained during routine clinic visits. We also evaluated the risk factors associated with albuminuria and the ACR threshold that predicts albuminuria persistence and how it relates to GFR decline and development of CKD.
Methods
Study design and participants
This was a prospectively designed, longitudinal, multicenter study to assess progression of sickle cell nephropathy. We enrolled children and adults with SCA (HbSS and HbSβ0 genotypes) at 11 centers in the United States and Jamaica between 2009 and 2017 using a predefined research protocol and a uniform sample processing plan and case report forms for all the sites. All analyses were performed at the primary site (Cincinnati Children’s Hospital Medical Center). Participants were enrolled from the following sites: Cincinnati Children’s Hospital Medical Center (primary site), University of Cincinnati Medical Center, University of the West Indies, University of Louisville, University of Illinois at Chicago, National Heart, Lung, and Blood Institute, Children’s Healthcare of Atlanta, Nationwide Children’s Hospital, Akron Children’s Hospital, and Children’s Hospital of Michigan. The institutional review board at each site approved the study. Informed consent was obtained from adult participants, and assent was obtained from minors, when applicable, with their legal guardians providing written permission.
We included individuals with HbSS or HbSβ0 thalassemia at steady state, defined by the absence of acute SCA-related events for 3 weeks before sample collection. Samples were obtained at least 3 months after an acute blood transfusion. Patients receiving a renin-angiotensin pathway inhibitor (eg, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker) were excluded. Blood and urine samples were prospectively collected (annually ± 4 months), coinciding with routine outpatient visits for up to 3 years (4 time points).
Additional clinical and laboratory data were obtained and updated from medical records during each visit when applicable: age, sex, genotype, blood pressure (BP), complete blood count, reticulocyte count, lactate dehydrogenase, electrolytes, blood urea nitrogen, serum creatinine, liver enzymes, serum bilirubin, concurrent SCA-modifying therapies (hydroxyurea or chronic transfusions), and last pretransfusion HbS or HbF levels.
Sample analysis
Urine osmolality was measured using a freezing point osmometer (Advanced Instruments 3250, Norwood, MA). Urine albumin was measured in spot urine samples by immunoturbidometry using Dimension Xp and clinical chemistry system (Siemens Healthcare Diagnostics, Tarrytown, NY). Levels of urine kidney injury molecule-1 (KIM-1; R&D Systems, Minneapolis, MN) were measured by enzyme-linked immunosorbent assay in duplicate as per manufacturer instructions using 50 μL per sample. Urine N-acetyl-β-D-glucosaminidase (NAG) activity was measured using a colorimetric assay (Roche Diagnostics) using 5 μL per sample. All urinary biomarker levels were normalized to urine creatinine and expressed as a ratio to urine creatinine. Urine creatinine was measured using an alkaline picrate assay (R&D Systems). Plasma cystatin C was measured by nephelometry using a clinical laboratory platform (BN ProSpec; Siemens Healthcare Diagnostics).
Definitions and outcome measures
Albuminuria was defined by urinary ACR ≥30 mg/g of creatinine. Microalbuminuria was defined by ACR 30 to 300 mg/g and macroalbuminuria as >300 mg/g. Participants with albuminuria for at least 2 annual measurements (2 years) were considered to have PA.25 Two methods were used to calculate estimated GFR (eGFR): serum creatinine based (eGFR-Cr), using Schwartz formula in children and CKD Epidemiology Collaboration (CKD-EPI) in adults, and cystatin C method (eGFR-CysC), using Larsson’s formula per manufacturer recommendations.26-28 Hyperfiltration was defined by eGFR-CysC ≥140 mL/min/m2.29,30 Rapid eGFR decline was defined as an eGFR decrease by ≥3 mL/min/m2 per year based on studies of renal function decline in African American and SCA individuals.31-33 CKD was defined using the Kidney Disease: Improving Global Outcomes CKD guideline (KDIGO), which incorporates eGFR and albuminuria.34
Statistical analysis
All categorical variables were summarized using count and percentage and continuous variables as mean (standard deviation [SD]). Mann-Whitney U test was used to compare 2 groups of continuous variables, and Fisher’s exact test was used for categorical variables. Linear regression was used to assess the univariate association between clinical and laboratory parameters and ACR at baseline. Variables of significant interest and with a P value <.05 from the univariate analysis were included in a multivariable linear mixed-effects model. R2 for the multivariate linear mixed model was calculated according to the method of Jaeger et al,35 using the R package r2glmm (https://cran.r-project.org/web/packages/r2glmm/index.html). For the longitudinal association of ACR with age, we applied the natural cubic spline method to age and then used the cubic splines for age as the independent variables to model the log ACR in a mixed-effects model. In the analysis of baseline ACR group and PA, only participants with at least 3 ACR measurements were included (2-year follow-up with baseline, year 1, and year 2 measurements). Statistically significant variables between participants with or without PA at baseline were included in a multivariate regression analysis. Variables with missing values >15% were excluded from multivariate analysis. Fisher’s exact test was used to compare baseline ACR with PA. Multiple linear regression was used to determine the association between PA and the last eGFR after adjusting for age. All P values were 2 tailed, and differences were considered significant when P < .05. Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA).
Results
Baseline characteristics
A total of 320 participants with SCA were enrolled. We included 303 participants with available ACR measurements in this study. The median follow-up was 23 months. Participants provided samples annually, with a 14.5- ± 8-month (mean ± SD) interval between sample collections and contributed 644 patient-years on the study. A total of 175 participants (58%) provided at least 3 annual samples and were included in the longitudinal analysis.
The demographic and baseline characteristics for the overall group are shown in Table 1. Participants were recruited from a wide age range (2-64 years; mean, 21 years; 46% adults), 172 (54%) were female, and 54 (18%) were recruited from Jamaica. Nearly two-thirds of the participants were on disease-modifying therapy (hydroxyurea, 45%; chronic transfusion therapy, 20%).
The prevalence of albuminuria (ACR ≥30 mg/g) at enrollment was 32% (97 of 303); 26% (80 of 303) had microalbuminuria (ACR 30-300 mg/g), and 6% (17 of 303) had macroalbuminuria (ACR >300 mg/g). PA was present in 30% (52 of 175) of participants included in the longitudinal analysis.
Correlates of albuminuria
In a univariate analysis, baseline ACR was positively associated with age (P < .001), systolic (P < .001) and diastolic BP (P = .005), serum creatinine (P = .002), serum potassium (P = .0001), serum chloride (P = .004), NAG (P < .0001), and KIM-1 (P < .0001) and was negatively associated with urine osmolality (P = .0001; supplemental Table 1). Also, baseline ACR was associated with female sex (P = .009), as well as lower Hb concentration (P = .01), hematocrit (P < .001), and HbF (P = .0005). Of the hemolysis surrogates, serum bilirubin was significantly associated with ACR (P = .001), but there was no association with reticulocyte count, lactate dehydrogenase, or aspartate aminotransferase. There was no association between ACR and the treating center.
In a multivariate analysis that included the statistically significant variables from the univariate analysis in addition to age-adjusted eGFR, only age (P = .03), female sex (P = .009), Hb concentration (P = .01), total bilirubin (P = .002), and KIM-1 (P < .0001) were significantly associated with ACR (Table 2). Hydroxyurea and chronic transfusions were introduced in 21 of 175 participants during the study period. The introduction of hydroxyurea or transfusions was not associated with a change in ACR in this small sample (P = .14).
Progression of albuminuria
We observed a strong association between age and ACR (P < .0001; Figure 1). The rate of linear ACR increase was 3.5 mg/g per year (95% CI, 1.8-5.3; Y = 3.5 × X + 5.6). Based on this rate of ACR increase, children are expected to develop albuminuria (>30 mg/g) by an average age of 7 years and high levels of albuminuria (≥100 mg/g) by the age of 27 years.
Longitudinal association of ACR with age. ACR progression with age including all participants in the study. Center line is a fitted regression line, with 95% confidence interval [CI] upper and lower bound lines. Each filled circle represents a single time point. Darker-appearing circles are the result of overlaying time points.
Longitudinal association of ACR with age. ACR progression with age including all participants in the study. Center line is a fitted regression line, with 95% confidence interval [CI] upper and lower bound lines. Each filled circle represents a single time point. Darker-appearing circles are the result of overlaying time points.
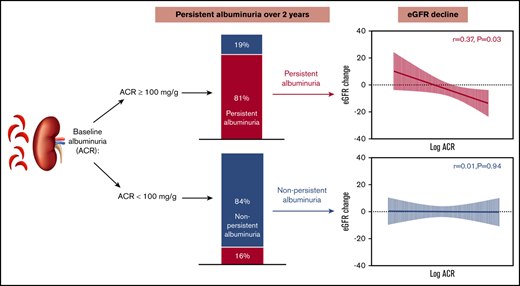
Higher baseline ACR was associated with an increased likelihood of PA (at least 2 annual measurements with albuminuria; P < .0001; Figure 2). Among participants with ACR ≥100 mg/g at baseline, 81% had PA, compared with 16% of participants with baseline ACR <100 mg/g (49% among those with ACR 30-100 mg/g and 7% among no-albuminuria group). All participants with macroalbuminuria at baseline had PA (Figure 2). Participants with baseline ACR ≥100 mg/g had a numerically steeper ACR increase, 6.5 mg/g per year, compared with the rest of the study participants.
Baseline ACR determines the probability of persistent albuminuria. The probability of PA based on baseline ACR.
Baseline ACR determines the probability of persistent albuminuria. The probability of PA based on baseline ACR.
In a comparison between individuals with PA and all other participants included in longitudinal analysis, those with PA were older (P < .0001) and had higher systolic and diastolic BP (P = .04 and P = .009, respectively; Table 3). Relative systemic hypertension in adults, defined by BP ≥120/70,36 was associated with PA; 77% of adults with BP ≥120/70 developed PA, compared with 42% of adult participants with BP <120/70 (odds ratio, 4.56; 95% CI, 1.22-16.88; P = .02).
Participants with PA also had higher serum creatinine (P = .03), potassium (P = .0007), NAG (P = .0005), and KIM-1 levels (P = .002) but had lower urine osmolality (P < .0001) compared with the rest of participants (Table 3). Participants with PA were less likely to be on disease-modifying therapy (hydroxyurea, P = .02; chronic transfusion therapy, P = .03) and had lower hematocrit (P = .005) and HbF (P = .01) but higher total serum bilirubin (P = .002). In a multivariate analysis, age, hematocrit, osmolality, baseline ACR, and NAG were associated with PA (Table 3).
eGFR and CKD
Using multiple linear regression, last eGFR-CysC was significantly associated with PA (coefficient, 16.6; P = .002), but not with baseline ACR. Overall, participants with PA were more likely to have rapid eGFR decline (>3 mL/min/1.73 m2 per year)31 compared with those with non-PA (48% vs 31%; P = .03). In addition, the association between eGFR and albuminuria was influenced by age; the average rate of eGFR-CysC decline was significantly higher among adults with albuminuria than adults without albuminuria (−5 mL/min/1.73 m2 per year vs −0.2 mL/min/1.73 m2 per year; P = .02), but the rate of eGFR-CysC decline among children with or without albuminuria was not different (P = .64; Figure 3A-B). We found similar results with eGFR-Cr (supplemental Figure 1). The rate of eGFR decline was significantly associated with baseline ACR in adults with PA (r = 0.37; P = .03), but not in adults with non-PA (P = .94; Figure 3C-D). The rate of eGFR-CysC decline was similar among male and female participants (P = .54).
Progression of eGFR-CysC. The rate of eGFR-CysC decline in children (A) and adults (B) based on presence or absence of albuminuria. Red lines represent participants with albuminuria, and blue lines represent participants without albuminuria. Filled circles represent mean eGFR value spanned by standard error of the mean. The median yearly eGFR decline (mL/min/1.73 m2 per year) is indicated for both groups. Association between change in eGFR-CysC and ACR as a continuous variable among adult participants with PA (C) and non-PA (D). Regression lines with 95% CIs are shown in red for adults with PA and black for adults with non-PA. (E) Linear regression of eGFR-CysC vs age in children (<18 years). (F) Linear regression of eGFR-CysC vs age in adults.
Progression of eGFR-CysC. The rate of eGFR-CysC decline in children (A) and adults (B) based on presence or absence of albuminuria. Red lines represent participants with albuminuria, and blue lines represent participants without albuminuria. Filled circles represent mean eGFR value spanned by standard error of the mean. The median yearly eGFR decline (mL/min/1.73 m2 per year) is indicated for both groups. Association between change in eGFR-CysC and ACR as a continuous variable among adult participants with PA (C) and non-PA (D). Regression lines with 95% CIs are shown in red for adults with PA and black for adults with non-PA. (E) Linear regression of eGFR-CysC vs age in children (<18 years). (F) Linear regression of eGFR-CysC vs age in adults.
Adult participants with CKD stage 2 or 3 (eGFR <90 mL/min/1.73 m2) were older (P < .001), had higher systolic BP (P < .001), higher BUN and serum creatinine (P < .001), and higher KIM-1 levels (P = .004), and were twice as likely to have PA than those with eGFR ≥90 mL/min/1.73 m2 (P = .02; Table 4). There was no difference in hematologic parameters between those with CKD and those without. No participant had ESRD in our study.
The association between eGFR and age differed between children and adults. Six (4%) pediatric patients had eGFR <90 mL/min/1.73 m2, but no pediatric patient had eGFR <60 mL/min/1.73 m2. The youngest patient with eGFR <90 mL/min/1.73 m2 was age 11 years. The frequency of distribution of eGFR among children and adults by age is shown in supplemental Figure 2.
We observed a stronger negative linear correlation between eGFR-CysC and age in adults compared with children (r = 0.36; P < .001 vs r = 0.18; P = .02, respectively; Figure 3E-F). This difference is possibly due to higher prevalence of hyperfiltration among children compared with adults (51% vs 44%). A similar proportion of participants with or without PA had hyperfiltration at baseline (40% vs 36%; P = .65). We observed similar results with eGFR-Cr (supplemental Figure 1C-D). In the overall group, participants with hyperfiltration at baseline had faster eGFR decline compared with those without hyperfitration (mean, −12 vs 0.3 mL/min/1.73 m2 per year; P < .001). We found a similar trend among adults with hyperfiltration compared with adults without hyperfiltration at baseline (mean, −5.4 vs −0.1 mL/min/1.73 m2 per year; P = .07).
When comparing GFR estimates by cystatin C and creatinine methods, eGFR-CysC and eGFR-Cr were poor surrogates for each other, and their agreement was influenced by hyperfiltration and age. In 527 pairs of eGFR measurements, overall, eGFR-CysC was higher than eGFR-Cr. Bland-Altman plots showed a positive bias (eGFR-CysC–eGFR-Cr) of 28.7 (95% CI of agreement, −48 to 105), with the highest bias among participants with the highest eGFR (bias ± SD, +65 ± 46 among participants within the fourth quartile for eGFR-CysC vs +2 ± 21 among those within the first quartile; supplemental Figure 3). The best agreement between both measurements was observed for a mean eGFR 123.3 ± 30 mL/min/1.73 m2. Finally, eGFR-CysC and eGFR-Cr measurements had better correlation among adults compared with children (r = 0.61 vs 0.25; supplemental Figure 4), likely because of higher prevalence of hyperfiltration among children.
Discussion
The natural history of albuminuria in SCA is not known, because most data on sickle nephropathy are inferred from retrospective and cross-sectional analyses. In this longitudinal, prospective multicenter study, we found that albuminuria was strongly associated with age and progressed by 3.5 mg/g per year. An ACR threshold ≥100 mg/g was predictive of the development of PA over the next 2 years, which was associated with CKD development in adults and a rapidly declining eGFR, the latter being an increasingly recognized high-risk factor for CKD progression.32,33 Thus, an ACR ≥100mg/g is a surrogate of persistent proteinuria, and both are superior to the traditional ACR threshold of 30 mg/g in predicting eGFR decline and CKD development in SCA.
The association between albuminuria and CKD in SCA is poorly understood. We found that low levels of microalbuminuria were less likely to be persistent, probably because of inherent fluctuations in albuminuria. Only persistent, and not transient, albuminuria has been linked to kidney diseases in children and adults without SCA.19-21 Previous longitudinal studies of sickle nephropathy showed that albuminuria measurements are variable22,23,25 ; almost one-third of patients with albuminuria had intermittent albuminuria over 2 years in 1 study, whereas only 9% of patients developed PA in another study.37,38 In our study, albuminuria was common overall; however, PA, and not merely the presence of albuminuria, was associated with a decline in eGFR and development of CKD in adults.
Recently, Lebensburger et al25 showed that in children with SCA, PA was associated with hyperfiltration. Although we did not find an association between hyperfiltration and PA, likely because of the broad age range in our study, we found that PA may be a marker of CKD in SCA, because it correlates with a rapid decline in eGFR. Rapid eGFR decline is an increasingly recognized high-risk factor for ESRD and mortality in non-SCA patients with CKD.39-41 Rapidly declining eGFR was observed in one-third of adults with SCA followed longitudinally in a recent study and was associated with proteinuria (dipstick-based assessment), severe SCA genotype, anemia, high systolic BP, higher platelet and reticulocyte counts, and lower body mass index.32 In our study, which included both children and adults, eGFR decline was associated with albuminuria in adults only, but PA was associated with rapid eGFR decline in all ages. Together, these results demonstrate that ACR >100 mg/g and PA may be better markers of eGFR decline in SCA than the mere presence of albuminuria.
The strong association between albuminuria and age suggests cumulative, progressive nephropathy and is consistent with data reported in cross-sectional studies.6 As a result, CKD and cardiopulmonary causes are the most common causes of premature mortality in young adults with SCA.42 Albuminuria is an early sign of glomerulopathy, and therapies that aim to prevent CKD in other diseases are typically considered when albuminuria is established. Based on the trajectory of ACR progression in our study, children as young as age 7 years may develop albuminuria and CKD as early as age 11 years. Our results highlight the importance of early detection of renal disease and provide insight into the use of ACR, BP, NAG, and KIM-1 in screening for sickle cell nephropathy. Prior studies showed that genetic modifiers confer increased risk for albuminuria and CKD progression.43,44 The presence of APOL1 risk alleles is associated with early onset of albuminuria, even in young children.45 Although we did not screen for these genetic modifiers in our study, incorporating these modifiers may further enhance the screening accuracy for early detection of renal disease. Although new therapies have recently shown promising results in small studies in halting the progression of albuminuria and sickle nephropathy,16,24,46,47 powered, controlled, randomized trials are still needed to evaluate the safety and efficacy of these therapies. Our study provides new insight into the natural progression of albuminuria in SCA that is needed for the successful design of such studies.
Our longitudinal study confirms previously reported findings and associations of sickle nephropathy.8,44,48-53 First, high baseline prevalence of albuminuria in a third of SCA patients was observed in our study.49-51,54 Second, an association was seen between albuminuria and anemia, an established marker of SCA severity, which may also be worsened by decreased erythropoietin production secondary to renal disease.55 Third, elevated systolic and diastolic BP were associated with baseline albuminuria and PA, although this association was not significant in a multivariate analysis. Despite apparent normal/low BP in individuals with SCA as a result of systemic vasodilation and endothelial dysfunction, elevated BP is associated with vasculopathy in SCA and the risk of sickle nephropathy.36,56 Relative systemic hypertension in adults, defined by systolic BP of 120 to 139 and diastolic BP of 70 to 89, is associated with increased risk of pulmonary hypertension and renal dysfunction in adults with SCA.36 Similarly, in our study, BP ≥120/70 in adults was associated with a significant risk of PA. Fourth, our study suggests a protective role for disease-modifying therapy (hydroxyurea and chronic transfusions) in sickle nephropathy. Hydroxyurea use, in isolation or combination with renin-angiotensin pathway inhibitors, is associated with less albuminuria and improvement in sickle nephropathy,38,46,57-59 and the early initiation of blood transfusions was renoprotective in children.60 Patients with PA in our study were less likely to be on chronic transfusion or hydroxyurea, and HbF and HbS levels were significantly lower and higher, respectively, in those who had PA. Only six participants were younger than age 5 years in our study, which suggests that response to hydroxyurea rather than age was the likely determinant of HbF. Finally, the association between albuminuria and hemolysis is controversial.49,52,53,56,61,62 In our study, albuminuria was associated with reticulocyte count and bilirubin at baseline and only with bilirubin in multivariate analysis.
We found a strong association between albuminuria and the tubular injury biomarkers NAG and KIM-1, and their levels among participants with albuminuria were nearly double those in participants without albuminuria. KIM-1 levels were also higher among adults with CKD. We and others have reported increased levels of urinary NAG and KIM-1 associated with albuminuria in individuals with SCA.63-66 The strong association between these biomarkers and albuminuria in this larger sample confirms these as valuable biomarkers of sickle nephropathy. Therapeutic studies should assess changes in levels of these biomarkers.
We also observed an interesting association between female sex and albuminuria in our study. Female sex was associated with baseline albuminuria in a multivariate analysis, but we did not find this association with PA. Relationship between sex and kidney disease is not straightforward. Although CKD affects more females than males, the rate of GFR decline and CKD progression is worse among males.67 The higher prevalence of kidney disease among females may explain the association between baseline albuminuria and female sex in our study, although the rate of eGFR decline was similar between both sexes.
In addition to the association between PA and eGFR, albuminuria was associated with attributes of renal tubular injury. Impaired medullary perfusion and ischemia in SCA results in the impaired free-water resorption and distal nephron dysfunction that manifest in urinary concentration defect and acidification defect, respectively.1,56 Hyperkalemia may also result from distal nephron resistance to aldosterone.68 In our study, albuminuria was associated with lower urine osmolality and higher serum potassium and chloride levels. Notably, urine osmolality in our study was measured without water deprivation; however, osmolality was overall low (mean, 430 mOsm/kg) and was associated with albuminuria. These associations, in addition to the association between albuminuria and the renal tubular injury markers NAG and KIM-1, reflect cortical and medullary involvement by sickle cell nephropathy. Measurement of low molecular weight proteins (eg, β2-microglobulin) that are normally reabsorbed in the proximal tubules can be valuable in assessing the severity of sickle cell nephropathy.65
Our study has several limitations. First, we had a relatively short follow-up period, and a longer follow-up may be more informative. Second, we did not use a gold-standard method to measure GFR. To account for this, we used 2 methods to estimate GFR, because the cystatin C method may be more sensitive to GFR decline in SCA.66 These estimates had the best agreement when eGFR was normal or low. Third, we used a single spot urine ACR measurement at each time point. Given ACR variability, measuring ACR in replicates may improve the accuracy of albuminuria classification. Our study was purposefully designed to have low burden on participants with sample collection during routine, steady-state visits. Fourth, we are unable to generalize these findings to all forms of sickle cell disease, because we excluded milder genotypes. We also excluded patients on renin-angiotensin pathway inhibitors, which may have biased the results by excluding adults with severe renal disease. Finally, clinical data (eg, number of vasoocclusive crises and degree of organ pathology) were only available for a subset of participants, which precluded the meaningful study of the correlation of sickle nephropathy with clinical data.
In conclusion, in a longitudinal, multicenter study, we found that the risk of eGFR decline and CKD in adults was associated with PA. Albuminuria in SCA progressed with age, and an ACR threshold >100 mg/g at baseline was associated with an increased risk of PA. PA was also associated with the features and biomarkers of renal tubular injury, and disease-modifying therapies seem to be protective against PA. Our findings have important implications for clinical decision making and improve the knowledge about the natural history of sickle nephropathy. These results provide critical information that will refine the design of future natural history and therapeutic studies of sickle nephropathy.
For original data, please contact omar.niss@cchmc.org or punam.malik@cchmc.org.
Acknowledgments
The authors thank Charles T. Quinn (Cincinnati Children’s Hospital Medical Center [CCHMC]), Marvin Reid (University of the West Indies), and Victor Gordeuk (University of Illinois at Chicago [UIC]) for their valuable advice and suggestions on the design and analysis of this study, as well as the following individuals for their significant contributions to recruitment, sample collection, study coordination, and assays: Megan Reynolds, Catherine Terrell, Amy Shova, and Michael Bennett (CCHMC); Bentley Chambers, Vikram Asnani, and Varma Thomas (University of the West Indies); Lani Krauz (UIC); Kate Roskom and Jim Nichols (National Institutes of Health); Heidi Ziegler, Veronica Brobbey, and Diana Glynn (Nationwide Children's Hospital); and Amanda Watt, Leann Schilling, and Natasha Morris (Children’s Healthcare of Atlanta).
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R34 HL 108752 and 1UO1 HL117709-01 (P.M.). O.N. was the recipient of a Translational Research Scholar Award (U01HL117709).
Authorship
Contribution: P.M. designed the study; O.N., M.R.A., M.E.Y., A.R., S.C., C.F., P.B., S.L.S., S.S., and P.M. recruited study participants and collected data; P.M. and P.D. supervised assay analysis; O.N. and A.L. analyzed the data; O.N. wrote the first draft; and all authors critically revised, edited, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Omar Niss, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 7015, Cincinnati, OH 45229; e-mail: omar.niss@cchmc.org; and Punam Malik, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 7013, Cincinnati, OH 45229; e-mail: punam.malik@cchmc.org.
References
Author notes
The full-text version of this article contains a data supplement.


![Longitudinal association of ACR with age. ACR progression with age including all participants in the study. Center line is a fitted regression line, with 95% confidence interval [CI] upper and lower bound lines. Each filled circle represents a single time point. Darker-appearing circles are the result of overlaying time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/7/10.1182_bloodadvances.2019001378/1/m_advancesadv2019001378f1.png?Expires=1765946733&Signature=dRXNF9V-sDVlS4biddDZhgP386MGOG4PrkndrXE3SEi4uaq~xqhqGqNsubdgg91bzcS20jeCbnMKTDZqCOjaaJiaGGL56pYY0F1MuptxG763LWCbM06J9cWtjlu8p~MMx6Vji~eiKWhlZPwR~gfEkoRyidfH-zg12EZMGRudK40WJKyxN4z1vvhM5e~aM89lTY7XjJOBlazMAmOj8SbiMHDHJYMkZLuS1TYlfzTx2cZorKQs61ZvNfAdUVmZA9XAvJ~PB3kHvhPE1eyKovL2nQl8Cas2UEGMTw5~vxs6MPCrpqsK4VOHMbh7GXcjNB~rdpBCIblxoYWKn~lXt44m4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Longitudinal association of ACR with age. ACR progression with age including all participants in the study. Center line is a fitted regression line, with 95% confidence interval [CI] upper and lower bound lines. Each filled circle represents a single time point. Darker-appearing circles are the result of overlaying time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/7/10.1182_bloodadvances.2019001378/1/m_advancesadv2019001378f1.png?Expires=1766181381&Signature=ADTOF2--22Bd~EJMDFX~26asgiIC6TYkEStfXJm9rOGpyB8cUP~YgOimr-pZ5KwzIIAuj4LSnYmdGiersDngIvCR2hZglTnpLTFw4C8QOeGIebwK~rEmi9wvGrhUB1XReVUyWN6ap0~QlUGykzGtXZ9rsXXGTrsv5VVS9VSvGzc5g0EIb6I5Ad8fGE~rhz9fizkZW7VOMWFwy6ic6vHq6LXtev~uLmFt788ighD8M0gmTnAl5VU~YXtdp9cS31v98hCKx7xFiRWaXeooh5Z2bvcSSYyEoQmtS-PRWoZSIvIMyyD~pkgx0Zn8JZFn2cgWU45BwkfR0pp5~qdYC3Z-Iw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

