Key Points
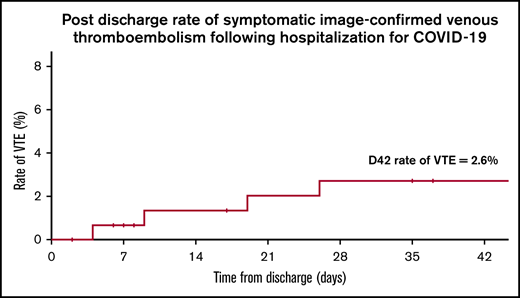
The rate of symptomatic VTE in patients after hospitalization for COVID-19 was 2.6% at 42 days after discharge.
Bleeding is a significant cause of morbidity in addition to thrombosis in patients admitted with COVID-19.
Abstract
Although COVID-19 has been reported to be associated with high rates of venous thromboembolism (VTE), the risk of VTE and bleeding after hospitalization for COVID-19 remains unclear, and the optimal hospital VTE prevention strategy is not known. We collected retrospective observational data on thrombosis and bleeding in 303 consecutive adult patients admitted to the hospital for at least 24 hours for COVID-19. Patients presenting with VTE on admission were excluded. Data were collected until 90 days after admission or known death by using medical records and an established national VTE network. Maximal level of care was ward based in 78% of patients, with 22% requiring higher dependency care (12% noninvasive ventilation, 10% invasive ventilation). Almost all patients (97.0%) received standard thromboprophylaxis or were already receiving therapeutic anticoagulation (17.5%). Symptomatic image-confirmed VTE occurred in 5.9% of patients during index hospitalization, and in 7.2% at 90 days after admission (23.9% in patients requiring higher dependency care); half the events were isolated segmental or subsegmental defects on lung imaging. Bleeding occurred in 13 patients (4.3%) during index hospitalization (1.3% had major bleeding). The majority of bleeds occurred in patients on the general ward, and 6 patients were receiving treatment-dose anticoagulation, highlighting the need for caution in intensifying standard thromboprophylaxis strategies. Of 152 patients discharged from the hospital without an indication for anticoagulation, 97% did not receive thromboprophylaxis after discharge, and 3% received 7 days of treatment with low molecular weight heparin after discharge. The rate of symptomatic VTE in this group at 42 days after discharge was 2.6%, highlighting the need for large prospective randomized controlled trials of extended thromboprophylaxis after discharge in COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), can result in pneumonia and acute respiratory distress syndrome with extrapulmonary symptoms.1 After the pandemic spread of SARS-CoV-2, anecdotal reports and early case series reported high rates of venous thromboembolism (VTE) associated with moderate and severe COVID-19. The highest rates of VTE, 18% to 85%,2-6 have been reported in association with admission to the intensive care unit (ICU), and patients in ICU have a 2 to 4 times greater risk of VTE than those managed on general wards.7-9 Arterial thrombosis has been reported with an incidence of 2% to 4%.2-4
Hospitalization as a result of medical illness is associated with increased risk of VTE, which is highest for the first 6 weeks and persists for 3 months.10-12 Thromboprophylaxis with low molecular weight heparin (LMWH) reduces the risk of VTE in acutely ill hospitalized medical patients by 50%,11-13 and all adult patients are routinely assessed for their risk of VTE and considered for LMWH prophylaxis on admission to our hospital. Meta-analysis of thromboprophylaxis for 28 to 45 days after discharge in medical patients (LMWH, rivaroxaban, betrixaban) has been shown to reduce the risk of VTE by 40%, but it doubles the risk of major or fatal bleeding14 and is currently not recommended at our hospital in line with national guidance.15
Although VTE incidence in patients admitted with COVID-19 seems to be higher than for patients with respiratory illness and acute respiratory distress syndrome,16 data on VTE incidence after hospitalization with COVID-19, which might be impacted by hospital VTE prevention strategies, remains limited. There is significant variability in reported VTE rates,2-5,7-9,17-19 reflecting relatively small patient numbers, screening strategies, and the proportion of patients in the ICU. Case series to date have only short follow-up and include VTE diagnosed within the first 24 hours of admission (accounting for up to 50% of reported events and not preventable by inpatient thromboprophylaxis).9,20 Interestingly, there is a marked predominance of reported pulmonary emboli (PE) compared with deep vein thrombosis (DVT), with many PEs being seen in segmental and subsegmental arteries only. In combination with reports of microvascular thrombosis from lung histology,21,22 this supports an emerging hypothesis that these may be in situ thromboses secondary to severe lung inflammation as opposed to an embolic event (immunothrombosis),23,24 and the effectiveness of standard thromboprophylaxis at reducing this risk is unknown. Reports on bleeding rates during hospitalization and VTE after discharge are limited.
The perceived high VTE rate in patients hospitalized for COVID-19 has resulted in hospitals world-wide considering increasing the intensity of inpatient thromboprophylaxis and considering extended thromboprophylaxis after discharge. Because of a limited evidence base, expert consensus guidelines currently give conflicting recommendations in these 2 key areas.25,26 Of note, although the American College of Chest Physicians (ACCP) guidelines25 do not recommend thromboprophylaxis after discharge because of the lack of available data after analyzing the major trials of extended thromboprophylaxis in medical patients, the panel states that “extended thromboprophylaxis would result in net benefit in patients with COVID-19 at low bleeding risk, if the risk of symptomatic VTE would be above 1.8% at 35 to 42 days after hospital discharge.”25
Oxford University Hospitals National Health Service (NHS) Foundation Trust is one of the largest teaching hospitals in the United Kingdom with ∼90 000 emergency admissions and 110 000 elective admissions each year. It has 1340 hospital beds, serves a local population of ∼800 000, and acts as a regional specialist center. Within the United Kingdom’s national VTE prevention program,27 Oxford has a strong local VTE system,28 and we are informed of patients who present to another hospital with VTE within 90 days of discharge from Oxford. The aim of this study was to determine the rate of VTE and bleeding during index hospitalization and the rate of VTE after discharge in a large tertiary center in the United Kingdom.
Methods
This study was designed when COVID-19 cases in Europe were rapidly increasing. Early reports of high thrombotic rates resulted in uncertainty regarding the effectiveness of standard thromboprophylaxis strategies. We designed this study to help inform urgent decision-making by collecting data on the rate of VTE and bleeding at 90 days from admission. Data cutoff at 90 days from admission was chosen because this is within the time-frame of established national reporting systems for hospital-associated VTE (usually 90 days from discharge, but because of the variability in length of index hospitalization, this was changed to allow collection of a complete data set within a specified time frame). The subgroup analysis of VTE rate at 42 days after discharge in patients discharged without anticoagulation treatment was specified during data collection, before analysis, following the ACCP guideline statement. This retrospective observational study was approved by London-London Bridge Research Ethics Committee (REC reference 20/HRA/2304; Protocol number 14937; IRAS project ID 282457).
Patients
All consecutive adult patients (age 18 years or older) were admitted to Oxford University Hospitals NHS Foundation Trust between 1 March and 14 April 2020. They had SARS-CoV-2 infection confirmed by reverse transcriptase polymerase chain reaction on a nose or throat swab or by a broncho-alveolar lavage sample, and they were identified by an electronic search of microbiology laboratory results. To analyze only events impacted by the hospital VTE prevention strategy, patients were excluded if the admission lasted less than 24 hours, if VTE was diagnosed within the first 24 hours after presentation (that is, likely present on admission and not preventable by inpatient thromboprophylaxis), or if patients were diagnosed with COVID-19 during their hospital stay for other medical conditions.
VTE prevention and diagnosis
The standard of care for thromboprophylaxis between 1 March and 14 April 2020, was to administer standard-dose LMWH dalteparin 5000 units subcutaneously to all adult patients admitted with COVID-19 unless there was a contraindication; the dose was adjusted for extremes of body weight (<40 kg and >120 kg), and anti-Xa monitoring was recommended at 10 days if creatinine clearance was <20 mL/min. Interim hospital COVID-19 VTE prevention guidelines were introduced on 25 April 2020, and they included intermediate-dose dalteparin prophylaxis (5000 units twice per day, adjusted at extremes of body weight) for patients in the ICU and 7 days of extended thromboprophylaxis on discharge with standard-dose dalteparin. A small proportion of this cohort therefore received adjusted VTE prevention measures from 25 April 2020. Patients were investigated for VTE with imaging (computed tomography pulmonary angiogram [CTPA], ultrasound Doppler) if they were symptomatic. Routine screening for VTE was not undertaken.
Data collection
Data were obtained retrospectively from electronic patient records until 90 days from admission or known death. Notably, the population admitted with COVID-19 was a local population and was likely to re-present to Oxford if they had significant complications after discharge. As part of the national VTE prevention program, we have established links with surrounding hospitals to inform us if a patient presents with VTE within 90 days of discharge from Oxford. We collected data on demographics, comorbidities, medication history, thromboprophylaxis regimen, arterial or venous thrombotic events, bleeding events, and mortality. Coagulation and inflammatory parameters were routinely collected at baseline and at time of bleeding events. Information on bleeding events was collected on the basis of the International Society on Thrombosis and Haemostasis (ISTH) definitions of major bleeding (MB) and clinically relevant nonmajor bleeding (CRNMB) in nonsurgical patients (see Table 1 for definitions).29,30
Bed capacity for higher-dependency patients was expanded during the height of the pandemic. For the purposes of this article, patients in ICU refers to those patients managed in traditional ICU areas, and those patients were the only ones who received invasive ventilation. High-dependency unit (HDU) care is defined as care provided for patients who received more advanced respiratory support than standard oxygen therapy alone, that is, high-flow nasal oxygen or noninvasive ventilation, but they were not in the ICU.
Outcomes
The primary outcome was the incidence of radiologically confirmed VTE within 90 days of index admission for symptomatic COVID-19 infection. Secondary outcomes included arterial thromboembolism (ATE) within 90 days of index admission, confirmed either by computed tomography imaging or magnetic resonance imaging or after typical electrocardiogram and cardiac enzyme changes; bleeding (MB or CRNMB) within 90 days of index admission; and VTE within 42 days of discharge in the subgroup of patients who were discharged without therapeutic anticoagulation.
Statistical analysis
Statistical analyses were performed using Excel (Microsoft Corporation, Redmond, WA), SPSS (IBM Corporation, Armonk, NY), and R (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were compared by using Fisher’s exact test or χ2 test, and continuous variables were compared using Mann-Whitney U test. Survival analysis with Kaplan-Meier estimation and univariable Cox proportional hazards regression model were performed to determine potential risk factors for primary and secondary outcomes. The threshold for statistical significance was 0.05. Multivariable analysis was attempted but did not yield valid results because of covariate collinearity and is not presented. No missing data were imputed. No corrections for multiple comparisons were performed. Graphs for figures were prepared using Excel and R.
Results
Between 1 March and 14 April 2020, 408 adult patients were diagnosed with COVID-19 in Oxford University Hospitals by viral polymerase chain reaction for SARS-CoV-2 on a nose or throat swab or broncho-alveolar lavage sample. Of these, 105 patients were excluded from further analysis: 56 were admitted for <24 hours, 5 were diagnosed with VTE within 24 hours of presentation, 1 patient was a hospital transfer with incomplete data, and 43 were hospitalized for unrelated medical problems. Therefore, 303 patients were included in the main analysis.
Characteristics
Patient characteristics are shown in Table 2. Median age was 73 years (interquartile range [IQR], 57-82 years), and 165 patients (55%) were male. Median body mass index was 27.0 kg/m2 (IQR, 23.7-31.5 kg/m2). At least 1 comorbidity was recorded in 253 patients (84%); 47 (16%) had an active malignancy, and 19 (6%) had a previous history of VTE. Therapeutic anticoagulation was given to 58 patients (19%) before admission (40 had atrial fibrillation, 13 had VTE, and 5 had other indications).
Index hospitalization
Inpatient pharmacologic thromboprophylaxis was administered to 294 patients (97%). Because of a change in local VTE prevention guidelines on 25 April 2 patients switched from standard-dose to intermediate-dose LMWH, and 5 patients received standard-dose LMWH for 7 days after discharge. On the basis of maximal intensity of thromboprophylaxis received while they were inpatients, 239 patients (81%) received standard-dose LMWH prophylaxis, 2 (1%) received intermediate-dose LMWH prophylaxis, and 53 (18%) received therapeutic anticoagulation. Pharmacologic thromboprophylaxis was not given to 9 patients (3%) for multiple reasons, including high risk of bleeding and patient preference.
Patients presented at a median of 5 days (IQR, 2-9 days) after onset of symptoms. Maximal level of care was ward based in 236 patients (78%); 67 (22%) required additional organ support in HDU (13 patients) or ICU (54 patients). Maximal respiratory support was noninvasive (high-flow nasal oxygen, continuous positive airway pressure ventilation, or bilevel positive airway pressure ventilation) in 36 patients (12%) and invasive in 30 (10%). Among those who were intubated for invasive ventilation, 24 (80%) required prone positioning, 17 (57%) required advanced cardiovascular support, and 12 (40%) required renal replacement therapy. No patients required extracorporeal membrane oxygenation. Median length of ICU stay was 9 days (IQR, 4-17 days), and median duration of hospitalization for these patients was 18 days (IQR, 10-25 days). Patients not admitted to the ICU had a median duration of hospitalization of 6 days (IQR, 4-11 days).
VTE.
Image-confirmed VTE was diagnosed in 18 patients (5.9%) during index hospitalization. Concurrent diagnosis of DVT and PE was classified as 1 VTE event. Thirteen patients had pulmonary tree occlusions on CTPA, 3 patients had concomitant proximal lower limb DVT. Five patients developed an isolated DVT: 3 upper limb DVTs (of which 2 were line associated) and 2 proximal lower limb DVTs. Of these patients, 17 received standard LMWH thromboprophylaxis during hospitalization and 1 did not receive LMWH (bleed on admission). Median time from index admission to VTE was 11.5 days (IQR, 8-17 days). Data on thrombosis within continuous venovenous hemofiltration circuits were not routinely collected, but multiple clots were noted in 2 patients within the hemofiltration circuit and 1 subsequently developed a line associated DVT.
Bleeding.
Bleeding events were recorded in 13 patients (4.3%; 95% confidence interval [CI], 2.5%-7.2%) during index hospitalization: MB 4 (1.3%; 95% CI, 0.5%-3.3%) and CRNMB 9 (3%; 95% CI, 1.6%-5.5%) (Table 1). The majority of patients who bled were on general wards (92%), and the commonest site of bleeding was the upper gastrointestinal tract. At the time of bleed, 6 patients were receiving therapeutic anticoagulation, 5 were receiving standard-dose thromboprophylaxis (2 were also receiving antiplatelet medication, and 2 were not receiving LMWH prophylaxis because of significant thrombocytopenia). Laboratory data at the time of bleeding showed thrombocytopenia was present in 7 patients (47%): 4 patients had a platelet count of 100 × 109/L to 150 × 109/L, 1 patient had a platelet count of 50 × 109/L to 100 × 109/L, and 2 patients had a platelet count of <50 × 109/L. The 3 patients with platelet counts <100 × 109/L were receiving chemotherapy for hematologic malignancy. Prothrombin time (PT) was mildly prolonged in 3 patients (PT ratio, 1.0-1.5) and was 4.5 in 1 patient receiving warfarin. Activated partial thromboplastin time (APTT) was prolonged in 4 patients, all with an APTT ratio of 1.0 to 1.5. D-dimer and Clauss fibrinogen levels were available for only 2 patients and were high in both cases. None of the bleeding events were fatal.
ATE and mortality.
ATE occurred in 4 patients (1.3%) during index hospitalization (see Table 3 for further details). Overall, 98 patients (32%) died. Autopsies were not performed, and cause of death was listed as COVID-19 infection in all patients. Median time to death from admission was 7 days (IQR, 4-12 days); median time from onset of symptoms to death was 12 days (IQR, 8-19 days).
Outcomes at 90 days after admission
Of the 205 patients discharged from index hospitalization, 149 were discharged without extended thromboprophylaxis, 5 were discharged with 7 days of standard-dose LMWH prophylaxis (discharged after new local COVID-19 guidelines were published), 2 were discharged on extended thromboprophylaxis because of recent total hip replacement surgery before subsequent admission for COVID-19 symptoms, and 51 discharged patients were receiving therapeutic anticoagulation (32 patients had a previous indication for anticoagulation, 13 had new VTE, and 6 had new atrial fibrillation).
VTE.
The overall rate of image-confirmed VTE within 90 days of index admission was 7.3% (22 patients), with a majority of VTEs (82%) occurring during index hospitalization. Of the 4 events that occurred after discharge, all were pulmonary tree occlusions on CTPA, with a median time from admission to diagnosis of 18 days (range, 9-34 days), and all occurred within 42 days of discharge (further details in the “VTE at 42 days after discharge (subgroup analysis)” section). Overall, 17 PEs and 8 DVTs were diagnosed (3 DVTs concomitant to PE). Of the 17 pulmonary arterial tree occlusions on CTPA, 3 (17.5%) were central, 3 (17.5%) were lobar, 10 (60%) were segmental, and 1 (6%) was subsegmental. The proportion of segmental and subsegmental occlusions diagnosed during index hospitalization compared with after discharge was not significantly different (P = .584). Patients who developed VTE compared with those who did not had significantly longer index hospitalization (median, 21 vs 7 days; P < .001), were more frequently treated on HDU or ICU (72.7% vs 27.3%; P < .001), and were significantly younger (median age, 57.5 vs 74 years; P = .002). None (0%) of the 58 patients who were receiving therapeutic anticoagulation before hospitalization developed VTE compared with 22 (9.0%) of the 245 patients who did not receive previous anticoagulation (P = .011).
ATE, bleeding, and mortality.
ATE occurred in 6 patients (2%) within 90 days of index admission: 4 (1.3%) were associated with index admission and 2 (1%) occurred after discharge (Table 3). MB or CRNMB occurred in 15 patients (5.0%; 95% CI, 3.0%-8.0%) at 90 days from index admission. The majority (87%) occurred during index hospitalization; 2 patients bled after discharge: 1 MB and 1 CRNMB (Table 1). After discharge, at least 15 more patients died (data are presumed to be incomplete because of the way hospital deaths were reported and cause of death was not available).
Predictors for thrombosis and bleeding at 90 days after index admission
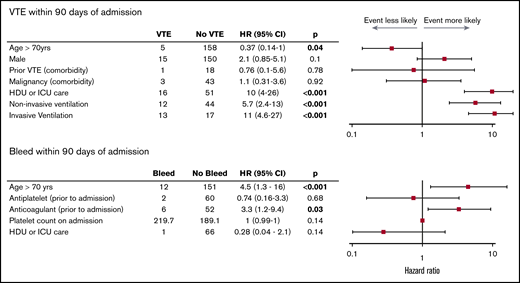
Survival analysis with Kaplan-Meier estimation (Figure 1) and univariable Cox proportional hazards regression analysis (Figure 2) were performed to identify risk factors for the primary and secondary outcomes. Many analyses had large CIs because of the small number of events.
Risk of VTE within 90 days of admission. Kaplan-Meier survival analysis comparing maximal respiratory support (A) and level of care (B). NIV, noninvasive ventilation.
Risk of VTE within 90 days of admission. Kaplan-Meier survival analysis comparing maximal respiratory support (A) and level of care (B). NIV, noninvasive ventilation.
Risk of VTE and bleeding at 90 days. Forest plot presenting HRs with 95% CIs calculated by univariable Cox proportional hazards regression analysis.
Risk of VTE and bleeding at 90 days. Forest plot presenting HRs with 95% CIs calculated by univariable Cox proportional hazards regression analysis.
Of continuous variables, elevated Clauss fibrinogen on admission (hazard ratio [HR], 3.3; 95% CI, 1-11; P = .014), and increasing length of hospitalization were associated with VTE (HR, 1; 95% CI, 1-1.1; P < .001). Patients requiring care in the HDU or ICU had an HR >4 for VTE (HR, 10; 95% CI, 4-26; P < .001), and invasive ventilation was associated with higher risk than noninvasive ventilation (HR, 11; 95% CI, 4.6-27; P < .001 vs HR, 5.7; 95% CI, 2.4-13; P < .001). Patients older than age 60 years were less likely to develop a VTE than those younger than age 60 years (HR, 0.4; 95% CI, 0.17-0.94; P = .033). Anticoagulation before admission (HR, 3.3; 95% CI, 1.2-9.4; P = .032) and age older than 70 years (HR, 4.5; 95% CI, 1.3-16; P < .001) were associated with bleeding. No other covariates, including VTE and arterial thrombosis, were associated with bleeding.
VTE at 42 days after discharge (subgroup analysis)
A secondary analysis was performed on the subgroup of patients who were discharged from the hospital without an indication for therapeutic anticoagulation. Of 154 patients, 2 were excluded because they were discharged on extended thromboprophylaxis for recent hip replacement surgery before hospitalization with COVID-19 (notably, neither patient had a VTE within 90 days of index admission). Of the 152 patients analyzed, 97% did not receive thromboprophylaxis after discharge, 5 (3%) received 7 days of treatment with LMWH on discharge after a change in local COVID guidelines. The records for all of these patients were reviewed up to 42 days after discharge. The median age was 61.5 years (IQR, 52-75 years), 16% had required care in the HDU or ICU, and median index hospitalization was 7 days (IQR, 4-13 days). Four patients (2.6%) developed a VTE (all PE) by 42 days after discharge. Median time from discharge to PE was 14 days (range, 4-26 days). None of these patients had required care in the HDU or ICU or had a previous history of VTE; 2 had an active malignancy (metastatic breast cancer or Waldenström macroglobulinemia) and were receiving chemotherapy. No bleeds and 9 deaths (5.9%) were recorded; cause of death was not available for these patients.
Discussion
There were 3 key findings in this cohort of 303 consecutive patients hospitalized for COVID-19 (78% did not receive care in the HDU or ICU, 12% received noninvasive ventilation, 10% received invasive ventilation), of whom almost all (97.0%) received standard LMWH thromboprophylaxis during hospitalization or were already receiving therapeutic anticoagulation (17.5%). In all, 5.9% were diagnosed with image-confirmed VTE during hospitalization, significant bleeding rate during hospitalization included patients on the general ward (4.3% overall), and the rate of VTE was 2.6% at 42 days after discharge in patients discharged without an indication for anticoagulation.
The incidence of image-confirmed symptomatic VTE in the 90 days after admission was 7.2% (23.9% in patients who received care in the HDU or ICU). The majority of events (82%) occurred during index hospitalization and were predominantly PEs. CTPA demonstrated 6 occlusions (35%) in central and lobar arteries, and 11 (65%) in segmental and subsegmental arteries alone (possible immunothrombosis). This VTE rate is lower than that in early reports,18 and in particular, in some of the early predicted cumulative incidences after a short duration of follow-up.7,9 Differences in rates of symptomatic VTE between case series can largely be explained by the proportion of patients treated in the ICU compared with those not treated in the ICU (especially the proportion of patients who received invasive ventilation) and the inclusion of VTE diagnosed at time of hospital admission. Our reported VTE rate is similar to that in a recently reported cohort by Al-Samkari et al8 : 400 patients (36% requiring invasive ventilation) with median follow-up of 10 days with a 4.8% rate of image-confirmed VTE.
Risk factors associated with VTE include increased length of hospitalization and admission to the HDU or ICU (HR, >4); invasive mechanical ventilation was associated with a higher risk than noninvasive ventilation. Patients who developed VTE were significantly younger (median age, 57.5 years) than those who did not (median age, 74 years) (P = .002). This may be because mortality was associated with increasing age and was therefore a competing risk and/or because younger patients who required admission had more severe COVID-19, which would predispose them to higher VTE risk (median age for ward-based care was 76 years [IQR, 59-84 years]) vs 62 years [IQR, 56-74 years] in patients receiving higher-dependency care [P < .001]). Notably, no patient who was receiving therapeutic anticoagulation before hospitalization developed VTE compared with 9% of patients who had not previously received anticoagulation (P = .011), even though the former group was likely to have a higher baseline risk of thrombosis. Although this may indicate that therapeutic anticoagulation has a protective effect against COVID-19–associated VTE, caution should be used for patients who have previously received anticoagulation because it was also associated with increased recorded mortality (HR, 2; 95% CI, 1.3-3; P = .002).
Al-Samkari et al8 reported a bleeding rate of 7.6% for hospitalized patients (2.3% MB overall; 5.6% MB for patients in the ICU). In this study, the incidence of bleeding during hospitalization was 4.3% (95% CI, 2.5%-7.2%) and MB was 1.3% (95% CI, 0.5%-3.3%). Interestingly, the majority of bleeds occurred in patients on the general ward which has not previously been highlighted and may reflect the proportion of general ward patients included in the study. Two bleeds were recorded after discharge, both of them in patients receiving therapeutic anticoagulation. Overall, approximately half the patients (53%) were receiving therapeutic anticoagulation at time of bleed. Receiving anticoagulation before admission (HR, 3.3; 95% CI, 1.2-9.4; P = .032) and age older than 70 years (HR, 4.5; 95% CI, 1.3-16; P < .001) were associated with bleeding events. Our study highlights a significant bleeding risk in these patients, including patients not in the ICU; caution should be practiced around any intensification of standard thromboprophylaxis outside randomized controlled trials (RCTs).
Of 152 patients discharged from the hospital without an indication for anticoagulation, the majority did not receive thromboprophylaxis after discharge (3% received 7 days of standard-dose LMWH prophylaxis). The incidence of symptomatic image-confirmed VTE in these patients at 42 days after discharge was 2.6%, and no bleeds were recorded. This is above the 1.8% threshold at which the recent ACCP guidance concluded that extended thromboprophylaxis would result in net benefit.25 Two observational case series of symptomatic VTE in patients not given thromboprophylaxis after discharge have recently been published. Patell et al31 reported 0.6% VTE at 30 days after discharge in 163 patients (MB, 0.7%; CRNMB, 2.9%), and Bourguignon et al32 reported 0.7% VTE in 140 patients (10% were receiving therapeutic anticoagulation). These lower rates may be explained by selection bias, differences in patient characteristics, comorbidities, or the severity of COVID-19, as well as potentially missed VTE events. Importantly, because all cohorts had relatively small numbers of patients, the difference in event rates could be by chance. Although Roberts et al33 reported only 0.48% VTEs at 42 days after discharge in a large cohort of 1877 patients, the proportion of patients admitted for symptoms of COVID-19 as opposed to those diagnosed with COVID-19 while they were in the hospital for other medical reasons is not stated, nor is the number of patients who were receiving therapeutic anticoagulation. In comparison, we report data on the specific group of patients in whom one would consider extended thromboprophylaxis after admission for symptoms of COVID-19.
Many RCTs examining increased intensity prophylactic anticoagulation (intermediate-dose LMWH, treatment dose anticoagulation, additional antiplatelets) in patients hospitalized with COVID-19 are currently ongoing (NCT02735707, NCT04359277, NCT04401293, NCT04362085, NCT04345848, NCT04366960). Fewer RCTs are currently under way to assess extended thromboprophylaxis after discharge (eg, 10 mg rivaroxaban for 28 days) (NCT04416048, NCT04508439) and early prophylactic LMWH in community COVID-19 infection (NCT04400799, NCT04492254). RCTs in these areas will be crucial for guiding optimal management of patients with COVID-19 at different time points.
The strengths of our single-center observational study are the 90-day duration of study follow-up for VTE and a detailed characterization of bleeding. Limitations include variability in clinical thresholds for suspecting VTE (and therefore requesting imaging), variations in patients seeking medical attention after discharge, and low-grade bleeds that may have been missed. Because patient interviews did not occur at 90 days, it is possible that events after discharge were underreported; this is particularly likely for deaths after discharge and also CRNMB. MB may also have been underreported, although because the majority of the population reside locally, the likelihood of re-presenting to Oxford with complications is high. It is unlikely that VTEs after discharge were missed because of established links with hospitals that are part of the national VTE prevention program.27,28 Relatively limited patient numbers (particularly for 42-day VTE analysis after discharge) result in imprecision of event rate. Events occurred in a small number of patients resulting in wide CIs for HRs that describe associations. Because laboratory data were not taken as part of a standardized protocol, there were missing data and variable time points, which limit analyses. No corrections for multiple comparisons were performed.
In summary, in addition to thrombotic complications, there is a significant bleeding rate in patients hospitalized for COVID-19, including in patients not in the ICU. Our findings highlight using caution when intensifying inpatient thromboprophylaxis outside of RCTs. The rate of VTE of 2.6% at 42 days after discharge in this cohort suggests that extended thromboprophylaxis may provide net benefit in patients at low risk of bleeding. In combination with other emerging observational data, it highlights the uncertainty regarding the optimal thromboprophylaxis strategy after discharge and the urgent need to support large prospective RCTs of extended thromboprophylaxis in this setting.
Reasonable requests for anonymized data should be sent to Susan Shapiro at susie.shapiro@ouh.nhs.uk.
Acknowledgments
The authors thank Louise Aaron, David Bruce, Harriet Fodder, Chloe Jacklin, and Charlotte Miles who helped with data collection, and all the staff at Oxford University Hospitals NHS Foundation Trust who looked after these patients.
The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, and by grants from the Medical Research Council (MR/T024054/1) (S.S.) and (MR/T023961/1) (N.C.). R.S. was supported by the Anya Sturdy Trust Foundation. A.S. was supported by an NIHR Doctoral Research Fellowship (NIHR-DRF-2017-10- 094).
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Authorship
Contribution: S.S. and N.C. conceived and designed the study; R.S., J.M., S.J., G.S., A.S., and P.U. collected data; R.S., V.I., and S.S. analyzed the data; R.S. and S.S. drafted the manuscript; and all authors critically revised the manuscript and approved the final version.
Conflict-of-interest disclosure: S.S. has received meeting sponsorship, speaker fees, and/or consultant fees from Bayer, Pfizer, NovoNordisk, Sobi, Chugai/Roche, and Shire/Takeda. N.C. has received 2 CSL Behring investigator-led grants, educational advisory board fees from LFB and Bayer; conference support from Bayer, Novo-Nordisk, CSL Behring, and Sobi; and speaker fees from Sobi. The remaining authors declare no competing financial interests.
Correspondence: Susan Shapiro, Oxford Haemophilia and Thrombosis Centre, Churchill Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, OX3 7LE, United Kingdom; e-mail: susie.shapiro@ouh.nhs.uk.