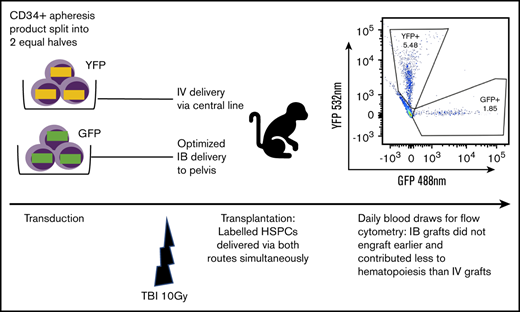
Key Points
IB transplant of CD34+ cells does not confer engraftment advantage even with optimized delivery in a competitive transplant RM model.
Abstract
Intrabone (IB) injection of umbilical cord blood has been proposed as a potential mechanism to improve transplant engraftment and prevent graft failure. However, conventional IB techniques produce low retention of transplanted cells in the marrow. To overcome this barrier, we developed an optimized IB (OIB) injection method using low-volume, computer-controlled slow infusion that promotes cellular retention in the marrow. Here, we compare engraftment of CD34+ cells transplanted in a myeloablative rhesus macaque (RM) model using the OIB method compared with IV delivery. RM CD34+ cells obtained by apheresis were split equally for transduction with lentiviral vectors encoding either green fluorescent protein or yellow fluorescent protein reporters. Following conditioning, one marked autologous population of CD34+ cells was injected directly IB using the OIB method and the other was injected via slow IV push into the same animal (n = 3). Daily flow cytometry of blood quantified the proportion of engrafting cells deriving from each source. Marrow retention was examined using positron emission tomography/computed tomography imaging of 89Zirconium (89Zr)-oxine–labeled CD34+ cells. CD34+ cells injected via the OIB method were retained in the marrow and engrafted in all 3 animals. However, OIB-transplanted progenitor cells did not engraft any faster than those delivered IV and contributed significantly less to hematopoiesis than IV-delivered cells at all time points. Rigorous testing of our OIB delivery system in a competitive RM myeloablative transplant model showed no engraftment advantage over conventional IV infusion. Given the increased complexity and potential risks of IB vs IV approaches, our data do not support IB transplantation as a strategy to improve hematopoietic engraftment.
Introduction
Umbilical cord blood (UCB) grafts are the only option for a significant minority of patients who require hematopoietic stem cell transplantation but lack a suitable related or unrelated donor. Although UCB can serve as an “off-the-shelf” graft for many patients, units with the best HLA match often contain low and sometimes insufficient numbers of CD34+ hematopoietic stem and progenitor cells (HSPCs) for use in transplantation, particularly for adult patients. Furthermore, thousands of collected units are discarded annually solely because they contain an insufficient number of HSPCs. Although UCB transplants have the advantage of expanding access to transplantation for patients without other donors, and are associated with a low risk of graft-versus-host disease, the historical use of grafts with suboptimal cell doses has resulted in slow immune recovery, delayed engraftment, and an increased risk of graft failure.
Recent improvements in graft selection to prioritize units with a higher CD34+ cell dose, and increased recipient immune suppression, have significantly improved the problem of graft rejection in centers with expertise in UCB transplantation.1 Furthermore, slow immune recovery has been mitigated by strategies that avoid the use of antithymocyte globulin.2 However, the challenge of delayed engraftment, particularly for those patients who only have access to units containing low CD34+ cell doses, remains.
A number of methods to increase the UCB unit cell dose have shown success. These include ex vivo expansion of UCB cells3-6 and the use of double UCB transplants.2,7,8 Bone marrow (BM), peripheral blood stem cell, and UCB grafts are usually injected IV, homing to the BM space after several hours in the circulation. Early following transplantation, a substantial number of CD34+ cells are lost in the lungs, liver, and spleen. Mouse and primate models demonstrate that <25% of CD34+ cells injected IV may actually make it to the BM.9-11
Investigators have sought to overcome the limitations of low cell dose in UCB grafts and loss of CD34+ cells in the circulation by injecting CD34+ cells directly into the BM space. Some clinical studies have reported beneficial outcomes from intrabone (IB) UCB stem cell delivery, with shorter engraftment times, and, in some reports, lower rates of graft-versus-host disease.12-14 However, other IB studies have shown similar engraftment times to standard IV delivery15-17 and, thus far, no randomized data comparing IB with IV administration are available. Therefore, despite the theoretical benefits of IB injection, the majority of UCB transplants continue to use an IV delivery route.
In an effort to understand why direct injection of stem cells to the target site of engraftment does not consistently improve transplant outcome, we conducted preclinical studies in animals to characterize the fate of hematopoietic progenitor cells injected using conventional IB delivery methods. Using a porcine model to evaluate an IB method used in an UCB transplantation trial in humans, we observed low-level retention of hematopoietic progenitor cells in the IB space with a substantial proportion of the injected cells leaking out of the bone.18 To overcome this, we developed an optimized IB (OIB) transplant method using computer-controlled low-pressure and low-volume injection (controlled infusion rate, <0.2 mL/min; total volume, <5 mL) under direct computed tomography (CT) guidance. In a porcine model, we have shown that OIB delivery of CD34+ cells dramatically improved IB retention, preventing the circulation of CD34+ cells through the lungs and other organs that occurred when conventional IB methods were used.18
Having confirmed that the OIB technique effectively enables retention of CD34+ cells in the IB space in a porcine model, we have now applied the OIB technique to rhesus macaques (RMs). This provides us with the opportunity to study the impact of IB transplantation in the context of myeloablative stem cell transplantation.19
Injecting stem cells directly into the BM can be associated with added risks, including but not limited to, pain, infection, and fat embolism and risks associated with the general anesthesia used in some centers.13 Therefore, before pursuing a clinical trial utilizing the OIB route for UCB transplantation, we conducted preclinical studies evaluating for in vivo evidence of an engraftment benefit using this approach. Hypothesizing that the OIB delivery method would result in superior engraftment compared with IV, we planned to conduct subsequent experiments to test whether reduced numbers of stem cells could be transplanted via the OIB method of delivery as a means to facilitate engraftment of UCB units containing suboptimal cell numbers. If successful, such a transplant approach could pave the way for making use of the many thousands of UCB units currently discarded each year due to cell counts being below the threshold considered safe for stem cell transplantation.1,20
In this regard, our preclinical animal studies used an autologous myeloablative RM transplant model to look at competitive repopulation, comparing engraftment of gene-marked CD34+ cells simultaneously transplanted IV and via the OIB delivery method.
Materials and methods
RM animal care and oversight
All procedures, housing, and handling relating to RMs conformed to regulatory standards, as specified in a protocol approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee. The RQ6686 male RM was enrolled in the positron emission tomography (PET)/CT study to confirm localization and retention of IB-injected CD34+ cells in the marrow space. DEFM, DFRM, and ZL45 male RMs were enrolled in the OIB study, weighing between 8 and 10 kg at the time of transplantation. RMs were healthy and specific pathogen free at the time of initiation of transplantation procedures. None had been previously enrolled in any other experimental study, undergone prior procedures, or been treated with any medications other than for routine clinical care. Animals were visually monitored daily and underwent regular full physical examinations. Monitoring of complete blood counts occurred daily and serum chemistries at least 3 times a week following transplantation until complete recovery, and then at least quarterly.
RM mobilization and HSPC collection
Prior to HSPC collection, an indwelling venous catheter was placed surgically into the internal jugular vein and tunneled under the skin to an exit site between the shoulder blades. This catheter remained in place until the animal recovered normal blood counts and activity posttransplantation. It was used for administration of medications and blood transfusions, and for blood drawing and apheresis.
RM mobilization, HSPC collection, and immunoselection were performed as previously described.19 Briefly, HSPCs were mobilized using granulocyte colony-stimulating factor (G-CSF; Amgen) 15 μg/kg per day subcutaneously for 6 days, and 1 dose of the CXCR4 antagonist AMD3100 (Plerixafor; Sigma) 1 mg/kg subcutaneously, 2 to 4 hours before apheresis. Apheresis was performed on day −2 using a Fenwal CS-3000 cell separator (no longer manufactured), using the central-line catheter as an inlet port and a saphenous vein temporary catheter as an outlet port. CD34+ immunoselection of the concentrated mononuclear cell product was performed using anti-mouse immunoglobulin M MicroBeads (Miltenyi Biotec) and the anti-CD34 hybridoma clone 12.8 (a murine immunoglobulin M anti-human CD34 cross-reactive with rhesus CD34). The antibody is not commercially available, but access can be arranged through Irwin Bernstein at the Fred Hutchinson Cancer Center, Seattle, WA.
OIB injection technique
To inject CD34+ cells IB, we developed and used an OIB transplant method using computer-controlled low pressure and low volume injection (controlled infusion rate, <0.2 mL/min; total volume, <5 mL) under CT guidance.18 Briefly, RMs were fasted overnight prior to the procedure, sedated with ketamine hydrochloride (10 mg/kg intramuscularly [IM]) or tiletamine and zolazepam (Telazol; 2-6 mg/kg IM), and glycopyrrolate (Robinul; 13-17 μg/kg IM), followed by intubation and general anesthesia using isoflurane. In the ventral recumbency position, the RMs were surgically prepared using 3× alternating betadine scrub and alcohol, followed by betadine solution sprayed topically and allowed to dry. Using both manual palpation then CT guidance, the posterior superior iliac spine injection site was identified, and the marrow space accessed using a truncated Jamshidi BM biopsy needle with a custom-threaded base (Cardinal Health, Dublin, OH). A 5-mL syringe containing CD34+ cells for OIB injection was connected to a computerized pump to allow cells to flow into the BM space at a controlled rate of 0.2 mL/min or lower. A hemostasis screw type Y connector (Cordis Corporation) was attached to the inserted BM needle, allowing passage of a 2-Fr Millar Mikro‐Tip pressure catheter (ADInstruments) into the BM needle to provide real‐time measurement of injection pressures. Infusion pressure was measured continuously during IB injection for DEFM and was compared with systemic arterial pressure and electrocardiography on a PowerLab data acquisition system (ADInstruments) to confirm that the controlled flow rate of 0.2 mL/min or lower maintained the pressure of the IB infusion below systemic blood pressure to reduce the risk of flow of cells from the IB space into the bloodstream. At the end of infusion, the tubing and needle were flushed with phosphate-buffered saline to ensure that cells were not retained in the tubing/needle, which was then removed from the IB space and a gauze and tape dressing applied. To ensure that there was no effect of the transduced reporter type on engraftment, we alternated the use of either green fluorescent protein (GFP) or yellow fluorescent protein (YFP) reporters to label IB-administered cells in different experiments.
Imaging with 89Zr-oxine–labeled CD34+ cells in RM
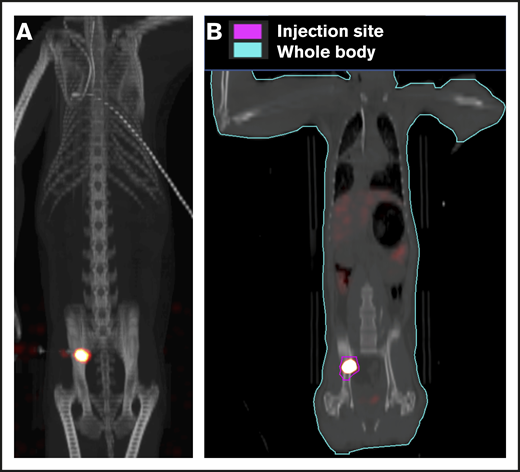
To track the retention of CD34+ cells injected IB, RM RQ6686 underwent a PET/CT imaging study. Purified CD34+ cells were labeled with 89Zirconium (89Zr)-oxine as previously reported.9,21 PET/CT scans were performed following labeled-cell transfer using a clinical PET/CT scanner (Gemini TOF; Philips Medical System, Cleveland, OH). Deferoxamine was infused continuously IV until day 8 (a 15 mg/kg per hour loading dose for 4 hours then 3 mg/kg per hour for the remainder of the study) to chelate any free 89Zr that may have been released from fragmented or dead cells. To quantitate 89Zr distribution, representing the distribution of injected cells, contours were drawn on the acquired PET/CT images delineating injection site in the ilium and whole-body, using MIMvista (MIM software). VivoQuant software (inviCRO LLC) was used to fuse the maximum-intensity projection PET images with CT. Standardized uptake values (SUVs) were calculated by the formula: SUV = [Bq/mL (decay corrected) in tissue] / [Bq of the cells injected/body weight (grams)]. The percentage of the injected dose (% ID) was calculated by decay-corrected activity in the tissue divided by the injected dose. In this experiment, the 10-kg RM received 48.1 kBq (1.3 µCi) of radiation in 2.9 × 106 89Zr-labeled CD34+ cells (0.29 × 106/kg).
Transduction of CD34+ cells
The apheresis collection was immunoselected to enrich for CD34+ HSPCs19 with product purity of 97% to 99% CD34+ cells in all experiments. Each collection of CD34+ cells was split into 2 equal portions for transduction with lentiviral vectors encoding either the GFP or YFP reporter, as previously described.22-24 We used a chimeric vesicular stomatitis virus glycoprotein pseudotyped HIV1-based lentiviral vector system (χHIV vector), which included simian immunodeficiency virus capsid instead of HIV1 capsid, for efficient transduction of rhesus CD34+ cells.22 After 3 days, transduction efficiency was assessed by flow cytometry (FACSCalibur; BD).
Autologous myeloablative transplantation
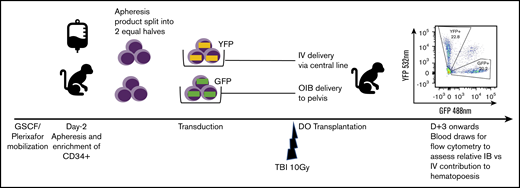
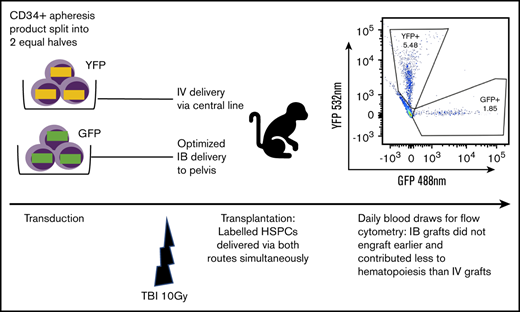
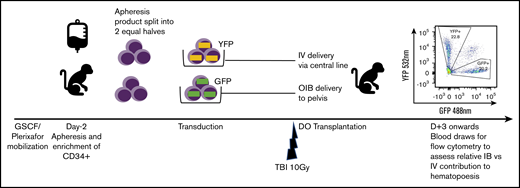
Fractionated total-body irradiation (TBI) at a dose of 5 Gy daily for 2 days (day −1 and day 0) for a total dose of 10 Gy was given at a dose rate of 0.5 Gy/min, using a cobalt source located at the Armed Forces Radiobiology Research Institute (Bethesda, MD). Following the second dose of TBI, separate aliquots of GFP- and YFP-transduced CD34+ cells were washed, resuspended in phosphate-buffered saline/2% fetal bovine serum/heparin (10 U/mL), and infused either IV via the central line or IB via the OIB technique. To ensure no effect of reporter type on engraftment, we altered the use of GFP- and YFP-transduced CD34+ cells for IB administration and IV administration in different experiments. Figure 1 provides a schema of the competitive transplantation experiment.
Schema for competitive engraftment RM transplantation experiments. A mobilized, autologous peripheral blood CD34+ cell apheresis product was split into 2 equal halves. Each half was transduced using a different marker (GFP or YFP). After 10 Gy of myeloablative total-body irradiation (TBI), gene-marked grafts were then delivered at the same time, either using the OIB technique or IV via a central line. Engrafted progeny were identified from the 2 different transplant methods to compare their relative contribution to hematopoiesis by using peripheral blood samples gated on granulocytes and analyzed for GFP and YPF by flow cytometry.
Schema for competitive engraftment RM transplantation experiments. A mobilized, autologous peripheral blood CD34+ cell apheresis product was split into 2 equal halves. Each half was transduced using a different marker (GFP or YFP). After 10 Gy of myeloablative total-body irradiation (TBI), gene-marked grafts were then delivered at the same time, either using the OIB technique or IV via a central line. Engrafted progeny were identified from the 2 different transplant methods to compare their relative contribution to hematopoiesis by using peripheral blood samples gated on granulocytes and analyzed for GFP and YPF by flow cytometry.
Flow cytometry of posttransplantation peripheral blood specimens
Post–stem cell transplantation, daily peripheral blood samples were collected. Red cells were lysed with ACK lysis buffer prior to sample collection on a BD LSR II cytometer. Granulocytes, monocytes, and lymphocytes were identified using forward and side scatter. To quantify the relative proportion of circulating peripheral blood mononuclear cells deriving from IB- vs IV-delivered CD34+ hematopoietic cells, GFP- or YFP-expressing cells were separated by the 488 (GFP) and 532 (YFP) lasers as previously reported.18 Peripheral blood samples from RM previously transplanted with GFP+ and YFP+ cells were used as controls to ensure accurate setting of voltages/collection gates. To independently validate our flow results, samples were analyzed on 2 different fluorescence-activated cell sorting machines by 2 different technicians, 1 of whom was blinded as to which marker was used to label the OIB-injected graft.
Results
OIB injection enabled CD34+ cell retention in the IB space
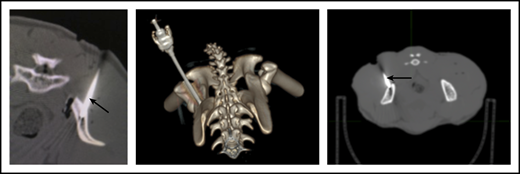
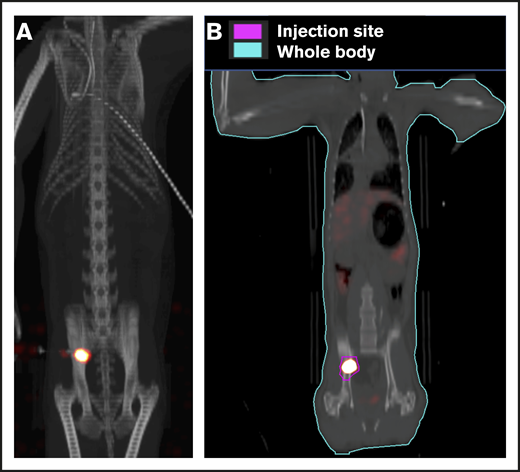
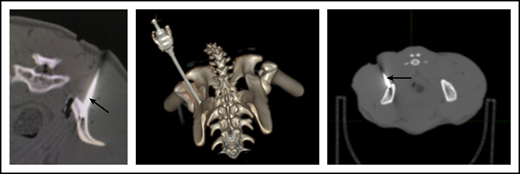
To ensure that CD34+ cells were injected directly into the IB space, CT imaging confirmed correct placement of the BM biopsy needle in all experiments. (Figure 2). To examine the retention of OIB-delivered cells in RMs, 89Zr-oxine labeled CD34+ cells were injected using the OIB technique (controlled infusion rate, <0.2 mL/min; total volume, <5 mL). Serial imaging with PET/CT was performed after cell transfer to quantify the proportion of labeled cells at each anatomical site, as a percentage of injected dose of the radioisotope. Figure 3 confirms retention of CD34+ cells at the site of injection immediately (10 minutes posttransfer corresponding to the midpoint of a 20-minute scan) after injection using the OIB technique. At 1 hour after OIB injection, 78% of the injected cells localized to the injection site, that is, remained in the right iliac IB space. Approximately 15% of infused cells leaked from the iliac IB space after IB injection and distributed to other organs, notably the liver and spleen, with the remaining radioactivity (∼7%) detected mainly in the kidneys and bladder, consistent with deferoxamine chelation and urinary excretion of free 89Zr.
CT imaging confirming successful localization of the BM needle (arrowed) into the RM right posterior superior iliac crest. Following 10-Gy TBI, RM were anesthetized, and a BM needle was placed into proper position in the IB marrow space prior to CD34+ cell infusion.
CT imaging confirming successful localization of the BM needle (arrowed) into the RM right posterior superior iliac crest. Following 10-Gy TBI, RM were anesthetized, and a BM needle was placed into proper position in the IB marrow space prior to CD34+ cell infusion.
Retention of cells in the IB space by PET/CT imaging following OIB injection with89Zr-oxine–labeled CD34+cells. Right posterior superior iliac spine of a 10 kg of RM was injected using the OIB method with 48.1 kBq (1.3 µCi)/2.9 × 106 (0.29 × 106/kg) labeled autologous CD34+ HSPCs. PET/CT imaging and quantification of isotope uptake from cells at the injection site and whole body was then performed. (A) Images at 10 minutes after OIB injection. (B) Regions of interest (ROIs) as a tool to calculate uptake at different anatomical sites as a percentage of injected dose: 1 hour after injection, 78% of injected cells were retained at the injection site with ∼15% distributed to liver/spleen/other marrow sites. The remaining 7% activity was detected mainly in the kidneys and bladder, consistent with deferoxamine chelation and urinary excretion of free 89Zr released from dead or dying cells.
Retention of cells in the IB space by PET/CT imaging following OIB injection with89Zr-oxine–labeled CD34+cells. Right posterior superior iliac spine of a 10 kg of RM was injected using the OIB method with 48.1 kBq (1.3 µCi)/2.9 × 106 (0.29 × 106/kg) labeled autologous CD34+ HSPCs. PET/CT imaging and quantification of isotope uptake from cells at the injection site and whole body was then performed. (A) Images at 10 minutes after OIB injection. (B) Regions of interest (ROIs) as a tool to calculate uptake at different anatomical sites as a percentage of injected dose: 1 hour after injection, 78% of injected cells were retained at the injection site with ∼15% distributed to liver/spleen/other marrow sites. The remaining 7% activity was detected mainly in the kidneys and bladder, consistent with deferoxamine chelation and urinary excretion of free 89Zr released from dead or dying cells.
Competitive transplantation model to quantify contribution to engraftment of IB vs IV cell delivery
As our aim was to compare OIB vs IV delivery, we set up an experimental model enabling direct comparison of their relative contribution to hematopoiesis in each individual animal as detailed in Figure 1. Briefly, we split the mobilized, CD34+ autologous graft into 2 equal halves. Each half was transduced using a different marker (GFP or YFP) before being transplanted at the same time, either OIB or IV via a central line. Subsequently, peripheral blood samples analyzed by flow cytometry for GFP and YFP enabled us to separately quantify and compare engrafted progeny from the 2 delivery methods.
Table 1 details characteristics of infused cell products. Grafts were >97% pure CD34+ cells, viability was 100%, and in vitro transduction efficiency of both markers was close to 40%, as assessed in vitro in an aliquot of cells analyzed 3 days following transduction via flow cytometry in all experiments.
Autologous CD34+ cells engrafted in RMs following myeloablative conditioning
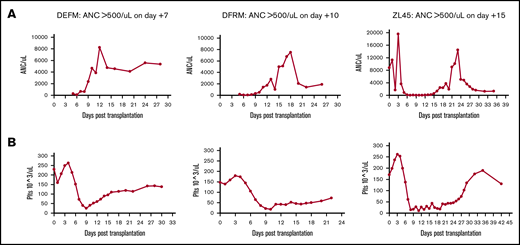
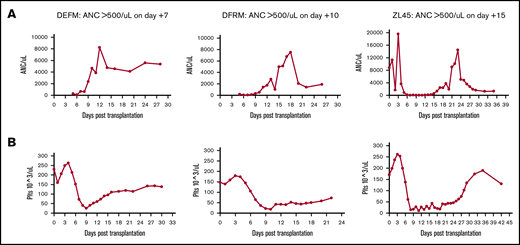
Following transplantation, we analyzed recovery of hematopoiesis via daily peripheral blood counts. These counts cannot distinguish IB vs IV source. As expected, neutrophil and platelet engraftment occurred in all animals (defined as the first of 3 days at which absolute neutrophil count [ANC] was >0.5 × 103/μL or platelets >20 × 103/μL independent of transfusion) (Figure 4). Median neutrophil engraftment occurred at day 10 (range, 7-15 days) and platelet engraftment at day 14 (range, 11-25 days), time frames that were consistent with previous data using IV-transplanted cells alone in this RM transplant model (median neutrophil engraftment on day 9).19
Complete blood count results showing engraftment times. ANC (A) and platelets (Plts) (B). Daily peripheral blood draws were performed on 3 RMs to track time to engraftment. Animals were given G-CSF daily until engraftment.
Complete blood count results showing engraftment times. ANC (A) and platelets (Plts) (B). Daily peripheral blood draws were performed on 3 RMs to track time to engraftment. Animals were given G-CSF daily until engraftment.
OIB-delivered CD34+ cells did not engraft earlier than cells delivered IV
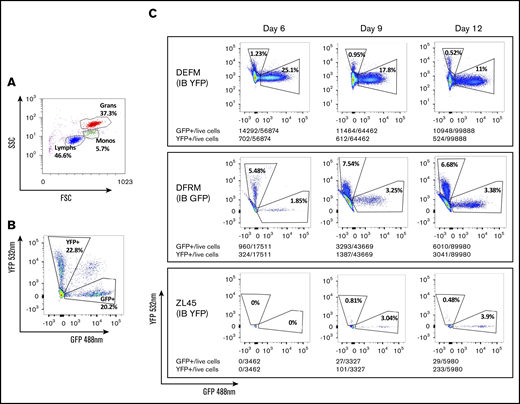
To understand and compare the relative contribution to hematopoiesis of circulating blood cells deriving from each source, we quantified GFP+ and YFP+ cells in peripheral blood by flow cytometry (Figure 5). Results between the 2 analyses correlated well (supplemental Figure 1). We detected small numbers of GFP+ and YFP+ neutrophils by flow cytometry several days before the peripheral blood ANC reached >0.5 × 103/μL. Neutrophils deriving from CD34+ cells injected IB did not appear earlier than those injected IV. Cells deriving from IB-delivered CD34+ cells appeared either at the same time or later than those derived from IV-delivered CD34+ cells.
Flow cytometry to identify progeny of GFP- or YFP-transduced CD34+cells transplanted via either IB or IV routes, respectively. Fluorescence-activated cell sorting plots showing: gating by forward (FSS) and side scatter (SSC) to identify cell types (A), separation of GFP+ and YFP+ granulocytes from a control RM previously transplanted IV with CD34+ cells transduced with equal numbers of both vectors (B), and proportions of circulating granulocytes deriving from each graft (IV and OIB) in 3 RMs at 6, 9, and 12 days posttransplantation (C).
Flow cytometry to identify progeny of GFP- or YFP-transduced CD34+cells transplanted via either IB or IV routes, respectively. Fluorescence-activated cell sorting plots showing: gating by forward (FSS) and side scatter (SSC) to identify cell types (A), separation of GFP+ and YFP+ granulocytes from a control RM previously transplanted IV with CD34+ cells transduced with equal numbers of both vectors (B), and proportions of circulating granulocytes deriving from each graft (IV and OIB) in 3 RMs at 6, 9, and 12 days posttransplantation (C).
OIB-delivered CD34+ cells contributed less to hematopoiesis than those administered IV and this contribution remained constant over time
Progeny of OIB-injected cells contributed less to hematopoiesis at all timepoints than progeny of IV-injected cells. Figure 6 summarizes the contribution to neutrophil engraftment of IB- vs IV-administered CD34 cells. Each animal had different overall marking levels due to variations in transduction efficiency and differing endogenous recovery, therefore graphs represent the percentage contribution of GFP+ or YFP+/% GFP+ + YFP+ marked cells. At day 30 following transplantation, the ratios of IV- to IB-derived cells for DEFM, DFRM, and ZL45, respectively, were 11:1, 2:1, and 5:1. In all 3 RMs, there was no engraftment advantage to injecting CD34+ cells directly into the BM space, compared with the traditional IV route. In all experiments, at least twice as many circulating peripheral blood cells derived from the IV-administered CD34+ cells were detected compared with the IB-administered cells.
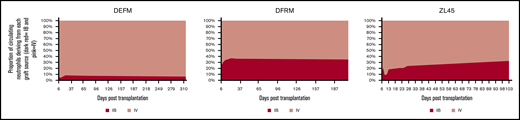
Contribution to hematopoiesis of the IV and IB graft remained constant over time with the IV graft predominating. Peripheral blood samples were analyzed by flow cytometry at serial time points up to a year following transplantation to assess the relative contribution of each graft to hematopoiesis over time. Graphs represent the percentage contribution of GFP+ or YFP+/% GFP+ +YFP+ marked cells. Each animal had different a overall marking level due to differing transduction efficiency and differing endogenous recovery.
Contribution to hematopoiesis of the IV and IB graft remained constant over time with the IV graft predominating. Peripheral blood samples were analyzed by flow cytometry at serial time points up to a year following transplantation to assess the relative contribution of each graft to hematopoiesis over time. Graphs represent the percentage contribution of GFP+ or YFP+/% GFP+ +YFP+ marked cells. Each animal had different a overall marking level due to differing transduction efficiency and differing endogenous recovery.
To assess the temporal dynamics between the IV- and IB-delivered grafts over time, peripheral blood samples were taken at regular intervals for up to a year following transplantation. Flow cytometry to assess relative contribution from each graft to hematopoiesis confirmed that the IV graft continued to dominate with the proportions remaining relatively stable over time (Figure 6).
OIB delivery of low CD34+ cell doses
To assess whether IB delivery would provide an engraftment advantage for a lower number of transplanted CD34+ cells, we transplanted 1 RM (ZL45) with less than half the total cell dose used for the other RMs (0.5 × 106/kg CD34+ cells) via the IB and the IV route (Table 1). As expected, this RM engrafted later than those receiving higher doses of CD34+ cells, with neutrophil engraftment being delayed to day +15. However, even using low cell doses, GFP+ and YFP+ cells appeared on the same day, demonstrating that while a lower dose of CD34+ cells delivered via the IB route did engraft, they did not engraft faster compared with IV.
Discussion
We tested here, in an RM model of autologous transplantation, a novel technique optimizing IB delivery using low-volume, computer-controlled injection of stem cells. We confirmed 78% retention of the injected cells in the IB space with minimal loss of retention over the subsequent week. These data compare favorably with our previous experiments injecting CD34+ cells via an IV catheter, in which ∼22% of 89Zr-oxine labeled CD34+ cells homed to and were retained in RM BM.9 However, despite optimal CD34+ cell retention in the BM space, we found no engraftment advantage with our OIB technique compared with standard IV delivery in 3 RM competitive transplantation experiments. Rather, we observed that IB delivery, even when optimized, conferred less effective stem cell engraftment than IV delivery.
There are a number of clinical studies demonstrating technical feasibility and a potential clinical benefit of IB injection,12-14 including a study of lentiviral hematopoietic stem cell gene transfer for transfusion-dependent β-thalassemia.25 Improvements in neutrophil recovery times following UCB transplantation of up to 5 days have been reported in both myeloablative and reduced-intensity–conditioned patients and there are phase 1/2 studies reporting fewer graft failures with IB12-14 compared with similar historical IV cohorts.26-28 However, when these data are compared with similar patients reported in the largest Eurocord/European Society for Blood and Marrow Transplantation (EBMT) cohort, outcomes actually appear very similar.29 Importantly, no randomized data comparing IB with IV administration currently exist to establish a definitive benefit of IB transplantation.
To date, published studies of IB injection have used a similar delivery technique, typically with 4 sites of injection, 2 in each pelvis. Our previous data using 89Zr-protamine sulfate complex–labeled CD34+ cells, injected IB with this method, identified a significant proportion of the graft leaking around the site of the first injection during injection into the second site,18 making it essentially an alternative method of IV administration. This is certainly a possible reason as to why many studies have not reported an engraftment benefit compared with IV-delivered cells.15-17 In support of this, the intraosseous route has long been used as a method to deliver fluids to the circulation in children with hypovolemic shock and is increasingly used in adults for this purpose.30
Another explanation as to why IB injection may not benefit engraftment is that it disrupts the architecture of the BM niche. Our hypothesis was that this would be less of a problem with low-pressure, controlled injection, but our results here suggest that this is not the case as engraftment was not improved. We know that sinusoidal integrity is crucial for normal hematopoiesis31 and possibly any disruption to the marrow architecture compromises the ability of injected cells to engraft when delivered via an IB route. Possible ways to mitigate stromal damage have been explored in mouse models in which concurrent IB injection of BM stromal cells at the time of hematopoietic stem cell transplantation improved hematopoietic reconstitution.32 However, this has yet to show benefit in clinical trials.
A further possible explanation for our finding no benefit to IB injection is that we used a single site for injection, and there may be insufficient niche capacity at a single site for the dose of cells injected. HSPCs may then die or differentiate in the absence of a supportive microenvironment. Although our previous data showing leakage of cells precluded our using 2 ipsilateral pelvic injection sites,18 it is possible that splitting the cell dose and infusing in 2 contralateral pelvic sites would improve IB engraftment. CD34+ HSPCs, once localized in the marrow, appear to have limited movement and spread is slow to other areas of the marrow microenvironment.33 In contrast, an IV graft will spread throughout several sites in the marrow space at time of initial seeding and may therefore be better positioned to maximize contact with the marrow microenvironment and contribute to active hematopoiesis.
Another possibility is that graft source may play a role and that UCB cells have particular homing deficiencies that benefit from IB administration.34 Our CD34+ cells were selected from peripheral blood stem cells derived from apheresis, which may having homing capabilities that differ compared with UCB cells. Indeed, the only beneficial studies of IB transplantation have used UCB grafts, with no benefit seen in a study of IB injection using BM grafts.16 We must also consider that the ability of the BM niche to support engraftment of CD34+ cells could be altered if it recently contained a hematological tumor, something not addressed with our non–tumor-bearing RM model.
In summary, although autologous CD34+ cells injected using the OIB transplant method were capable of long-term engraftment in RMs following myeloablative conditioning, they did not engraft faster and contributed less to hematopoiesis than CD34+ cells injected IV. Our data raise the question of whether IB injection, even when optimized, has utility in improving CD34+ cell engraftment. Although theoretically appealing, IB delivery is not supported by the results of this RM animal model, and, in the context of recent data showing improved outcomes with conventional UCB transplantation approaches, may no longer be warranted.
For original data, please contact childsr@nih.gov.
Acknowledgments
The authors thank Philip C. Eclarinal and Alicia Forest for acquiring PET/CT images, and Kenneth R. Jeffries, Karen J. Keeran, Arthur Zetts, Sandra D. Price, and Shawn Kozlov for animal care.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute, and the Commissioned Corps of the US Public Health Service.
Authorship
Contribution: J.K.D.-M., R.F.H., and R.W.C. wrote the study protocol; K.S. performed the experiments and wrote the manuscript; J.K.D.-M., J.M.P., R.F.H., T.J.H., R.R.C., A.K., M.E.M., A.C.B., J.F.T., N.U., L.N.R., T.E., and R.R. performed experiments; N.S. and P.L.C. provided 89Zr-oxine labeling, imaging expertise, and imaging analysis; W.T. provided flow expertise; and C.E.D. and R.E.D. provided RM transplantation expertise.
Conflict-of-interest disclosure: R.W.C., R.F.H., J.K.D.-M., T.J.H., R.R.C., J.M.P., and P.L.C. have filed a US patent application for the OIB technique used in this study. N.S. and P.L.C. possess a US patent for the 89Zr-oxine complex cell-labeling method and have filed US divisional and international patent applications for generation and application of the 89Zr-oxine complex. The remaining authors declare no competing financial interests.
Correspondence: Richard W. Childs, Laboratory of Transplantation Immunotherapy, National Institutes of Health, 10 Center Dr, Bethesda, MD 20892; e-mail: childsr@nih.gov.
References
Author notes
The full-text version of this article contains a data supplement.