Abstract
Mesenchymal stromal cells (MSCs) are widely recognized to possess potent immunomodulatory activity, as well as to stimulate repair and regeneration of diseased or damaged tissue. These fundamental properties suggest important applications in hematopoietic cell transplantation. Although the mechanisms of therapeutic activity in vivo are yet to be fully elucidated, MSCs seem to suppress lymphocytes by paracrine mechanisms, including secreted mediators and metabolic modulators. Most recently, host macrophage engulfment of apoptotic MSCs has emerged as an important contributor to the immune suppressive microenvironment. Although bone marrow–derived MSCs are the most commonly studied, the tissue source of MSCs may be a critical determinant of immunomodulatory function. The key application of MSC therapy in hematopoietic cell transplantation is to prevent or treat graft-versus-host disease (GVHD). The pathogenesis of GVHD reveals multiple potential targets. Moreover, the recently proposed concept of tissue tolerance suggests a new possible mechanism of MSC therapy for GVHD. Beyond GVHD, MSCs may facilitate hematopoietic stem cell engraftment, which could gain greater importance with increasing use of haploidentical transplantation. Despite many challenges and much doubt, commercial MSC products for pediatric steroid-refractory GVHD have been licensed in Japan, conditionally licensed in Canada and New Zealand, and have been recommended for approval by an FDA Advisory Committee in the United States. Here, we review key historical data in the context of the most salient recent findings to present the current state of MSCs as adjunct cell therapy in hematopoietic cell transplantation.
Introduction
Human mesenchymal stromal cells (MSCs), previously referred to as mesenchymal stem cells, were first described in bone marrow in 1968.1,2 Since then, MSCs have been isolated from a striking array of fetal and adult tissues, suggesting that they may reside in virtually every tissue in the human body.3-9 Depending on the tissue of origin and the ex vivo expansion protocol, MSCs have been shown to exhibit a variety of morphological and physiological characteristics. This variability, as well as considerable in vitro plasticity, has confounded efforts to assign a precise phenotype to define the identity of MSCs.10
Given the ability of MSCs to differentiate into osteoblasts, chondrocytes, and adipocytes in culture, many investigators have proposed that MSCs are stem cells or progenitors that give rise to specialized mesodermal cell lineages during development or throughout the process of tissue regeneration.11-15 However, there is a conspicuous lack of evidence that MSCs physiologically perform this function in vivo. Indeed, MSCs present in some tissues could be multipotent and directly perform stem cell–like functions, although in vivo data are lacking. It is more likely, however, that MSCs indirectly facilitate endogenous cellular mechanisms that result in tissue repair and regeneration, giving the impression of stem cell–like activity.16 Regardless of the mechanism, MSCs have been shown to promote tissue repair in various damaged or inflamed sites in the laboratory and in clinic trials.17
MSCs are also known to exert strong immunosuppressive activity on the adaptive and innate immune systems.18-21 MSCs have been reported to inhibit proliferation of T and B lymphocytes via contact-dependent and secretory mechanisms and to promote anti-inflammatory pathways in vitro and in vivo.19,22-24 As a result, the therapeutic properties of MSCs in combating various human diseases that are impacted by the immune system, such as graft-versus-host disease (GVHD), have been examined in many preclinical studies and several clinical trials.10 Although clinical trials have generated mixed results in treating GVHD, Mesoblast Limited has recently produced promising results in a clinical trial for their off-the-shelf MSC therapy RYONCIL (remestemcel-L).25
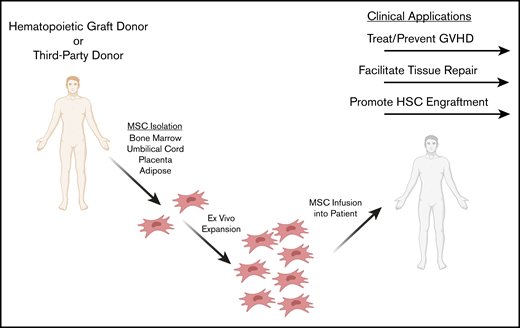
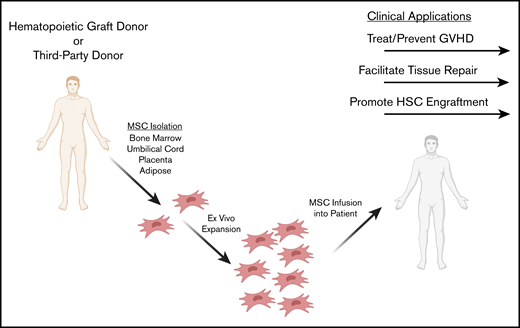
Indeed, the immunomodulatory properties of MSCs present clinical advantages in treating and preventing GVHD, as well as promoting tissue repair and engraftment during hematopoietic cell transplantation (HCT). In this review, we describe in vitro, in vivo, and clinical studies in which MSCs have been applied as immunomodulatory cell therapies during HCT to prevent and treat GVHD, repair damaged tissue, and facilitate hematopoietic stem cell engraftment (Figure 1).
Potential clinical applications of MSCs as adjunct cell therapies in HCT. MSCs may be isolated from a third-party or HLA-matched donor. A variety of MSC tissue sources are being explored for ex vivo expansion, including bone marrow, adipose, umbilical cord, and placenta. MSCs are ex vivo expanded and infused IV into the patient in the context of HCT. Clinical application during HCT includes preventing and treating GVHD, repairing tissue damaged from the conditioning regimen, and facilitating hematopoietic cell engraftment.
Potential clinical applications of MSCs as adjunct cell therapies in HCT. MSCs may be isolated from a third-party or HLA-matched donor. A variety of MSC tissue sources are being explored for ex vivo expansion, including bone marrow, adipose, umbilical cord, and placenta. MSCs are ex vivo expanded and infused IV into the patient in the context of HCT. Clinical application during HCT includes preventing and treating GVHD, repairing tissue damaged from the conditioning regimen, and facilitating hematopoietic cell engraftment.
Ex vivo preparation of MSCs
MSCs are obtained for research and clinical studies from an array of tissue sources. Viable MSCs have been isolated from bone marrow, adipose tissue, amniotic membrane and fluid, placental and fetal tissues, umbilical cord tissues, endometrium, blood, and synovial fluid. However, MSCs isolated from bone marrow are most commonly studied clinically.10 In addition, there is increasing evidence that stromal cells derived from decidua placental tissues may have more immunosuppressive activity and therapeutic potential than bone marrow–derived MSCs (BM-MSCs).26-29 BM-MSCs and MSCs collected from various other tissues are more effectively isolated via a Ficoll density gradient by plating the Ficoll-purified suspension in a culture dish, washing off adherent hematopoietic cells, and collecting the remaining adherent spindle-shaped cells.9,30-32 The efficacy of other MSC-isolation methods, including a bone marrow filtration system, is also being investigated.33
Upon isolation, MSCs must be expanded in culture ex vivo, because cell numbers are very limited in human tissues. MSCs may be effectively cultured in several media, although Dulbecco’s modified Eagle media and αMEM are the most common. Although serum-free media for tissue culture of MSCs is under development, current protocols require some serum supplementation. For ex vivo expansion of MSCs in animal studies, fetal bovine serum or human platelet lysate may be used.10 For preclinical and clinical applications, human platelet lysate may be more suitable to avoid xenoprotein exposure.34
The impacts of cryopreservation and freeze-thaw cycles on MSC immunomodulatory activity remain speculative.35,36 Previous studies have demonstrated that a freeze-thaw cycle may reduce MSC immunosuppressive activity in vitro, in vivo, and in clinical studies.35-38 However, other studies have shown in vitro and in vivo immunosuppressive activity to be maintained after thawing cryopreserved MSCs.39,40 A multitude of factors, including passage number, tissue source, thawing conditions, cell culture media used, biological activity, and disease application, likely contribute to the efficacy of cryopreserved MSCs compared with cells maintained in culture. Most importantly, a randomized study comparing the efficacy of freshly prepared and cryopreserved/thawed MSCs has not been reported.
Immunomodulatory properties of MSCs
The immunomodulatory properties of MSCs have been extensively investigated in vitro and in vivo (Table 1), providing significant implications toward applying MSCs to HCT. These immunomodulatory properties can effectively regulate the adaptive and innate immune responses, although additional research is required to understand which mechanisms occur in vivo. Focusing on T cells, MSCs have demonstrated the ability to suppress the proliferation of CD4+ and CD8+ activated T cells and inhibit the differentiation of CD8+ cytotoxic T cells.22,23 Because these suppressive effects have been observed in cocultures when MSCs are separated from peripheral blood mononucleocytes via a Transwell membrane, MSC-modulated lymphocyte suppression seems to be due to paracrine mechanisms, including secreted mediators (eg, transforming growth factor-β, hepatocyte growth factor, prostaglandin E2), as well as metabolic activity (eg, indoleamine 2,3-dioxygenase).18-21,41,42 Interestingly, it has been shown that MSCs only display suppressive activity against T lymphocytes when first primed with interferon-γ (IFN-γ), suggesting that this is the “active form” of MSCs for T-cell suppression.43,44 The exact mechanism(s) of action of MSC-induced T-cell suppression in vivo is poorly understood for GVHD or any given clinical indication. Most likely, not all immune-suppressive activity of MSCs is relevant to every clinical setting. The most recognized current concepts are that MSCs inhibit proliferation by arresting T cells in the G0/G1 phases of the cell cycle and perhaps by inducing T-cell apoptosis.41,42,45-47 Indoleamine 2,3-dioxygenase seems to play a prominent role by depleting tryptophan or producing kyneurine.43,44 Finally, there is substantial evidence that MSCs induce polarization of T cells toward a regulatory phenotype, which may also impede inflammation.48
MSCs have also been implicated in the suppression of B lymphocytes.43,44 For instance, after being activated with anti-CD40L and interleukin-4 (IL-4), B-cell proliferation of murine MSCs is suppressed.49 Likewise, human MSCs consistently suppress B-cell proliferation in vitro in the presence of anti-immunoglobulin antibodies, soluble CD40L, and various other cytokines (eg, IFN-γ).44 Although B cells contribute to the pathogenesis of GVHD,50-52 it is not known whether MSCs impact this particular B-cell activity.
MSCs interact with the innate immune system, specifically by signaling with monocytes and macrophages to promote pro- or anti-inflammatory pathways and repair damaged tissues.19,24 For example, upon being primed by proinflammatory cytokines (such as IFN-γ, tumor necrosis factor, and Toll-like receptor ligands) or in the presence of monocytes in coculture, MSCs become immunosuppressive and promote the polarization of M2 macrophages.19,21,53 Additionally, MSCs can facilitate wound and tissue repair by recruiting monocytes and macrophages to inflamed sites through the secretion of chemokine ligands and other cytokines.54 Thus, a component of MSC-mediated action against GVHD could be attributed to the polarization of M2 macrophages, which reduce inflammation and promote wound healing.
There is also evidence that inactive (apoptotic) MSCs and MSCs engulfed by phagocytes (eg, monocytes) or inactivated via recipient cytotoxic cells promote host immunosuppressive mechanisms in vitro and in mouse models.55-57 In fact, Galleu et al recently reported that only GVHD patients with high cytotoxic activity against MSCs responded to MSC infusions, suggesting that MSC cell death pathways stimulated by the innate immune response may contribute to MSC-mediated immunosuppresion.56
Prevention and treatment of GVHD
Overview of GVHD pathogenesis
Although detailed mechanisms of GVHD have been described, Ferrara and colleagues proposed what has become the classic 3-stage conceptualization of the pathogenesis of acute GVHD (aGVHD).58 In stage 1, the conditioning regimen, radiation, and/or chemotherapy injures nonhematopoietic tissue, resulting in inflammation and secretion of inflammatory cytokines. Stage 2 ensues after the hematopoietic graft has been infused when the adoptively transferred donor T cells are activated by host antigen-presenting cells, which is potently promoted by the inflammatory cytokines resulting from the injured tissues. In stage 3, activated donor T cells target host tissue and initiate alloreactive cytotoxic mechanisms.
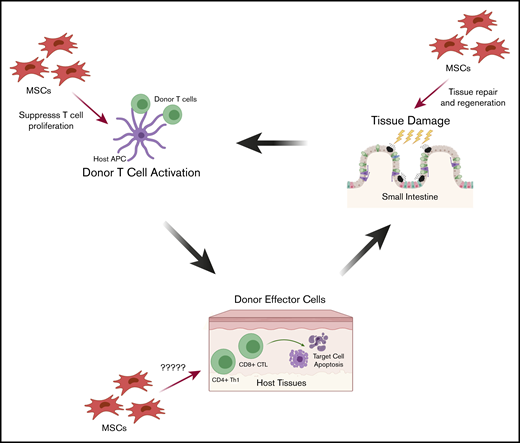
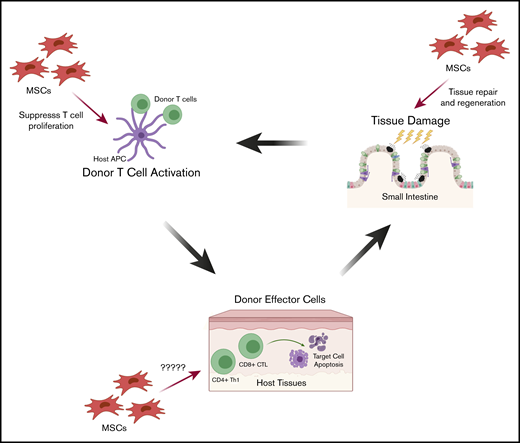
This 3-stage schema can illustrate the opportunities for IV-infused MSC prophylaxis and treatment of aGVHD (Figure 2). In stage 1, MSCs may migrate to sites of inflammation and limit tissue injury and hasten healing by their tissue-regeneration activity. In stage 2, MSCs may traffic to sites of alloactivation and inhibit the proliferation of activated T cells. In stage 3, MSCs may migrate to sites of donor T-cell host-tissue interaction and inhibit the immune response. For established GVHD, MSCs could migrate to the sites of host tissue, target and suppress alloreactive T-cell cytotoxicity, and promote tissue healing.
Proposed mechanisms by which MSCs may treat and prevent aGVHD during pathogenesis. The 3 stages of aGVHD pathogenesis provide opportunities for IV-infused MSCs for prophylaxis and treatment, through cell-cell contact or secreted mediators. First, tissue injury induced by the conditioning regimen (radiation and/or chemotherapy) causes inflammation and secretion of inflammatory cytokines. MSCs migrate to these sites of inflammation where they may limit tissue damage and promote healing and tissue regeneration. Second, following HCT, donor T cells are activated by host antigen-presenting cells (APCs), and MSCs may traffic to sites of alloactivation and suppress proliferation of activated T cells. Third, activated donor T cells target host tissue immune injury. Although less is known about how MSCs act in the third stage, MSCs could migrate to sites of graft-versus-host interactions and inhibit the local immune response. CTL, cytotoxic T lymphocyte.
Proposed mechanisms by which MSCs may treat and prevent aGVHD during pathogenesis. The 3 stages of aGVHD pathogenesis provide opportunities for IV-infused MSCs for prophylaxis and treatment, through cell-cell contact or secreted mediators. First, tissue injury induced by the conditioning regimen (radiation and/or chemotherapy) causes inflammation and secretion of inflammatory cytokines. MSCs migrate to these sites of inflammation where they may limit tissue damage and promote healing and tissue regeneration. Second, following HCT, donor T cells are activated by host antigen-presenting cells (APCs), and MSCs may traffic to sites of alloactivation and suppress proliferation of activated T cells. Third, activated donor T cells target host tissue immune injury. Although less is known about how MSCs act in the third stage, MSCs could migrate to sites of graft-versus-host interactions and inhibit the local immune response. CTL, cytotoxic T lymphocyte.
Although less understood than aGVHD, chronic GVHD (cGVHD) is proposed to occur as a result of thymic damage and impaired T-cell–negative selection, poor regulatory T-cell function, production of autoantibodies, and profibrotic lesions.59 Although MSCs have been studied as therapy for cGVHD, the underpinnings of a successful therapy are less certain.
MSCs as immunosuppressive treatments for GVHD
Given that interest in clinical applications began with hematologists who focus on HCT, it is not surprising that GVHD has commanded considerable attention.60 In preclinical animal models, MSCs isolated from different sources and/or cultured under different conditions have been shown to have varying effects on GVHD.61 In a murine study, C57BL/6 and BALB/c mice were injected with BM-MSCs and T cells in a bone marrow transplant at a ratio previously demonstrating effective suppression of T-cell proliferation in vitro, although the transplant did not effectively reduce GVHD or increase survival.62 In a more recent study, human BM-MSCs significantly reduced GVHD and increased survival in irradiated B6D2F1 mice.63 Polchert et al were the first to show that when murine BM-MSC infusions are activated with IFN-γ, survival is increased, and GVHD may be prevented and treated.64 Weekly doses of MSCs isolated from human umbilical cord tissue were recently shown to prevent, but not reduce, GVHD in peripheral blood mononucleocyte–transplanted NOD/SCID mice.65 Despite these variations observed between animal studies, a recent meta-analysis of 50 studies demonstrated robust prophylaxis of clinical aGVHD and increased survival in rat and murine allogeneic HCT models.66 Interestingly, cell dose and administration time were not significant predictors of aGVHD-associated mortality, whereas MSC source showed strong correlations with treatment outcome; of note, BM-MSCs and umbilical cord blood–derived MSCs demonstrated high efficacy, whereas adipose-derived MSCs did not differ from control groups.
Indeed, preclinical animal studies have presented mixed results, underscoring the importance of appreciating the nuances of a specific model and experimental conditions. A consistent finding among animal and clinical studies is that MSC infusions seem to be safe, laying the foundation for the plethora of clinical trials. With regard to clinical efficacy, MSCs applied as adjunct therapies for aGVHD in the context of HCT have also generated mixed results.67-70 In a recent systematic review and meta-analysis of 9 studies and 309 patients, Kallekleiv et al reported that MSCs did not have a significant effect on aGVHD.67 However, other meta-analyses show that MSCs are promising candidates for treatment of aGVHD.68,69 For example, Chen et al proposed that patient age, skin involvement, lower-grade disease, and infusion number are significant prognostic factors for the efficacy of MSC treatments for steroid-refractory aGVHD.68 Given that there is high heterogeneity in clinical study design, and few randomized clinical trials that meet inclusion criteria for many systematic reviews,70 meta-analyses on the topic of MSC-based therapies for GVHD should be interpreted with caution.
Le Blanc et al were the first to demonstrate BM-MSCs as an effective treatment of GVHD; a 9-year-old boy diagnosed with grade IV refractory aGVHD in the liver and gut following unrelated donor allogeneic HCT responded rapidly to treatment with maternal MSCs.71 Interestingly, these investigators did not have preclinical animal data prior to treating this patient. In 2008, these investigators reported a phase 2 clinical study applying ex vivo–expanded BM-MSCs as a treatment for severe refractory aGVHD.72 In this work, 55 patients were administered 1 to 5 doses of MSCs donated from HLA-identical sibling donors, haploidentical donors, or third-party HLA-mismatched donors. No MSC-related toxicity was associated with these infusions; 30 patients demonstrated a complete response to treatment, including increased survival, and 9 showed improved symptoms. Response rate was not correlated with donor HLA match status, suggesting that MSCs expanded from a third-party donor are equally effective as MSCs donated by HLA-compatible donors.
More recently, Salmenniemi et al surveyed 30 patients (22 adults and 8 children) diagnosed with steroid-refractory aGVHD or cGVHD, following treatment with 6 weekly or biweekly doses (2 × 106 cells per kg) of third-party BM-MSCs expanded in human platelet lysate.73 On day +28 postinfusion, 62% of aGVHD patients had successfully responded to treatment. Four of the 22 adult patients suffered from cGVHD and did not respond to treatment, 3 of whom had died 3 months into the study. Although the response rate did not differ statistically between adult (50%) and pediatric (88%) patients experiencing aGVHD, survival was significantly higher (P = .003) in children (88%) than in adults (22%) at median follow-up on day +767. In 2016, a phase 2/3 clinical trial for grade 3/4 steroid-refractory aGVHD reported 24% and 36% complete responses and partial responses, respectively, at 4 weeks postinfusion of BM-MSCs and ∼80% complete responses by week 8.74
The first large-scale industry phase 3 randomized clinical trial (NCT00366145; Prochymal [remestemcel-L]) for allogeneic third-party donor BM-MSCs as a treatment for refractory aGVHD was reported in 2009 by Osiris Therapeutics. Overall, patients suffering from steroid-refractory aGVHD did not show a statistically significant improvement following infusion of the cell therapy compared with placebo; however, the response in children on day +28 was clinically interesting, but the study was not powered to prove a benefit in this subgroup.75 Importantly, this was the first study to suggest that MSCs may be more effective in children. Despite an array of mixed results reported since this trial began, other phase 3 studies have resulted in clinical approval of commercial MSC products for the treatment of aGVHD in Japan (TEMCELL), as well as conditional approval for pediatric aGVHD in Canada and New Zealand (Prochymal).76 Of note, Mesoblast Limited obtained Prochymal from Osiris Therapeutics, has rebranded the MSC product (remestemcel-L) as RYONCIL, and continues to develop it in clinical trials.25,75-77 In a recent United States phase 3 clinical trial (NCT02336230) reported in March 2020 by Mesoblast Limited, RYONCIL/remestemcel-L was infused IV into 55 children with steroid-refractory aGVHD at a dose of 2 × 106 MSCs/kg twice a week for 4 weeks. It resulted in a day +28 overall response of 69.1%, as well as 74.5% and 69.5% survival at day +100 and day +180, respectively.25 Mesoblast Limited recently submitted a Biologics License Application to the US Food and Drug Administration (FDA) for RYONCIL. The FDA agreed to fast-track consideration of the product for treating pediatric steroid-refractory aGVHD and the Oncologic Drugs Advisory Panel recommended approval; however, in October 2020 the FDA issued a Complete Response Letter requesting addtional data.
The specific tissue sources of MSCs are of particular interest in developing a cell product for the treatment of GVHD. Although MSCs derived from bone marrow are the most well studied, recent clinical trials have shown that MSCs derived from placental tissue, placenta-derived decidua stromal cells (DSCs), are able to suppress aGVHD, possibly with greater efficacy than BM-MSCs. Although the immunologic role of BM-MSCs in situ has not been fully elucidated, DSCs physiologically reside at the maternal-fetal interface and contribute to the immunologic barrier preventing maternal cell–mediated immunity from attacking the fetus.78,79 Considering this immunologic barrier, Ringden and colleagues propose that DSCs may have heightened immunosuppressive activity compared with MSCs harvested from other organs (eg, bone marrow), although no mechanism of action has yet to be discovered.26,27,29
In a 2018 phase 1/2 clinical study (NCT02172937), DSCs isolated from term placentas were infused into patients with severe or steroid-refractory aGVHD.26 In the severe aGVHD group, all patients responded to treatment (48% partial; 52% complete), with a 76% 1-year survival rate when cells were thawed into 5% albumin in phosphate-buffered saline/EDTA buffer. In the steroid-refractory aGVHD group, the investigators reported 1-year survival as high as 73% compared with 20% with BM-MSCs and 3% in historical controls. Additional aGVHD clinical studies have shown that DSC infusions appeared safe and demonstrated survival rates as high as 90% at 1-year postinfusion.27,29 Despite these encouraging findings, there is only 1 published clinical study comparing DSCs with BM-MSCs26 ; additional research and randomized trials are necessary to determine whether DSCs or MSCs are more effective. Table 2 summarizes relevent clinical trials.
Although the effect of MSCs, particular BM-MSCs, has been well studied in aGVHD, data on cGVHD are less prevalent. In a preliminary study, 4 sclerodermatous cGVHD patients were infused with third-party BM-MSCs (1.0-2.0 × 107 cells) via intra–bone marrow infusion.81 Although a complete response was not observed in this trial, the investigators report a gradual improvement in symptoms over the course of 123 days, as well as an increased ratio of helper T lymphocyte 1/helper T lymphocyte 2 cells. In contrast, Peng et al reported, in a larger prospective clinical study, that 87% (N = 23) of cGVHD patients demonstrated a complete or partial response 12 months after infusion of third-party BM-MSCs (1 × 106/kg), most notably in skin, oral mucosa, and liver.82 Moreover, increased IL-10–producing CD5+ B cells and reduced T-cell inflammatory cytokine production were observed in patients who exhibited a response to treatment, perhaps providing insight into an MSC-induced mechanism of action against cGVHD.
Altogether, these studies suggest that ex vivo–expanded BM-MSCs are safe and have the potential to treat aGVHD. In addition, MSCs derived from HLA-matched siblings or third-party donors seem to perform equally well. Interestingly, the efficacy of MSCs as a treatment for aGVHD appears to be greater in pediatric patients than in adult patients, although there is currently no experimental evidence to provide insight into the underlying mechanism of this observation. Because of its selective success in children, Prochymal is only approved for pediatrics, and RYONCIL is under FDA consideration only for pediatric indications. Although off-the-shelf MSC products have demonstrated efficacy, the level of efficacy is variable between studies, suggesting that further research is required to determine the optimal cell dosage, treatment timeline, patient age, and disease presentation to treat. Moreover, additional clinical research is needed to define the long-term effectiveness, the therapeutic mechanism of action in vivo, and the efficacy of different MSC tissue sources. Finally, placenta-derived DSCs present a promising therapy for aGVHD and should be investigated extensively in randomized clinical trials.83
Tissue repair and regeneration
MSCs act directly on the immune system, and their regenerative potential could play a role in repairing tissue damage during HCT, because such injury is the first stage in the progression of aGVHD (Figure 2). MSCs are known to traffic to damaged and inflamed tissue sites, and it has been suggested that they may promote repair of damaged tissues or tissue regeneration.18,19,84 In a recent preclinical study, BALB/c mice with inflammatory bowel disease were infused with 1.0 × 106 MSCs and found to exhibit improved intestinal lesions; in a coinfusion of MSCs and endothelial progenitor cells, animals showed an even greater response to treatment.85 In other mouse models, MSCs have been shown to repair a variety of inflamed and damaged tissues in select diseases, including acute renal failure, peritoneal sepsis, lupus, multiple sclerosis, acute lung injury, and asthma.86
MSC-induced tissue repair after HCT is a current avenue of research in the clinical arena. In a retrospective clinical study, 7 patients with severe late-onset hemorrhagic cystitis (HC) were infused with ≥1 dose (median 1.0 × 106 cells/kg) of Wharton’s jelly umbilical cord–derived MSCs, isolated from a third-party donor.87 A significant response to treatment was observed in 5 of the patients, with 3 patients having a complete response after only 1 infusion. Similarly, Hassan et al reported that HC may be successfully treated with third party BM-MSCs, noting gross hematuria receding a median of 3 days postinfusion.88
In another clinical report, 10 patients (ages 13-64 years) developed tissue toxicity after allogeneic HCT and were treated with HLA-mismatched BM-MSC infusions.89 Seven patients developed HC (grades II-V), 2 developed pneumomediastinum, and 1 developed perforated colon and peritonitis. Gross hematuria subsided after a median of 3 days in 5 of the 7 HC patients, with the remaining 2 patients dying of multiorgan failure. Both patients with pneumomediastinum responded completely to treatment; the patient experiencing perforated colon and peritonitis initially responded to treatment, but after remission, a second infusion again reversed the disease.
Although the notion of tissue injury is an established component of GVHD pathogenesis, and the regenerative actions of MSCs have been amply demonstrated, the regenerative functions of MSCs may take on greater significance.58,90-94 Wu and Reddy have proposed the concept of tissue tolerance, the notion that a tissue’s capacity to withstand the damaging effects of the conditioning regimen contributes to the severity of aGVHD.95 MSCs may be administered to limit tissue damage and/or foster repair and regeneration, effectively increasing tissue tolerance. The idea of tissue tolerance predicts that this would mitigate the severity of GVHD. Although not yet explored, the mechanisms of MSC efficacy as prophylaxis and treatment of aGVHD may, in part, involve modulation of tissue tolerance.
Facilitating engraftment
In addition to the prevention and treatment of GVHD, MSCs may facilitate hematopoietic stem cell (HSC) engraftment by secreting hematopoietic cytokines and/or suppressing the residual host immunity, which can reject the transplanted graft. In animal systems, it has been reported that MSCs facilitate HSC engraftment and reconstituting of hematopoiesis by secreting cytokines, (eg, granulocyte-macrophage colony-stimulating factor and IL-6).96,97 Noort et al coinfused MSCs expanded from fetal lung tissue and umbilical cord–derived CD34+ cells and noted improved bone marrow engraftment of human HSCs in NOD/SCID mice.97 MSCs have also been shown to effectively improve bone marrow HSC engraftment in an autologous mouse transplantation model.98 Recently, Yin et al demonstrated that infusions of PDGFB-transduced MSCs significantly improved human HSC engraftment in immunodeficient mice, indicating that MSCs engineered to express relevant cytokines, receptors, or growth factors may further enhance HSC engraftment.99
In clinical studies, MSCs have also been used to improve HSC engraftment efficiency (Table 3). In the first phase 1/2 clinical trial applying MSCs to facilitate HSC engraftment, 28 breast cancer patients were given peripheral blood progenitor cells and host-expanded BM-MSC IV infusions following high-dose chemotherapy; they experienced rapid hematopoietic recovery with no observable toxicity.100 In another phase 1/2 trial, 14 children receiving haploidentical HSC transplants from relatives were given 2 infusions of donor BM-MSCs (target dose 1-5 × 106/kg).101 The investigators reported increased lymphocyte recovery and a complete graft acceptance compared with a 15% rejection rate in historical controls. However, the kinetics of neutrophil recovery was not observed.
Graft failure after autologous HCT is an especially serious complication; MSC therapies have demonstrated the potential to rescue hematopoiesis after autologous HCT graft failure. In a randomized clinical trial (NCT01763099) of 22 patients (aged 14-60 years old) receiving autologous HCT, Xiong et al reported that BM-MSCs were equally as effective as BM-MSCs infused with cord blood in rescuing hematopoietic reconstitution after autograft failure.102
MSCs have additionally been studied in the field of umbilical cord blood transplantation (UCBT). MacMillan et al reported a phase 1/2 clinical trial in which 15 pediatric leukemia patients received an UCBT, 8 of whom were simultaneously infused with BM-MSCs expanded from haploidentical parental donors.103 The treatment resulted in hematopoietic recovery and successful neutrophil engraftment at a median of 19 days in all 8 patients, as well as an increased survival probability relative to controls. Conversely, in another pediatric phase 1/2 trial, Bernardo et al did not observe any effect of parental BM-MSC infusions on allogeneic UCBT engraftment or hematopoietic recovery, notwithstanding that treatment was correlated with a reduced risk for aGVHD.104 Adult-only clinical trials testing the efficacy of MSCs at preventing UCBT graft failure and GVHD have also proved inconclusive.105 In sum, these findings suggest that MSC infusions used to facilitate HCT and UCBT engraftment are safe, yet overall efficacy remains controversial, especially in the adult setting.
Conclusions and future directions
MSCs are unambiguously immunosuppressive in vitro, as well as in preclinical animal models and clinical studies. However, the most appropriate clinical indications and the extent of therapeutic benefit remain to be firmly established. Although MSCs have great potential to become a standard therapeutic option for patients undergoing HCT, these cells should not be envisioned as a “magic bullet” that will rapidly and fully remedy GVHD or any other toxicity of HCT. Rather, MSCs should be considered a new agent that may have important advantages compared with current therapeutic options.
The cornerstone of MSCs as a cellular therapy may be the combination of demonstrated efficacy and the outstanding clinical safety profile. Indeed, immune-suppressive activity without significant toxicity is a major advantage over pharmaceuticals, such as calcineurin inhibitors and JAK inhibitors, and could justify more widespread use in patients. Even extracorporeal photopheresis, with relatively few associated toxicities, requires placement of a dialysis-quality central venous catheter and several hours of treatment. By contrast, MSCs can be infused through peripheral IV access over minutes, not hours. Moreover, unlike many other immune-suppressive agents, there is no evidence that MSCs increase the risk of leukemic relapse or opportunistic infection.
The development of MSCs as therapy has seen many setbacks; nonetheless, recent scientific advances and well-designed clinical trials underscore the unrealized therapeutic potential and suggest that MSCs will become an important component of our armamentarium in HCT. To fulfill this prediction, 3 great challenges must be addressed. First, the basic science and complete mechanism of specific therapeutic activity must be understood. MSCs exhibit many potentially immune-modulating activities; however, it is unlikely that the cells use all possible pathways in all settings. Hence, the distinct mechanism of an explicit activity, eg, treatment of GVHD or stimulating tissue repair, must be elucidated. Such scientific knowledge will inform the development of greatly needed clinically relevant potency assays, as well as strategies to enhance MSC potency, including genetically modifying MSCs,106,107 and goals to optimize manufacturing protocols, all of which are critical elements of ultimate success. The second challenge is translational science: investigators must understand the activity of MSCs in patients. Every phase I and II clinical trial should be accompanied by an extensive battery of correlative laboratory studies to understand the impact of MSCs in human subjects. Moreover, it is especially important that these studies seek to understand why MSCs are seemingly more effective in children and whether the mechanisms can be leveraged to increase MSC efficacy in adults. The third major challenge is clinical science: well-designed adequately powered clinical trials with appropriate study populations and relevant and reasonable end points should be implemented. Only through properly designed clinical trials may optimal MSC tissue sources, cell preparations, cell dose and treatment regimen, the appropriate patient age range, and severity of a given disorder for treatment be defined.
Sixteen years since MSCs were first reported to be effective for GVHD, the first MSC product has been recommended for approval by an FDA Advisorey Committee, although the Agency requested additional data. The MSC field is entering a new era in which more sophisticated science will undoubtedly lead to new breakthroughs and establish new cellular therapies to improve outcomes of patients.
Acknowledgment
This work was funded, in part, by the National Institutes of Health, National Heart, Lung, and Blood Institute award R56 HL147867 (E.M.H.).
Authorship
Contribution: A.J.B. and E.M.H. conceived and designed the review and created the figures; A.J.B. wrote the manuscript; and E.M.H. and L.P.D.-B. edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin M. Horwitz, Emory Children’s Center, Office 424, 2015 Uppergate Dr, Atlanta, GA 30322; e-mail: edwin.horwitz@emory.edu.