Key Points
FA rescue following MTX GVHD prophylaxis does not decrease regimen-related toxicity or affect transplantation outcomes.
Abstract
The use of methotrexate (MTX) for graft-versus-host disease (GVHD) prophylaxis is associated with increased rates of organ-specific toxicities. Despite limited data, the European Society for Blood and Marrow Transplantation-European LeukemiaNet working group recommend the use of folinic acid (FA) rescue to reduce MTX toxicity after allogeneic hematopoietic cell transplantation (allo-HCT). In a multicenter, double-blind, randomized, controlled trial, we explored whether FA rescue reduces MTX-induced toxicity. We enrolled patients undergoing allo-HCT with myeloablative conditioning with peripheral blood stem cell grafts, with GVHD prophylaxis consisting of cyclosporine and MTX. Patients were randomized to receive FA or placebo starting 24 hours after each MTX dose and continuing over 24 hours in 3 to 4 divided doses. The primary end point was the rate of grades 3 and 4 oral mucositis. After enrollment of 52 patients (FA, n = 28; placebo, n = 24), preplanned interim analysis revealed similar rates of grade 3 and 4 (46.6% vs 45.8%; P = .97) and grades 1 to 4 (83.3% vs 77.8%; P = .65) oral mucositis. With a median follow-up of 17 (range, 4.5-50) months, there was no difference in the rates of acute and chronic GVHD, disease relapse, nonrelapse mortality, and overall survival. These interim results did not support continuation of the study. We conclude that FA rescue after MTX GVHD prophylaxis does not decrease regimen-related toxicity or affect transplantation outcomes. This study was registered at clinicaltrials.gov as #NCT02506231.
Introduction
The most widely used approach to preventing graft-versus-host disease (GVHD) comprises a calcineurin inhibitor (CNI) with a short course of methotrexate (MTX).1,2 Whereas the CNI dose is adjusted by predose CNI blood concentrations, MTX is given at 3 or 4 fixed doses (15 mg/m2 on posttransplantation day +1 and 10 mg/m2 on days +3 and +6, with or without an additional treatment on day +11). However, its use may be associated with increased rates of oral mucositis, delayed engraftment, hepatotoxicity, and nephrotoxicity.3-6 Oral mucositis has been shown to be associated with increased mortality and morbidity (mainly from infection); significant pain; dysgeusia; difficulty speaking; difficulty receiving nutrition, hydration, and oral medications; prolonged hospitalization; and increased costs of care.7-10 Doses of MTX are commonly reduced and even omitted because of regimen-related toxicities. However, dose reduction of MTX may be associated with increased risk of acute GVHD and early death.11,12 A canine model and several nonrandomized studies have shown that folinic acid (FA; leucovorin) administration may reduce MTX toxicity.13-17 Nevertheless, the efficacy and safety of its administration remain controversial. Despite limited data, the European Society for Blood and Marrow Transplantation (EBMT)-European LeukemiaNet (ELN) working group recommended the use of FA rescue and proposed a uniform policy of FA rescue 24 hours after each MTX dose.18 Yet, according to several surveys, only half of bone marrow transplantation (BMT) centers use post-MTX FA rescue.2,10,19 In this report, we detail the results of a prospective, randomized, multicenter, double-blind trial undertaken to determine whether FA rescue reduces the rate of MTX-induced toxicity and/or affects outcomes in patients undergoing myeloablative conditioning (MAC) for allogeneic hematopoietic cell transplantation (allo-HCT) with posttransplantation MTX GVHD prophylaxis.
Patients and methods
Inclusion and exclusion criteria
This was a prospective, randomized, double-blind study conducted in 3 transplant centers in Israel (Rabin Medical Center, Tel-Aviv Sourasky Medical Center, and Rambam Medical Center). The study was approved by the institutional review boards, and all participants gave written informed consent. Patients were eligible if they had undergone allo-HCT and met all the following criteria: (1) ≥18 years of age, (2) with hematological malignancies in complete remission or with myelodysplastic syndrome (MDS), (3) receiving a MAC regimen and (4) a transplant from an HLA-matched or 1-antigen mismatched sibling donor (MSD) or mismatched unrelated (MUD) donor, (5) of a peripheral blood stem cell graft (6) without ex vivo T-cell depletion, and (7) with GVHD prophylaxis consisting of cyclosporine and short-course MTX in doses of 15 mg/m2 on posttransplantation day +1 and 10 mg/m2 on days +3 and +6 and (8) provision of signed informed consent. Patient were excluded if they met 1 or more of the following criteria: (1) transplantation for a nonmalignant hematological disease; (2) transplantation for a hematological malignancy not in complete remission, except for MDS; (3) treatment with reduced-intensity conditioning (RIC) or a nonmyeloablative conditioning regimen; (4) transplantation from a haploidentical donor or cord blood; and (5) baseline alanine aminotransferase >3 times the upper limit of normal or creatinine >1.4 mg%.
Study design and procedures
Eligible patients were randomized 1:1 by concealed assignment to receive oral FA or placebo. The patients were stratified by transplant center and conditioning regimen intensity (standard MAC vs reduced-toxicity MAC). FA or placebo administration started 24 hours after each MTX dose and was given during 24 hours: 15 mg 3 times daily after MTX administration on posttransplantation day +1 and 4 times daily after MTX administration on days +3 and +6. FA and placebo were provided by Superpharm Professional Laboratory (Petach Tikva, Israel). Patients who received transplants from unrelated donors received anti-T-cell globulin (Fresenius, Germany) at a dose of 5 mg/kg on days −3 to −1. All patients were evaluated daily from randomization through discharge, and oral mucositis was graded and documented by a transplant team physician.
Supportive care
All patients received filgrastim (granulocyte colony-stimulating factor) at a dose of 5 μg/kg per day from day 7 until neutrophil engraftment, infection prophylaxis according to institutional guidelines, and a ursodeoxycholic drug for prevention of sinusoidal obstruction syndrome (SOS).20 Concomitant cryotherapy was not allowed. Total parenteral nutrition (TPN) was given at the physician’s discretion when nausea, vomiting, or oral mucositis prevented adequate nutritional intake.
End points
The primary end point was the proportion of patients with grade 3 or 4 oral mucositis and its duration. The secondary end points were the proportion of patients with grades 1 to 4 oral mucositis, adherence to MTX doses, time to neutrophil and platelet engraftment, need for an IV opioid analgesic, administration of TPN and its duration, incidence and severity of SOS, incidence of febrile neutropenia and bloodstream infections from transplantation through first hospital discharge, time from transplantation to first hospital discharge, cumulative incidence of acute and chronic GVHD, cumulative incidence of relapse and nonrelapse mortality (NRM), and overall survival (OS).
Definitions
Disease risk was scored according to the Disease Risk Index for allogeneic stem cell transplantation.21 Comorbidities were scored according to the Hematopoietic Cell Transplantation Comorbidity Index.22 Standard MAC regimens consisted of IV cyclophosphamide 120 mg/kg plus either total body irradiation 12 Gy or IV busulfan 12.8 mg/kg. Reduced toxicity MAC consisted of IV fludarabine 160 mg/m2 plus IV busulfan 12.8 mg/kg or IV fludarabine 150 mg/m2 plus treosulfan 36 to 42 g/m2. Adherence to the MTX schedule was defined as the ratio of the actual drug dose given to the dose planned × 100. Oral mucositis was graded according to the World Health Organization Oral Mucositis Grading Scale (grade 0, none; grade 1, oral soreness and erythema; grade 2, oral erythema and ulcers with solid diet tolerated; grade 3, oral ulcers and liquid diet only; grade 4, oral alimentation impossible).23,24 Neutrophil and platelet engraftments were defined as the first of 3 days with absolute neutrophil counts ≥0.5 × 103/μL and the first of 7 days with an unsupported platelet count ≥20 × 103/μL, respectively. Veno-occlusive disease (VOD) was graded according to the EBMT diagnostic and severity criteria for SOS/VOD.25 Acute GVHD was assessed by using the consensus grading system,26 and chronic GVHD was assessed according to the National Institutes of Health consensus criteria.27
Statistical analysis
The study was designed to enroll 116 patients (58 in each arm), which would provide a power of 80% to detect a 50% reduction in the rate of severe oral mucositis from an anticipated 50% in the placebo arm to 25% in the FA arm. A futility analysis was included in the study design and was performed after roughly half of the target events (ie, severe oral mucositis) occurred. After the interim analysis, the investigators would stop active accrual if the power to reject the null hypothesis decreased below .2. Rules for cessation were based on the 1-sided Lan-DeMets method.28
Data are presented as frequencies (percentages) for categorical variables and as median (range) for continuous variables. The χ2 or Fisher’s exact test was used to compare categorical variables and the Mann-Whitney U test to compare medians. To define variables that predict oral mucositis, a logistic regression model was applied, with age, sex, conditioning regimen, and FA as covariates. The odds ratio was estimated by the exp(β), and the Hosmer-Lemeshow test was used to estimate the goodness of fit of the model. Probability of OS was estimated by using the Kaplan-Meier method, whereas NRM, relapse, and GVHD were estimated by using cumulative incidence analysis, considering competing risks. Univariate comparison was performed with the log-rank test for OS and Gray’s test for GVHD, relapse incidence, and NRM. Death was treated as a competing risk in the analyses of relapse and GVHD, whereas relapse was treated as a competing risk in the analysis of NRM. Statistical analyses were performed with SPSS, version 21 (Chicago, IL), and Prism, version 5.0 (GraphPad, San Diego, CA).
Results
Based on a planned interim analysis, we closed the study to further accrual after enrollment of 52 patients (45% of the planned sample size) because of futility analysis results. The following report is based on the interim analysis.
From February 2016 through July 2019, a total of 52 patients with acute myeloid leukemia (n = 32; 61%), acute lymphoblastic leukemia (ALL; n = 13; 29%), MDS (n = 6; 11.5%), or non-Hodgkin lymphoma (n = 1; 2%) were enrolled and randomized to the FA (n = 28) or placebo (n = 24) arms. The median age at the time of enrollment was 49 (range, 20-71) years and 44% were women. The conditioning regimen intensity was standard MAC in 31% of patients and reduced-toxicity MAC in 69%. Thirty-two percent of the patients received transplants from matched sibling donors (MSDs), 62% from matched unrelated donors (MUDs), and 6% from 1-antigen mismatched unrelated donors. The 2 cohorts were balanced in terms of patient, disease, and transplant characteristics (Table 1). The median follow-up was 17 (range, 4.5-50) months.
Overall compliance with the protocol was good, as most (n = 49; 94%) patients received 100% of the planned study drug doses, and there were no differences between the study groups (FA, 27 of 28 [96%] vs placebo, 22 of 24 [92%]; P = .46). Twelve percent of the patients received doses of MTX reduced by 10% to 30% because of mucositis, and there were no differences between the study groups in this respect (FA, 4 of 28 [14%] vs placebo, 2 of 24 [8.5%]; P = .5).
Overall, 24 (46%) patients developed severe (grade 3 or 4) oral mucositis, and 44 (85%) patients developed oral mucositis of any grade (1-4). Importantly, the rate (46.6% vs 45.8%; P = .97) and duration (4 vs 4 days) of severe oral mucositis and the rate of oral mucositis of any grade (83.3% vs 77.8%; P = .65) were similar in the FA and placebo groups, respectively. Furthermore, time to neutrophil (12 vs 12 days; P = .6) and platelet (13 vs 13 days; P = .4) engraftment, rates of febrile neutropenia (57% vs 58%; P = .94), bloodstream infections (10% vs 16%; P = .5), VOD (7% vs 12%; P = .5), need for opioid analgesics (78% vs 67%; P = .3), administration of TPN (14% vs 25%; P = .3) and time from HCT to discharge (18.5 vs 19 days; P = .95) were not significantly different between the study groups (Table 2).
In both univariate and multivariate analyses, the patient’s age, sex, conditioning regimen intensity (standard vs reduced-toxicity MAC), and FA treatment (vs placebo) had no impact on the rate of oral mucositis or its severity.
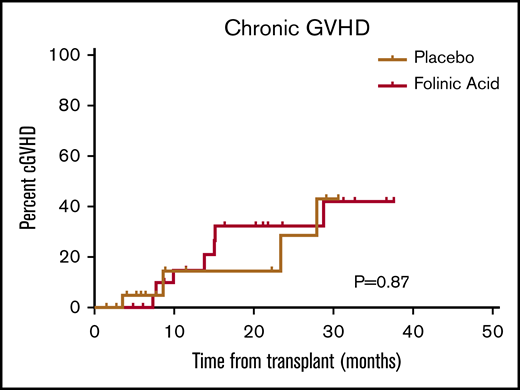
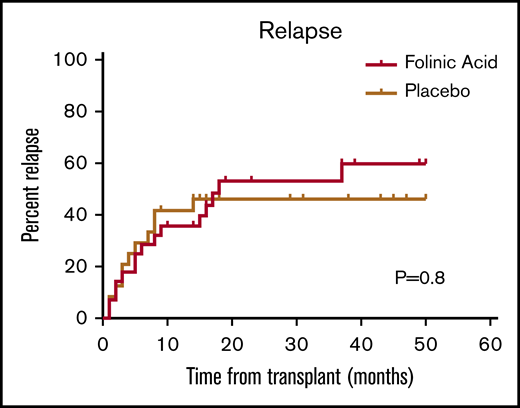
The cumulative incidences of grades 2 to 4 acute GVHD (46% vs 50%; P = .78) and grade 3 or 4 acute GVHD (18% vs 17%; P = .91) by day 100 and the cumulative incidences of chronic GVHD by 2 years (35% vs 32%; P = .87) were similar in the FA and placebo groups (Figure 1). There was also no difference between the 2 groups in cumulative incidence of NRM (Figure 2) and disease relapse (Figure 3). Overall, the comparable clinical courses translated into similar OS (Figure 4).
When patients with severe oral mucositis were compared with those with nonsevere oral mucositis, patients with severe oral mucositis more often had febrile neutropenia (71% vs 46%; P = .07) and VOD (17% vs 3.6%; P = .1), were more often given TPN (29% vs 10.7%; P = .09) and opioid analgesics (100% vs 50%; P = .0001), and had a significantly longer median time from transplantation to first hospital discharge (19.5 vs 17 days; P = .06).
Discussion
Oral mucositis is a debilitating adverse effect, affecting most patients who undergo allo-HCT.3-6 Although it is largely triggered by the chemoradiotherapy conditioning regimen, oral mucositis of significantly higher incidence and severity is associated with MTX for GVHD prophylaxis than with other immunosuppressive drugs after MAC regimens.3-6 The protective effect of FA against oral mucositis reported in patients who receive low and high doses of MTX in autoimmune disorder and chemotherapy protocols, respectively, provided the rationale for exploring the use of FA as a means reducing MTX toxicity in the transplantation setting.29-32 Several series and nonrandomized clinical trials have suggested that FA administration may reduce MTX toxicity after GVHD prophylaxis.14-17 Based on these data, the EBMT-ELN working group recommended the use of FA rescue to reduce MTX toxicity after allo-HCT.18 However, this hypothesis has never been evaluated in a prospective randomized trial. Another important point to keep in mind is that theoretically, the neutralizing effect of FA may abrogate the MTX-induced anti-GVHD effect. In the present randomized, double-blind, controlled study the hypothesis regarding the benefits of FA was not validated, because the FA and placebo cohorts were similar in both primary and secondary end points, namely in the rate and duration of grade 3 or 4 oral mucositis, rate of grades 1 to 4 oral mucositis, time to neutrophil and platelet engraftment, rates of febrile neutropenia and bloodstream infections, rate of VOD, need for opiate analgesics and TPN, and time from HCT to discharge. These unequivocal interim results led to our decision to discontinue the study.
Overall, 85% of our patients had grades 1 to 4 oral mucositis and 46% had grade 3 or 4. These rates are in line with previous reports of the incidence of oral mucositis among patients treated with MTX for GVHD prophylaxis.3-6 Like others, we found that patients with grade 3 or 4 oral mucositis had delayed engraftment, more infections, required TPN more often, and had a longer duration of treatment with opioid analgesics and longer duration of hospitalization.3-6
Despite the concern that the neutralizing effect of FA could abrogate the MTX-induced anti-GVHD effect, we did not observe any difference in the incidence of acute and chronic GVHD in the FA and placebo cohorts.33
Over several years, it has been debated whether administration of FA can reduce the curative rate of hematologic malignancies by selectively rescuing leukemic cells. Indeed, higher FA doses during high-dose MTX treatment were associated with increased risk of relapse and reduced cure rates in childhood ALL.34 However, in the pediatric transplantation setting, FA seemed safe with regard to relapse in malignant disease.35 Nevertheless, FA supplementation in higher doses (>185 mg/m2) and RIC were recognized as risk factors for graft rejection in nonmalignant disease.35 Thus, in children who undergo allo-HCT, FA treatment should be used with caution, especially in patients with nonmalignant conditions and those receiving RIC regimens to avoid graft rejection.
Serum MTX levels were not monitored in the present study. In fact, we were unable to find data on the proportion of transplant centers that routinely monitor serum MTX levels after MTX GVHD prophylaxis. Although both levels and duration of MTX exposure were shown to be strongly associated with MTX toxicity,36 there is no reason to suspect that our 2 study groups were imbalanced in their serum MTX levels. Furthermore, the low doses of MTX provided as GVHD prophylaxis was shown to be undetectable in the serum of 40% of patients as early as 24 hours after MTX administration and below 0.1 µmol/L in most of the remaining patients, making it impossible to correlate drug levels and mucositis severity in this context.37
This study was confined to adult patients who underwent allo-HCT with MAC regimens only, whereas patients with RIC regimens were excluded. Although similar high incidence and severity of oral mucositis have been reported after MAC and RIC regimens,6 a separate study in the RIC setting is needed, to determine the impact of FA rescue on MTX-induced toxicity.
Several strategies have been developed to prevent or minimize chemotherapy-induced mucositis. However, only a few of them have shown efficacy in the allo-HCT setting. Although the quality of evidence derived from randomized trials is limited, updated evidence-based clinical practice guidelines for oral mucositis developed by the Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) identified oral cryotherapy and photobiomodulation as beneficial in the prevention of oral mucositis in patients receiving high-dose chemotherapy with or without total body irradiation as conditioning for auto- and allo-HCT.38 In contrast, randomized trials failed to show any benefit of prophylactic palifermin and calcium phosphate (Caphosol) in allo-HCT.38-40 Interestingly, in a double-blind, randomized, placebo-controlled trial, N-acetyl-cysteine was associated with lower incidence, severity, and duration of oral mucositis in patients who underwent allo-HCT with MAC.41
In summary, the results of the present study indicate that FA rescue has no significant effect, favorable or unfavorable, on regimen-related toxicity and transplant outcomes following MTX GVHD prophylaxis after allo-HCT with MAC regimens. The present role and recommendations of FA rescue in this setting should be reconsidered.
Original data are available by e-mail request to the corresponding author, Moshe Yeshurun (moshe.yeshurun@gmail.com).
Acknowledgment
This work was supported by a grant from the Israel Society of Hematology and Transfusion Medicine (M.Y.).
Authorship
Contribution: M.Y. designed and performed the study, analyzed the data, and wrote the manuscript; L.S.-A. designed and performed the study and wrote the paper; U.R. designed the study, analyzed the data, and wrote the manuscript; O.P., O.A., T.Z., A.P., M.R., and M.S.-N. performed the study; R.R. performed the study and wrote the manuscript; and P.R. and O.W. wrote the manuscript.
Conflict-of-interest disclosure: M.Y is the medical director and a stock option holder in Kalytera Therapeutics and STERO Biotechs. The remaining authors declare no competing financial interests.
Correspondence: Moshe Yeshurun, Institution of Hematology, Davidoff Cancer Center, Rabin Medical Center, 39 Jabotinski St, Petah Tikva 49100, Israel; e-mail: moshe.yeshurun@gmail.com.