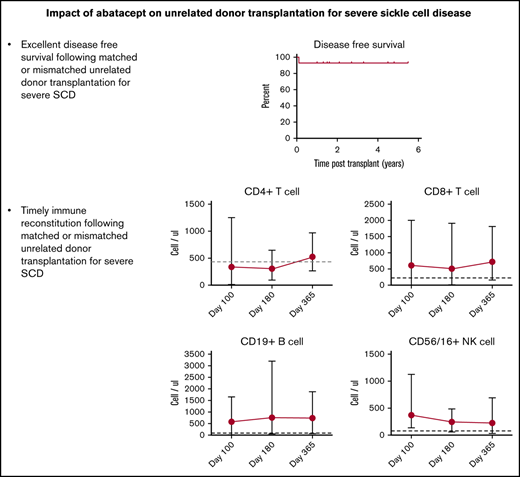
Key Points
Strategies to minimize GVHD are essential if URD SCT is to be considered with curative intent in SCD.
Abatacept-inclusive GVHD prophylaxis protects against transplant complications that contribute to morbidity/mortality after URD SCT in SCD.
Abstract
We report results of a phase 1 multicenter stem cell transplantation (SCT) trial from HLA-matched (n = 7) or one-antigen–mismatched (n = 7) unrelated donors (URD) using bone marrow or cord blood as stem cell source, following reduced-intensity conditioning (RIC) in severe sickle cell disease (SCD). Conditioning included distal alemtuzumab, fludarabine, and melphalan (matched donors), with thiotepa (mismatched donors). Abatacept, a selective inhibitor of T cell costimulation, was added to tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis to offset GVHD risks, and was administered for longer duration in bone marrow recipients than in cord blood recipients because of increased incidence of chronic GVHD with bone marrow. Median age at transplant was 13 years (range, 7-21 years). The incidence of grades II to IV and grades III to IV acute GVHD at day +100 was 28.6% and 7%, respectively. One-year incidence of chronic GVHD was 57% and mild/limited in all but 1 patient who received abatacept for a longer duration. Only 1 patient developed reversible posterior encephalopathy syndrome and recovered. With a median follow-up of 1.6 years (range, 1-5.5 years), the 2-year overall and disease-free survival was 100% and 92.9%, respectively. The encouraging results from the phase 1 portion of this RIC SCT trial, despite risk factors such as older age, URD, and HLA-mismatch, support further evaluation of URD SCT in clinical trial settings. The phase 2 portion of the trial is in progress. This trial was registered at www.clinicaltrials.gov as NCT03128996.
Introduction
Allogeneic stem cell transplantation (SCT) from HLA-matched sibling donors has excellent disease-free survival (DFS) of >90% in children with sickle cell disease (SCD) but is limited by donor unavailability in >80% of eligible patients.1 Unrelated donors (URD) are potential alternate donor sources. Patients of African origin have a 19% chance of finding fully (8/8) HLA-matched, but a 57% chance of finding minimally mismatched (7/8), URD in donor registries.2 However, SCT from URD bone marrow has been associated with high risks of acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD) and consequent mortality in the first URD SCT trial for SCD performed following reduced-intensity conditioning (RIC) designed to offset high-dose chemotherapy-related organ toxicities. The 2-year overall survival (OS) was 79% (95% confidence interval [95% CI], 59%-90%) suggesting caution for this approach.3 aGVHD also complicated unrelated cord blood transplantation with RIC.4,5 Further, graft-versus-host disease (GVHD) risks rise exponentially with recipient age >12 years irrespective of donor source.3,6,7 African Americans, encompassing most SCD patients, have a higher risk of GVHD.8-10 GVHD prophylaxis or treatment with corticosteroids also increase hypertension-related complications such as posterior reversible encephalopathy syndrome (PRES) and cerebral hemorrhage, complications that SCD patients are highly susceptible for at baseline.3,11 Thus, it is critically important to prevent GVHD and limit complications to successfully expand this curative therapy in SCD patients.
Abatacept is a fusion protein that selectively inhibits T‐cell costimulation by binding to CD80/CD86 on antigen‐presenting cells and blocking CD28-mediated signaling.12 A first-in-human trial of abatacept prophylaxis after SCT for malignant disorders safely protected against aGVHD, earning the agent a breakthrough designation for this indication from the US Food and Drug Administration and provided background for this approach.13 In the phase 2 portion of the trial, grade 3 to 4 aGVHD decreased to 2.3% following URD SCT when short-term abatacept was administered until day +28 in 4 doses, but no benefit was noted on cGVHD following HLA-mismatched SCT compared with controls14 . We report the results of the phase 1 portion of a trial (NCT03128996) of URD SCT in recipients ≤21 years with severe SCD using the RIC previously reported, followed by tacrolimus, methotrexate, or mycophenolate (in cord blood recipients), and abatacept as GVHD prophylaxis.5 The primary end points were engraftment, OS, and DFS at 1 year. Secondary end points included the kinetics of engraftment, nature and severity of GVHD, disease symptoms, organ toxicities, infections, and immune reconstitution for 2 years.
Methods
The institutional review board at each participating institution approved the protocol. Between August 2016 and June 2018, 14 SCD patients, age 7 to 21 years (median, 13 years), with stroke (n = 6), ≥3 severe vaso-occlusive pain crises per year (n = 6), or ≥2 acute chest syndrome episodes per year (n = 5) underwent URD SCT at 5 participating centers after consent. Seven received 8/8 HLA-matched (-A, -B, - C, and -DRB1) bone marrow, 5 received 7/8 matched bone marrow, and 2 received 5/6 matched (-A, -B, and –DRB1) cord blood (Table 1). Hemoglobin S was <30% prior to conditioning in all patients. RIC included hydroxyurea (days −50 to −21), distal alemtuzumab (days −22 to −19), fludarabine (days −8 to −4), and melphalan (day −3) as previously described.3 Thiotepa (8 mg/kg) on day −4 was added to the regimen for mismatched bone marrow and cord blood products.5 GVHD prophylaxis included tacrolimus and short-course methotrexate (bone marrow) or mycophenolate mofetil (cord blood) as previously described.3 Abatacept (10 mg/kg) was administered intravenously on days −1, +5, +14, +28, +100, +180, +270, and +365. Cord blood transplants reported in this study received abatacept until day +28 (4 doses). After April 2019, as a result of aGVHD risks we reported with unrelated cord blood in the first few months post-SCT but lower cGVHD risks, the protocol was changed so that abatacept doses are continued only until day +100 in cord blood transplants.5,15,16 Abatacept dosing was extended in bone marrow transplant recipients as an amendment after the first 2 patients, to evaluate the possible benefit of extended use on cGVHD, a complication to which this population is highly susceptible (Table 1). The interval between doses with the extended use was based on the assumption that proliferation of alloreactive T cells in damaging numbers would have a lag period after wash-out of a previous dose, and balancing the interval between doses would be advantageous for immune reconstitution. Supportive care included hypertension control, seizure prophylaxis, and hemoglobin/platelet count maintenance.3 GVHD was graded according to previously published criteria.17 The probability of OS and DFS was estimated from the time of transplantation using Kaplan-Meier product-limit estimates.
Patient characteristics and outcomes
| Patient . | Age, y/sex . | Indication . | Stem cell source/HLA match . | Number of abatacept doses received . | Myeloid chimerism at last evaluation, % . | aGVHD/cGVHD (cGVHD NIH grade) . | Follow up, y . | Organ toxicity/outcome . | Systemic immune suppression (time withdrawn) . | Performance score pre/at last follow up . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/F | Stroke | Bone marrow/A antigen | 4* | 95 | 0/limited (mild) | 5.5 | SJS/resolved | Off (1 y, 9 mo) | 100/100 |
| 2 | 13/F | VOE | Bone marrow/DRB1 antigen | 4* | 100 | Gr 1/extensive (severe) | 4.8 | PRES/resolved | On | 100/70 |
| 3 | 21/M | Stroke | Bone marrow/none | 8 | 95 | 0/limited (mild) | 4.5 | Pancreatic insufficiency/ongoing | Off (1 y) | 100/100 |
| 4 | 18/F | VOE | Bone marrow/ C allele | 8 | 100 | 0/limited (mild) | 3.3 | None | Off (1 y, 1 mo) | 80/100 |
| 5 | 19/M | VOE, ACS | Bone marrow/A allele | 8 | 100 | Gr 2/0 | 2.9 | AKI/resolved | Off (1 y, 1 mo) | 90/100 |
| 6 | 7/M | ACS | Bone marrow/none | 8 | 96 | 0/limited (mild) | 2.1 | None | Off (1 y, 5 mo) | 90/100 |
| 7 | 18/F | VOE, ACS | Bone marrow/A antigen | 8 | 100 | Gr 2/extensive (severe) | 1.6 | Peripheral neuropathy/resolved | On | 90/80 |
| 8 | 16/F | VOE, RBC antibodies | Bone marrow/none | 8 | 96 | 0/limited (mild) | 1.5 | None | Off (1 y) | 100/100 |
| 9 | 9/M | VOE, ACS | Cord blood/DRB1 | 4 | 100 | Gr2/0 | 1.5 | Transient red cell aplasia, AKI, cholelithiasis/resolved | Off (1 y, 6 mo) | 90/90 |
| 10 | 8/F | Stroke | Bone marrow/B allele | 8 | 100 | Gr 4/limited (mild) | 1.5 | None | On | 80/90 |
| 11 | 13/F | Stroke | Bone marrow/none | 4 | 0 | NE | GR | None | NE | 90/90 |
| 12 | 21/M | Stroke | Bone marrow/none | 8 | 100 | 0/0 | 1.4 | None | Off (1 y) | 100/100 |
| 13 | 2/M | ACS | Cord blood/B antigen | 4 | 100 | 0/0 | 1 | AKI/resolved | Off (1 y) | 100/100 |
| 14 | 7/M | Stroke | Bone marrow/A antigen | 8 | 100 | 0/0 | 1 | None | Off (1 y) | 70/90 |
| Patient . | Age, y/sex . | Indication . | Stem cell source/HLA match . | Number of abatacept doses received . | Myeloid chimerism at last evaluation, % . | aGVHD/cGVHD (cGVHD NIH grade) . | Follow up, y . | Organ toxicity/outcome . | Systemic immune suppression (time withdrawn) . | Performance score pre/at last follow up . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/F | Stroke | Bone marrow/A antigen | 4* | 95 | 0/limited (mild) | 5.5 | SJS/resolved | Off (1 y, 9 mo) | 100/100 |
| 2 | 13/F | VOE | Bone marrow/DRB1 antigen | 4* | 100 | Gr 1/extensive (severe) | 4.8 | PRES/resolved | On | 100/70 |
| 3 | 21/M | Stroke | Bone marrow/none | 8 | 95 | 0/limited (mild) | 4.5 | Pancreatic insufficiency/ongoing | Off (1 y) | 100/100 |
| 4 | 18/F | VOE | Bone marrow/ C allele | 8 | 100 | 0/limited (mild) | 3.3 | None | Off (1 y, 1 mo) | 80/100 |
| 5 | 19/M | VOE, ACS | Bone marrow/A allele | 8 | 100 | Gr 2/0 | 2.9 | AKI/resolved | Off (1 y, 1 mo) | 90/100 |
| 6 | 7/M | ACS | Bone marrow/none | 8 | 96 | 0/limited (mild) | 2.1 | None | Off (1 y, 5 mo) | 90/100 |
| 7 | 18/F | VOE, ACS | Bone marrow/A antigen | 8 | 100 | Gr 2/extensive (severe) | 1.6 | Peripheral neuropathy/resolved | On | 90/80 |
| 8 | 16/F | VOE, RBC antibodies | Bone marrow/none | 8 | 96 | 0/limited (mild) | 1.5 | None | Off (1 y) | 100/100 |
| 9 | 9/M | VOE, ACS | Cord blood/DRB1 | 4 | 100 | Gr2/0 | 1.5 | Transient red cell aplasia, AKI, cholelithiasis/resolved | Off (1 y, 6 mo) | 90/90 |
| 10 | 8/F | Stroke | Bone marrow/B allele | 8 | 100 | Gr 4/limited (mild) | 1.5 | None | On | 80/90 |
| 11 | 13/F | Stroke | Bone marrow/none | 4 | 0 | NE | GR | None | NE | 90/90 |
| 12 | 21/M | Stroke | Bone marrow/none | 8 | 100 | 0/0 | 1.4 | None | Off (1 y) | 100/100 |
| 13 | 2/M | ACS | Cord blood/B antigen | 4 | 100 | 0/0 | 1 | AKI/resolved | Off (1 y) | 100/100 |
| 14 | 7/M | Stroke | Bone marrow/A antigen | 8 | 100 | 0/0 | 1 | None | Off (1 y) | 70/90 |
ACS, acute chest syndrome; AKI, acute kidney injury; F, female; Gr, grade; GR, graft rejection; M, male; NE, not evaluable; NIH, National Institutes of Health; RBC, red blood cell; SJS, Stevens-Johnson syndrome; VOE, vaso-occlusive episodes.
The first 2 patients were treated on an earlier version of the protocol and received 4 doses of abatacept. Abatacept administration was extended to 8 doses in bone marrow transplants subsequently.
Results and discussion
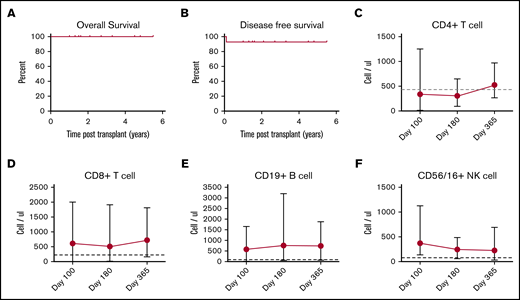
Patient demographics, transplantation details, and outcomes are summarized in Table 1. All patients had risk factors for transplant-related morbidity and/or mortality on the basis of age (n = 10), URD source, and HLA-mismatch (n = 7).18-21 The median number of total nucleated and CD34+ cells per kilogram of body weight was 3.0 × 108 (range, 0.75 × 108 to 5.5 × 108) and 3.36 × 106 (range, 0.19 × 106 to 4.5 × 106), respectively. At a median follow up of 1.6 years (range, 1-5.5 years), 13 of 14 recipients had stable donor engraftment. One patient with cytomegalovirus (CMV) reactivation had secondary graft rejection on day +30. This patient subsequently rejected another URD graft following myeloablative conditioning and received a haploidentical transplant with donor engraftment at early follow-up. The 2-year probability of OS and DFS was 100% and 92.9%, respectively (Figure 1A-B). The median time to neutrophil and platelet (>50 × 103/μL) engraftment was 14 days (range, 10-24 days) and 19.5 days (range, 16-39 days), respectively. All engrafted patients had >95% donor chimerism at their last evaluation. No patient had SCD manifestations such as vaso-occlusive episodes, acute chest syndrome, or central nervous system events or radiologic progression in the follow-up period post-SCT.
Probability of survival and serial immune reconstitution. (A) Five-year OS, 100%. (B) Five-year DFS, 92.9%. Dots depict a censored event. Recovery of CD4+ T cells (C), CD8+ T cells (D), CD19+ B cells (E), and CD16/56+ NK cells (F) presented as absolute numbers on days +100, +180, and +365. Cut offs for normal values are indicated with horizontal dashed lines.
Probability of survival and serial immune reconstitution. (A) Five-year OS, 100%. (B) Five-year DFS, 92.9%. Dots depict a censored event. Recovery of CD4+ T cells (C), CD8+ T cells (D), CD19+ B cells (E), and CD16/56+ NK cells (F) presented as absolute numbers on days +100, +180, and +365. Cut offs for normal values are indicated with horizontal dashed lines.
The incidence of grade II to IV aGVHD at day +100 was 28.6%. Three patients developed grade 2 aGVHD involving the skin, and were treated with topical therapy. One developed grade 4 GVHD of the gut, and required systemic therapy until resolution. All patients continued GVHD prophylaxis as dictated by the trial. Seven patients developed localized skin rash or oral plaques in the first year that responded to topical therapy. Systemic immune suppression wean was commenced 9 to 12 months post-SCT successfully per protocol. Only 1 of 9 patients who received URD bone marrow and abatacept doses beyond day +100 developed extensive cGVHD affecting the lung; that patient continues systemic immune suppression. One patient developed PRES that was self-limiting, in contrast to 34% reported previously with URD SCT.3
The sharp decrease in complications is attributed to changes in GVHD prophylaxis (tacrolimus weaned 9-12 months post-SCT, deletion of corticosteroid prophylaxis, and addition of abatacept). GVHD prophylaxis was modified on the basis of advantages provided by abatacept’s mechanism of action and low toxicity in primate models and subsequent clinical trials in malignant disorders.12,14 The continued risk of cGVHD in the first 2 participants in this trial prompted an amendment to extend costimulation blockade with abatacept to 1 year posttransplant in SCD patients receiving bone marrow products, though discontinued on day +100 following cord blood transplants. Another trial is exploring the benefits of intermediate-duration abatacept on the risk of GVHD in SCD SCT.22 If successful, this strategy of GVHD prophylaxis avoids exposure to chemotherapy-based prophylaxis such as high dose post-SCT cyclophosphamide.23
Abatacept did not hinder donor engraftment. T-cell subsets gradually recovered after day +100 to the normal range by 1 year (Figure 1C-F). The pace of immune reconstitution was robust after 6 months despite the use of abatacept. Asymptomatic CMV reactivation, noted in 8 patients prior to day +180, responded to antiviral therapy and was concomitant with graft rejection in 1 patient. Thus, susceptible patients (recipient seropositive) were at high-risk for early CMV reactivation during the most severe period of immune incompetence and could benefit from prophylaxis if at risk.24 EBV replication noted in 1 patient was asymptomatic and treated with 2 doses of rituximab. One was treated with 2 doses of rituximab, and the other received no treatment. EBV replication subsided in both without symptoms. Additional infections in single patients included adenovirus viremia, BK viremia, influenza, Salmonella enteritis, line infection with Fusarium, and methicillin-resistant Staphylococcus aureus (Table 2); all responded to treatment. The low GVHD incidence that successfully allowed taper of systemic immune suppression (Table 1) likely enhanced immune reconstitution and recovery from infectious complications. Though infection risks seemed to abate after the initial few months post-SCT, continued monitoring is prudent until immune suppression is discontinued.
Etiology and timing of infections posttransplant
| Infection type . | Infections recorded . | ||
|---|---|---|---|
| 0-100 d posttransplant . | 101-180 d posttransplant . | 181-365 d posttransplant . | |
| Bacterial | Pantoea (1) | Klebsiella (1) | |
| Acinetobacter (1) | Staphylococcus (1) | ||
| Staphylococcus (1) | |||
| Salmonella (1)* | |||
| Clostridium (1) | |||
| Fungal | Candida (1) | Fusarium (1) | |
| Viral | CMV | Adenovirus (1) | Influenza (2) |
| Reactivation (7) | Parainfluenza (2) | Parainfluenza (1) | |
| BK virus (1) | EBV reactivation (2) | ||
| Adenovirus (1) | Influenza (1) | ||
| CMV reactivation (2) | |||
| Infection type . | Infections recorded . | ||
|---|---|---|---|
| 0-100 d posttransplant . | 101-180 d posttransplant . | 181-365 d posttransplant . | |
| Bacterial | Pantoea (1) | Klebsiella (1) | |
| Acinetobacter (1) | Staphylococcus (1) | ||
| Staphylococcus (1) | |||
| Salmonella (1)* | |||
| Clostridium (1) | |||
| Fungal | Candida (1) | Fusarium (1) | |
| Viral | CMV | Adenovirus (1) | Influenza (2) |
| Reactivation (7) | Parainfluenza (2) | Parainfluenza (1) | |
| BK virus (1) | EBV reactivation (2) | ||
| Adenovirus (1) | Influenza (1) | ||
| CMV reactivation (2) | |||
The numbers in parentheses indicate number of patients with the infection.
Except for Salmonella enteritis, the other bacterial infections were bacteremias diagnosed by blood culture.
The most frequent organ toxicity noted was renal dysfunction (n = 3), which resolved with weaning calcineurin inhibitors. One patient developed pure red aplasia that was treated with immunoglobulin, bortezomib, and prednisolone, with resolution. One patient required cholecystectomy for cholelithiasis. No patient had SCD-related symptoms post-SCT.
Our preliminary results suggest that this RIC regimen combined with abatacept-inclusive GVHD prophylaxis protects against transplant complications that contribute to morbidity/mortality after URD SCT. These results support further study of URD SCT in clinical trial settings. Phase 2 trials of URD SCT, adding abatacept to standard GVHD prophylaxis are underway. Safely expanding the donor pool so SCD patients can benefit from curative intervention with minimal treatment-related morbidity remains a goal for hematology and transplant teams.
Study protocol synopsis and individual participant data (after deidentification) that underlies the result reported in this article will be made available on request to the corresponding author, Alexander Ngwube (e-mail: angwube@phoenixchildrens.com), after the publication of this manuscript.
Acknowledgments
The authors thank research coordinators Lisa Murray, Kara Kronemeyer, and Alexa Yarish, who collected the data for the trial.
The Children’s Discovery Institute, Washington University, and St. Louis Children’s Hospital (St. Louis, MO) provided support for the trial.
Authorship
Contribution: A.N., N.S., and S.S. designed the trial, analyzed the data, and drafted the manuscript; and all authors enrolled patients, collected data, critically reviewed, and approved the final manuscript.
Conflict-of-interest disclosure: M.L.H. has a spouse employed by Pfizer, Inc.; receives research funding from Global Blood Therapeutics; and has a consultancy with bluebird bio. The remaining authors declare no competing financial interests.
Correspondence: Alexander Ngwube, Center for Cancer and Blood Disorder, Phoenix Children’s Hospital, 1919 East Thomas Rd, Phoenix, AZ 85016; e-mail: angwube@phoenixchildrens.com.