Key Points
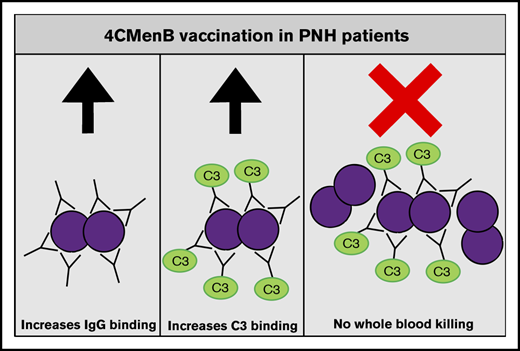
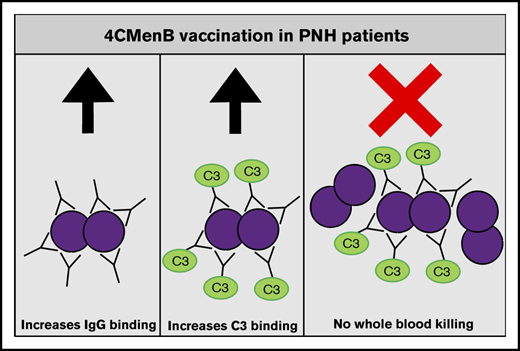
4CMenB vaccination induces MenB-specific IgG levels that are able to initiate complement activation similarly to healthy controls.
Despite vaccine-induced MenB-specific IgG levels and complement C3 deposition, eculizumab impairs whole blood killing of MenB.
Abstract
Complement C5 inhibitor eculizumab has a great impact on the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH). However, this treatment success has a major drawback: a substantially increased susceptibility for life-threatening Neisseria meningitidis infections. Therefore, N meningitidis vaccination is strongly advised before initiating complement C5–blocking therapy. In this study, we show that the multicomponent N meningitidis serogroup B (4CMenB) vaccination of PNH patients treated with eculizumab results in a significant increase in anti–N meningitidis serogroup B (MenB) plasma immunoglobulin G (IgG) levels. Anti-MenB IgG was able to bind to the bacterial surface and initiate complement activation; however, inhibition of the membrane attack complex formation completely blocked whole blood–mediated killing of MenB. This would suggest that, despite 4CMenB vaccination, PNH patients taking C5 inhibitors are not sufficiently protected against MenB infection, which is in line with the fact that vaccinated PNH patients still experience meningococcal infections.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare clonal hematopoietic stem cell disorder that is characterized by hemolytic anemia due to uncontrolled complement activation, leading to lysis of erythrocytes. Humanized monoclonal antibodies eculizumab and ravulizumab block C5 cleavage into C5a and C5b, which prevents the formation of membrane attack complex (MAC), thereby blocking complement-mediated hemolysis.1-3 A major risk for the use of complement C5 inhibitors is increased susceptibility for Neisseria meningitidis infections4 ; therefore, N meningitidis serogroup ACWY vaccination and, when available, N meningitidis serogroup B (MenB) vaccination is strongly advised.5-7
Beginning in July of 2018, we started offering the multicomponent N meningitidis serogroup B (4CMenB) vaccination for all PNH patients treated with complement C5 inhibitors. However, a recent study showed that eculizumab eliminated vaccine-induced complement-mediated protection (ie, opsonophagocytic killing) in in vitro experiments using whole blood from 4CMenB-vaccinated healthy individuals.8 In this study, we determined the effects of 4CMenB vaccination of PNH patients treated with eculizumab on the MenB-specific immunoglobulin G (IgG) levels in plasma, the initiation of complement activation, and whole blood–mediated killing of MenB.
Methods
Study design
Sixty-three patients with PNH, and who were taking complement C5 inhibitors, were vaccinated with 4CMenB using a 2-dose schedule with a 12-week interval (Figure 1A). Eight patients were excluded because they did not sign informed consent for research, and 9 patients were excluded because they used other C5 inhibitors in the context of clinical trials. Blood was collected before the first vaccination, before the second vaccination, and 12 weeks postvaccination (Figure 1A). Three patients were excluded because blood collection was not performed at all time points. In total, 43 patients were included in this study; their characteristics are summarized in Table 1.
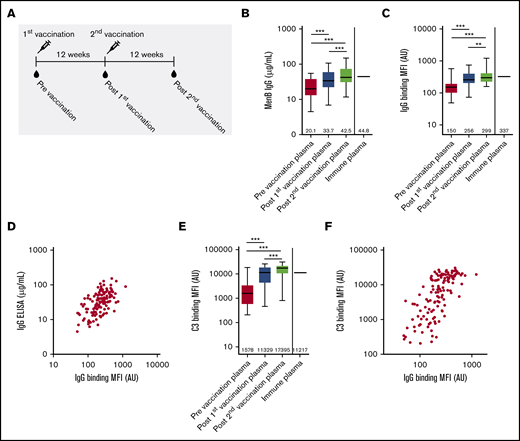
Characterization of functional antibody responses induced by 4CMenB vaccination. (A) Schematic representation of vaccinations and blood draws. MenB IgG level (B), IgG binding to the bacterial surface of MenB (C), and complement C3 binding to the bacterial surface of MenB (D) using PNH patient plasma from before and after the first and second 4CMenB vaccinations and immune plasma from healthy individuals. Figures show boxplots with minimum/maximum whiskers; horizontal lines and values indicated in the figures are medians. Spearman correlation for IgG binding to the bacterial surface with MenB IgG concentration in serum (r = +0.5865; P < .0001) (E) and C3 binding to the bacterial surface (r = +0.7217; P < .0001) (F). Statistical analyses were performed with Prism version 5.03 for Windows (GraphPad Software, La Jolla, CA). Repeated measures analysis of variance with Tukey’s posttest was used to determine statistical significance. **P < .01, ***P < .001. AU, arbitrary unit; MFI, median fluorescence intensity.
Characterization of functional antibody responses induced by 4CMenB vaccination. (A) Schematic representation of vaccinations and blood draws. MenB IgG level (B), IgG binding to the bacterial surface of MenB (C), and complement C3 binding to the bacterial surface of MenB (D) using PNH patient plasma from before and after the first and second 4CMenB vaccinations and immune plasma from healthy individuals. Figures show boxplots with minimum/maximum whiskers; horizontal lines and values indicated in the figures are medians. Spearman correlation for IgG binding to the bacterial surface with MenB IgG concentration in serum (r = +0.5865; P < .0001) (E) and C3 binding to the bacterial surface (r = +0.7217; P < .0001) (F). Statistical analyses were performed with Prism version 5.03 for Windows (GraphPad Software, La Jolla, CA). Repeated measures analysis of variance with Tukey’s posttest was used to determine statistical significance. **P < .01, ***P < .001. AU, arbitrary unit; MFI, median fluorescence intensity.
Ethics
The collection of plasma from patients was part of the protocol Biobank Hematology, which was approved by the ethics committee of Radboudumc (#2013-064). Collection of blood was performed for routine clinical diagnostics. Written informed consent was obtained from healthy volunteers, and the experimental guidelines of the ethics committee of Radboudumc were observed. All experiments were carried out in accordance with local guidelines and regulations and complied with the Declaration of Helsinki and Good Clinical Practice guidelines.
Bacterial strain
Plasma collection
Blood was collected in Hirudin blood collection tubes (Roche) and immediately put on ice. Plasma was collected by pelleting the cells for 10 minutes at 1000g, followed by a second centrifugation at 16 100g. Plasma was stored at −80°C within 2 hours of blood collection. Immune serum was made by pooling post-4CMenB vaccination plasma from 3 vaccinated healthy individuals.
N meningitidis IgG ELISA
Enzyme-linked immunosorbent assay (ELISA) was performed, as described previously,11 with some minor modifications. MenB was grown in TSB to an optical density at 620 nm (OD620) = 0.5. Bacteria were washed with phosphate-buffered saline (PBS) and suspended in PBS to OD620 = 0.1. Bacteria were heat killed for 30 minutes at 56°C, and MaxiSorp 96-well plates (BD Biosciences) were coated with 100 µL heat killed bacteria per well and dried overnight at 37°C. The following day, plates were blocked with 200 µL of PBS + 2% bovine serum albumin (BSA) at room temperature for 2 hours. Plates were washed 5 times with PBS + 0.1% Tween 20. Plasma was diluted 100-fold, 500-fold, or 25 000-fold in PBS + 2% BSA, and 100 μL was added to the plates and incubated for 1 h at 37°C. Plates were washed 5 times with PBS + 0.1% Tween 20. Alkaline phosphatase–conjugated mouse anti-human IgG was diluted 1000-fold in PBS with 2% BSA, and 100 µL was added to the plates and incubated at room temperature for 1 hour. Alkaline phosphatase activity was detected by the addition of 1 mg/mL p-Nitrophenyl Phosphate (Calbiochem) in 10 mM diethanolamine, pH 9.5 containing 500 µM MgCl2 buffer for 20 minutes and subsequently read on an ELISA microplate reader at 405 nm and 690 nm. A human IgG standard was used as reference.
Serum bactericidal activity assay
Plasma was heat inactivated (HI) for 30 minutes at 56°C and diluted in PBS in a twofold series from 1:2 to 1:256. MenB was grown in TSB to OD620 = 0.23 and diluted 100-fold in PBS. Ten microliters of bacteria was mixed with 20 µL of HI plasma. Lastly, 10 µL of pooled human serum was added as a complement source, and the samples were incubated for 60 minutes at 37°C. Colony-forming units (CFU) were counted by plating 10 µL of suspension on GC Agar plates with IsoVitaleX and incubating overnight at 37°C. SBA titers were based on the initial serum dilution that showed killing of ≥90% of colonies compared with colonies surviving in serum from a healthy unvaccinated individual with no bactericidal activity.
Flow cytometry
MenB was grown in TSB to OD620 ∼ 0.3. Bacteria were washed with Hanks balanced salt solution (HBSS) + Ca2+/Mg2+ + 0.1% gelatin (HBSS3+) and suspended in HBSS3+ to OD620 = 0.1. Twenty-five microliters of bacteria was mixed with 25 µL of 10% plasma (C3) or 25 µL of 10% HI plasma (IgG) diluted in HBSS3+ and incubated for 30 minutes at 37°C with 5% CO2. Bacteria were pelleted by centrifugation at 3200g, and supernatant was removed by decanting. Bacteria were fixed in 100 µL of 2% paraformaldehyde in PBS for 20 minutes at room temperature. Bacteria were pelleted by centrifuging at 3200g for 5 minutes, and supernatant was removed by decanting. All antibody incubations were performed for 15 minutes at room temperature in 50 µL of PBS + 2% BSA. Surface-bound complement C3 was detected with 1:500-diluted FITC-labeled polyclonal goat anti-human C3 (MP Biomedicals). Surface-bound IgG was detected with 1:500-diluted Fcγ fragment–specific PE-labeled AffiniPure Goat anti-Human IgG (Jackson ImmunoResearch). Surface binding of C3 and IgG was determined by flow cytometry using a BD LSR II instrument (BD Biosciences) and expressed as mean fluorescence intensity in arbitrary units (AU). Data were analyzed using FlowJo version 10.4.1. Flow cytometry was repeated 3 times, and the average IgG binding was calculated.
Whole blood–killing assay
MenB was thawed and washed with PBS. Blood from healthy donors was collected in Hirudin blood collection tubes (Roche). Cells were pelleted by centrifuging at 1000g for 10 minutes, and plasma was removed. Cells were washed by suspending them in PBS and pelleted by centrifuging at 1000g for 10 minutes; PBS was removed. Bacteria were added to the blood (∼2 × 106 CFU/mL); 50 µL of blood was mixed with 50 µL of 10% plasma or 10% HI plasma diluted in HBSS3+ and incubated for 60 minutes at 37°C with 5% CO2. After incubation, 10-fold dilutions were made, and 2 droplets of 10 µL of each dilution was plated on GC Agar plates and incubated overnight at 37°C with 5% CO2. The CFU count was determined the following morning. MenB survival percentage was determined as numbers of CFU after exposure to 10% plasma/CFU after exposure to 10% HI plasma. Whole blood killing assay was repeated 3 times for all plasma samples, and the average survival (percentage) was calculated.
Results
Effective 4CMenB-vaccination responses in PNH patients using eculizumab
Anti-MenB plasma IgG level increased from 20.1 µg/mL to 33.7 and 42.5 µg/mL after the first and second 4CMenB vaccinations, respectively (Figure 1B). Postvaccination anti-MenB plasma IgG levels in PNH patients were similar to those found for pooled immune plasma made from 3 healthy individuals vaccinated with 4CMenB (Figure 1B). MenB-specific IgG binding to the bacterial surface was determined by flow cytometry and increased from 150 AU to 256 AU and 299 AU after the first and second 4CMenB vaccinations, respectively (Figure 1C). Binding of MenB-specific IgG postvaccination of PNH patients was similar to that found for pooled immune plasma made from 3 healthy individuals vaccinated with 4CMenB (Figure 1C). Total MenB-specific IgG antibody level correlated significantly with the binding of anti-MenB IgG to the bacterial surface (Figure 1D), indicating that the increased level in plasma increased binding to the bacterial surface of MenB. MenB-specific antibodies were detected in prevaccination plasma, which might indicate previous exposure or cross-reactivity with other Neisseria species.
Binding of complement C3 to the bacterial surface of MenB increased significantly from 1578 AU to 11 329 AU and 17 395 AU after the first and second 4CMenB vaccinations, respectively (Figure 1E). Binding of complement C3 postvaccination of PNH patients was similar to that found for pooled immune plasma made from 3 healthy individuals vaccinated with 4CMenB (Figure 1D). Binding of complement C3 to the bacterial surface correlated significantly with the binding of IgG to the bacterial surface (Figure 1F), indicating that 4CMenB vaccine–induced IgG levels increased complement activation on the bacterial surface of MenB.
PNH patients using eculizumab show no 4CMenB vaccination–induced protection in a whole blood–killing assay
The correlate of protection against MenB is serum bactericidal antibody (SBA) activity using exogenous pooled human serum as complement source.12 However, the presence of eculizumab in plasma from PNH patients inhibited complement activity of exogenous pooled human serum in the SBA assay; therefore, no SBA titers could be determined because no killing was observed (data not shown).
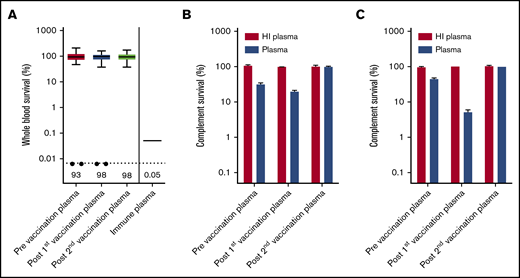
Eculizumab inhibits formation of MAC by preventing C5 cleavage into C5a and C5b, but binding of IgG and complement C3 to the bacterial surface could initiate opsonophagocytosis in whole blood.13 In a recent study, we showed that patients with C8 deficiency, who are not able to form an effective MAC, showed 4CMenB-induced killing in whole blood.14 However, no whole blood killing was observed with plasma from PNH patients taken before and after 4CMenB vaccination, whereas survival was only 0.05% with immune plasma from 4CMenB-vaccinated healthy individuals (Figure 2A). Killing of MenB was observed in 2 patients (Patient 1 and Patient 54); however, this was abrogated after subjecting plasma to heat inactivation, indicating that this killing was complement dependent (Figure 2B-C). It is possible that the eculizumab trough concentration (Ctrough) was not sufficient to completely block C5 cleavage in these patients. Therefore, we conclude that, despite effective IgG and complement C3 opsonization of MenB, plasma from PNH patients taking eculizumab does not result in opsonophagocytic-mediated killing.
Determining opsonophagocytic response induced by 4CMenB vaccination. (A) Whole blood-mediated killing of MenB using PNH patient plasma from before and after the first and second 4CMenB vaccinations and immune plasma from healthy individuals. Figure shows boxplots with minimum/maximum whiskers; horizontal lines and values indicated in the figure are medians. Statistical analyses were performed with Prism version 5.03 for Windows. Repeated measures analysis of variance with Tukey’s posttest was used to determine statistical significance. No statistically significant differences were found. The filled circles represent killing of MenB with plasma from patient 1 and patient 54. Complement survival of MenB in HI plasma and normal plasma taken before and after the first and second 4CMenB vaccinations: Patient 1 (B) and Patient 54 (C).
Determining opsonophagocytic response induced by 4CMenB vaccination. (A) Whole blood-mediated killing of MenB using PNH patient plasma from before and after the first and second 4CMenB vaccinations and immune plasma from healthy individuals. Figure shows boxplots with minimum/maximum whiskers; horizontal lines and values indicated in the figure are medians. Statistical analyses were performed with Prism version 5.03 for Windows. Repeated measures analysis of variance with Tukey’s posttest was used to determine statistical significance. No statistically significant differences were found. The filled circles represent killing of MenB with plasma from patient 1 and patient 54. Complement survival of MenB in HI plasma and normal plasma taken before and after the first and second 4CMenB vaccinations: Patient 1 (B) and Patient 54 (C).
Discussion
Using a whole blood killing assay to address opsonophagocytic killing of MenB, we did not observe 4CMenB vaccine–induced protection in vitro, despite IgG and complement C3 opsonization. These results corroborate the results from in vitro experiments described by Konar and Granoff, who showed that eculizumab eliminated killing in whole blood from 4CMenB-vaccinated healthy individuals.8 It is important to note that we used 10% plasma in our whole blood–killing assay vs undiluted plasma that was used by Konar and Granoff.8 Despite this lower plasma concentration, effective killing of MenB was observed with immune plasma from 4CMenB-vaccinated healthy individuals, indicating that 10% plasma can be effective in killing MenB.
However, our results are in contrast with patients with C8 deficiency, who cannot form MAC, but did show killing of MenB in whole blood–killing experiments.14 The release of C5a following cleavage of complement C5a is lacking in patients taking complement C5 inhibitors in comparison with patients with C8 deficiency. Blocking C5a receptor signaling abrogated phagocytosis of Escherichia coli in whole blood and reduced the ability of neutrophils to generate reactive oxygen species.15 In addition, blocking complement activation or C5a receptor signaling reduced the uptake of MenB by neutrophils.16 Therefore, the lack of C5a generation in the whole blood–killing assay might contribute to the inability to kill MenB, as observed in PNH patients.
Despite the lack of whole blood–mediated killing of MenB after 4CMenB vaccination of PNH patients, it is important to note that vaccination might prevent N meningitidis infection through other immune mechanisms that we have not addressed in this study. For instance, MenB outer membrane vesicle vaccination was shown to increase proliferative T-cell responses17,18 ; however their contribution to 4CMenB vaccine–induced protection is not known. During infections, breakthrough hemolysis due to increased complement activity has been observed,19 which, in the context of a vaccinated individual, might initiate killing of MenB.
Because there is no solid evidence that PNH patients using C5 inhibitors are protected against MenB infections after 4CMenB vaccination, patients and physicians still need to be aware of increased susceptibility to severe invasive infections.20 The US Food and Drug Administration recommends the use of prophylactic antibiotics until 2 weeks after vaccination.6 The European Society of Clinical Microbiology and Infectious Diseases recommends prophylactic antibiotics for 4 weeks following vaccination.21 We offer antibiotic prophylaxis for 2 weeks according to the US Food and Drug Administration recommendation and prescribe antibiotics for immediate administration at home upon start of fever; however, these directives differ per country. In a recent abstract for the 61st ASH Annual Meeting, Patriquin et al showed that rates of meningococcal infections in patients with or without prophylactic antibiotic use were similar, although the number of patients (n = 7) was limited.22 Altogether, it appears that vaccination and antibiotic prophylaxis do not prevent all cases of meningococcal disease in PNH patients taking eculizumab; heightened awareness and seeking early care remain essential.
This study has a few limitations. We did not include a control group of 4CMenB-vaccinated healthy individuals; however, to compare PNH patients with healthy individuals, we included pooled immune plasma from 4CMenB-vaccinated healthy individuals. Based on these data, anti-MenB plasma IgG levels and binding of IgG and C3 to the bacterial surface with plasma from 4CMenB-vaccinated PNH patients were similar to the levels found for immune plasma from 4CMenB-vaccinated healthy individuals. Another limitation is that all experiments were performed with strain H44/76, from which only 1 of the vaccine antigens, factor H binding protein, was derived.10 Therefore, IgG levels, complement deposition, and killing might not be representative of the complete immunological response to N meningitidis after 4CMenB vaccination. However, this does not change our conclusion, because whole blood killing in the presence of MenB-specific IgG showed effective killing of strain H44/76 with pooled immune plasma made from 3 healthy individuals vaccinated with 4CMenB, as well as in previous studies.8,14
In conclusion, we show that PNH patients taking eculizumab have 4CMenB vaccine–induced levels of anti-MenB plasma IgG that are able to bind to the bacterial surface and initiate complement activation. However, no increase in whole blood–mediated killing was observed after 4CMenB vaccination. Therefore, despite 4CMenB vaccination, patients and physicians need to be aware that there is no solid evidence that PNH patients taking C5 inhibitors are protected against MenB infections after 4CMenB vaccination.
Data sharing requests should be sent to Jeroen D. Langereis (jeroen.langereis@radboudumc.nl).
Acknowledgment
The authors thank all patients who participated in this study.
Authorship
Contribution: J.D.L., M.I.d.J., and S.L. conceived the study; S.F. collected patient materials; J.D.L. and B.v.d.B. designed, performed, and analyzed laboratory experiments; J.D.L. wrote the first draft of the manuscript; and all authors reviewed and edited the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeroen D. Langereis, Section of Pediatric Infectious Diseases, Laboratory of Medical Immunology, Radboudumc, P.O. Box 9101, 6500HB Nijmegen, The Netherlands; e-mail: jeroen.langereis@radboudumc.nl.