Key Points
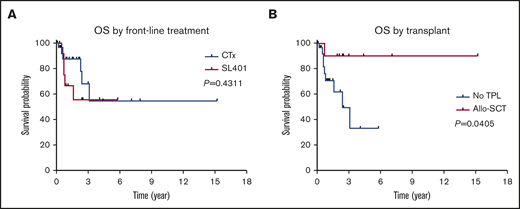
There was no significant OS difference between BPDCN patients treated with SL-401 vs other chemotherapies as their first-line treatment.
Patients who received an allo-SCT had significantly longer OS.
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy with dismal clinical outcomes. Conventional chemotherapies such cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with high-dose cytarabine and methotrexate (CVAD) have been commonly used for the BPDCN treatment until a recent study showed promising outcomes in patients treated with SL-401 (Tagraxofusp). In this single-institution retrospective study, we identified a total of 49 consecutive BPDCN patients. Among 42 patients who received treatment, hyper-CVAD regimen was associated with higher complete response rate compared with CHOP-based regimens or SL-401 (91% vs 50% vs 50%), although the difference did not achieve statistical significance. Furthermore, there was no significant overall survival (OS) difference between patients treated with SL-401 vs other chemotherapies as their first-line treatment (hazard ratio = 1.597; 95% CI, 0.460-5.548; P = .431). Of note, patients who received allogeneic stem cell transplant (allo-SCT) had significantly longer OS (hazard ratio = 0.160; 95% CI, 0.0453-0.56; P = .041). Extent of disease (skin vs bone marrow vs both) or younger age (<60 years old) did not have significant prognostic impact on OS. Collectively, our study confirmed the survival benefit of allo-SCT and suggests that conventional and intensive chemotherapies such as CHOP and hyper-CVAD as well as SL-401 would be comparable first-line choice for the BPDCN patients.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy with an aggressive disease course and dismal clinical outcome.1-3 Although genomic studies have demonstrated similarity in somatic mutation profiles between BPDCN and other malignancies such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML),4 recent studies have shown that BPDCN cells originate from plasmacytoid dendritic cells or their precursors.5,6 BPDCN is characterized by common involvement of skin, bone marrow (BM), lymph node (LN), spleen, and other extramedullary organs and by its unique immunophenotype that is universally positive for CD4, CD56, and CD123; frequently positive for CD36, CD38, CD43, CD45RA, TCL1, TCF4, CD303/BDCA-2, and TdT; and negative for CD34, CD3, CD13, CD16, CD19, CD20, lysozyme, and MPO.7 Of note, there is a small subset of cases showing CD4 or CD56 negativity; however, it should not be excluded when 2 or more PDC-associated markers (CD123, TCL1, BDCA2, CD2AP, TCF4) are expressed.8 Accordingly, BPDCN is classified as an independent category from MDS, AML, and chronic myelomonocytic leukemia (CMML) based on 2016 World Health Organization (WHO) classification.9 Conventional chemotherapies such as cyclophosphamide, doxorubicin hydrochloride, oncovin, and prednisone (CHOP); hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with high dose cytarabine and methotrexate (hyper-CVAD); and 7+3 (cytarabine, anthracycline) that are commonly used for non-Hodgkin lymphoma, acute lymphoblastic leukemia, or AML were previously shown to have some clinical efficacy; however, long-term outcomes of BPDCN patients treated with these regimens remain poor.3,10,11 Although allogeneic hematopoietic stem cell transplantation (allo-SCT) was shown to be associated with significant survival improvement, especially in those allografted in their first complete remission,10,12 this approach is very limited given that the median age of BPDCN diagnosis is ∼70 years, and thus many BPDCN patients are not eligible for a transplant.
Previous studies have shown that SL-401 (tagraxofusp), a CD123-directed cytotoxin consisting of recombinant human interleukin-3 fused to a truncated diphtheria toxin, has promising anti-BPDCN activity.13-15 In a recent clinical trial, SL-401 demonstrated an overall response rate of 90% in previously untreated BPDCN patients.16 Despite promising outcomes observed in SL-401 studies, there remains a lack of data regarding the optimal first-line therapy. In this single-institution retrospective study, we explored the survival outcomes based on front-line treatment and allo-SCT. Furthermore, we investigated other potential prognostic factors in BPDCN.
Materials and methods
Patient population
This study was approved by the institutional review board and Scientific Review Committee. Eligible patients were age 18 years and older with pathologic diagnosis of BPDCN based on WHO classification.9 Study subjects were retrospectively identified at H. Lee Moffitt Cancer Center database from January 2001 to December 2019 by performing a computer search with diagnostic names that include “blastic NK-cell lymphoma,” “blastic NK-cell leukemia,” “NK-cell lymphoma,” “NK-cell leukemia,” “agranular CD4+ NK-cell leukemia,” “CD4+/CD56+ hematodermic neoplasm,” “blastic plasmacytoid dendritic cell neoplasm,”, and “BPDCN.” The original slides from BM or/and skin biopsy samples including the corresponding flow cytometry and immunohistochemical stain results were individually reviewed by experienced hematopathologists (L.Z. and A.I.) to screen cases that meet the morphologic and immunophenotypic criteria of BPDCN diagnosis (supplemental Table 1). Aggressive NK cell leukemias and extranodal NK cell lymphomas according to WHO criteria were excluded.9 Additional immunohistochemical stains for TCL1 and TCF4 were performed on a small subset of cases when the diagnosis of BPDCN was equivocal. Treatment regimens were captured for all patients and included CHOP-based therapy, hyper-CVAD, AML induction chemotherapy, SL-401, radiation therapy, stem cell transplants, and other modalities. At our cancer center, either allo-SCT or autologous stem cell transplant (auto-SCT) has been consistently recommended for the eligible patients in their complete response (CR) or partial response (PR) status regardless of their first-line or salvage regimen. Other clinical variables including age, sex, cytogenetics, mutation profile, prior and/or concurrent hematologic malignancies were captured at the time of diagnosis.
Conventional karyotyping/fluorescence in situ hybridization
Karyotyping was assessed by routine cytogenetic analysis using standard trypsin-Giemsa banding technique by Laboratory Corporation of America (Burlington, NC) on all patients with sufficient BM aspirate specimen and results were reported in accordance with an International System for Human Cytogenetic Nomenclature, 2016 (Karger). Fluorescence in situ hybridization (FISH) was routinely conducted with probe sets designed for MDS [del(5q)/-5, del(7q)/-7, +8, del(17p)/-17, and del(20q)/-20] based on the manufacturer’s instructions (Vysis, Downers Grove, IL).
Next-generation sequencing and mutation analysis
Next-generation sequencing (NGS) was performed as previously described.17 The NGS panel used in our study encompasses a total of 54 genes. Sequencing was performed using an Illumina NextSeq 500 sequencer (Illumina, San Diego, CA). For our pathogenic vs nonpathogenic call algorithm, we used a modification of the American College of Medical Genetics and Genomics classification scheme that was developed for germline variants for the classification of somatic sequence variants.18 Variants were selected if they included a read depth of greater than 500×, a quality score >30, and variants of allele frequency of 5% or greater.
Statistical analysis
The primary end point of the current study was overall survival (OS) and the secondary end points were treatment response and progression-free survival (PFS) by front-line therapy (chemotherapy vs SL-401). OS was defined as the time from BPDCN diagnosis to the date of death or last follow-up. Kaplan-Meier method was used to estimate the PFS and OS distributions, which were compared using the log-rank test. Univariate and multivariate Cox regression models were used to assess the impact of treatment regimens (chemotherapy vs SL-401), allogeneic stem cell transplant (no transplant vs transplant), and disease involvement (skin only, BM only, both skin and BM) on OS outcomes. Response to therapy was assessed according to criteria proposed by Pemmaraju et al.16 In patients with cutaneous involvement, modified Severity Weighted Assessment Tool (mSWAT) score was used for the response of skin lesions. In patients who did not have enough information for the retrospective mSWAT scoring, the total skin examination before and at the end of treatment and repeat biopsy results of the equivocal residual skin lesions were used for the response assessment. In addition, contrast computed tomography (CT) or positron emission tomography/CT scan were used for the response evaluation of both systemic and cutaneous disease. For the statistical analyses of response assessment, Fisher’s exact test was used. Unpaired Student t test was used to compare the mean ages at diagnosis. All tests were 2-sided and P < .05 was used to declare statistical significance. Analyses were performed using GraphPad Prism (version 7.03) and SPSS (version 24.0).
Results
Patient characteristics
A total of 49 patients with confirmed diagnosis of BPDCN were included in the study (Table 1; supplemental Table 1 for immunophenotypic findings). Median age at diagnosis was 71.4 (32.0-91.4) years with a male predominance (82%) and there was no significant difference in age at diagnosis between patients treated with SL-401 vs chemotherapy (P = .0992) (supplemental Figure 1). Among these, 5 (10%) patients had preceding hematologic malignancies (CMML, n = 2; MDS, n = 2; essential thrombocythemia [ET], n = 1) and 2 of them were treated with anagrelide and decitabine for ET and CMML, respectively, before their BPDCN diagnosis. Further, 11 (22%) patients had concurrent hematologic neoplasms at the time of BPDCN diagnosis based on their BM assessment (CMML, n = 5; MDS, n = 3; AML, n = 1; PTCL, n = 1; ET, n = 1) (Table 1). For the initial assessment of disease involvement, BM biopsy was performed in 44 (90%) patients and CT (n = 22) or positron emission tomography/CT (n = 18) was performed in 40 (82%) patients at the time of diagnosis. All patients underwent full skin examination; any suspicious lesions based on image studies were biopsied except spleen. The most common site of disease involvement was skin (n = 42, 86%) followed by BM (n = 32, 65%), LN (n = 13, 27%), and nasopharynx (n = 2, 4%) with 14 (29%) and 5 (10%) patients having skin and BM involvement only, respectively (Table 1). Of note, 17 (35%) patients had both skin and BM disease and 8 (16%) had simultaneous skin, BM, and LN involvement. Splenomegaly was observed in 7 (14%) patients. Other detailed demographic profiles and clinical parameters are summarized in Table 1.
A total of 42 (86%) patients received some form of treatments and 7 (14%) opted for the best supportive care or hospice care. In the front-line setting, CHOP-based regimens and hyper-CVAD were used in 10 (20%) and 11 (22%) patients, respectively (Table 1). In 12 (24%) patients, SL-401 was used as first-line therapy. Among these patients, the median number cycles of SL-401 treatment was 6 (1-73). One patient received AML induction regimen (cladribine, cytarabine, granulocyte colony-stimulating factor) and 5 patients received radiation up-front for the cutaneous lesion. A total of 19 (39%) patients received second-line treatments: CHOP-based regimens in 2 (11%), hyper-CVAD in 5 (26%), and SL-401 in 3 (16%) patients. CHOP-based regimens included CHOP, cyclophosphamide, mitoxantrone, vincristine, prednisone (CNOP), and etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (EPOCH). Of note, 15 patients received SL-401 per trial protocol (NCT02113982); 1 of these patients transitioned to commercial product after US Food and Drug Administration approval of SL-401. Allo-SCT was performed in 10 (20%) patients and 4 (8%) patients received auto-SCT. Transplant was performed following first-line therapy in 11 patients (CR, n = 10; PR, n = 1) and second-line therapy in 3 patients (CR, n = 3). Among 21 vs 12 patients who received chemotherapy (CHOP-based regimen or hyper-CVAD) vs SL-401 as their first-line therapy, a total of 11 (52%) and 3 (25%) patients underwent transplant. Detailed treatment regimens are summarized in Table 1. The median follow-up from the initial diagnosis was 18.9 (1.4-182.1), 12.1 (4.1-48.8), and 10.5 (0.1-182.1) months for chemotherapy treated patients, SL-401 treated patients, and all patients (including patients who were not treated), respectively.
Spectrum of gene mutations and cytogenetics
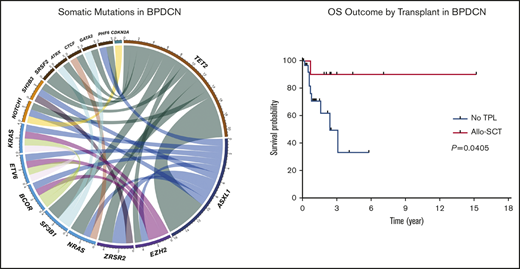
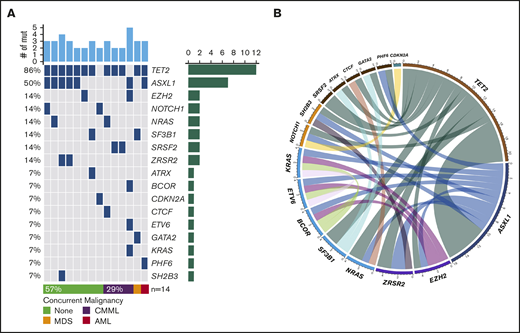
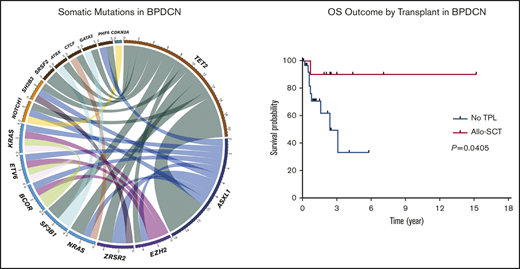
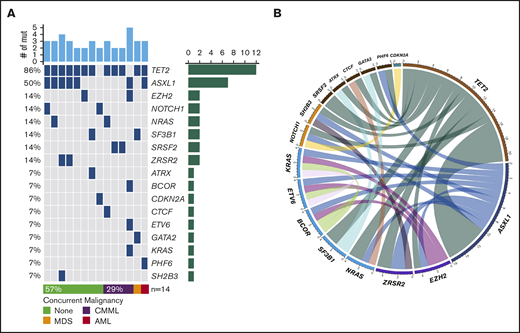
NGS assessment was performed in 14 (29%) patients using BM samples at the time of initial BPDCN diagnosis; all patients were found to have at least 2 mutations. The median number of mutations was 3 (range, 2-5). Of note, 6 of the 14 patients had concurrent myeloid neoplasms. Among somatic mutations identified, the most common mutation was TET2 (n = 12, 86%) followed by ASXL1 (n = 7, 50%), NRAS (n = 2, 14%), SRSF2 (n = 2, 14%), SF3B1 (n = 2, 14%), ZRSR2 (n = 2, 14%), NOTCH1 (n = 2, 14%), and EZH2 (n = 2, 14%). One-half of the patients with TET2 mutations also had mutated ASXL1 (Figure 1). Cytogenetic studies were performed using BM aspirate specimen and a total 29 (59%) patients had results available. The most common cytogenetic results were normal karyotype (n = 27) followed by del(7) (n = 1) and del(20q) (n = 1) based on conventional karyotyping and FISH interphase analyses.
Landscape of somatic mutations and cytogenetics. (A) Mutated genes in BPDCN patients (n = 14). Each column represents individual patient and somatic mutations are highlighted in blue in each gene (rows). Top bar plot indicates number of somatic mutations in individual patient and side bar plot indicates the number of patients who are positive for individual somatic mutation. Bottom bar represents concurrent hematologic malignancy. (B) Comutations between genes in patients with BPDCN (n = 14).
Landscape of somatic mutations and cytogenetics. (A) Mutated genes in BPDCN patients (n = 14). Each column represents individual patient and somatic mutations are highlighted in blue in each gene (rows). Top bar plot indicates number of somatic mutations in individual patient and side bar plot indicates the number of patients who are positive for individual somatic mutation. Bottom bar represents concurrent hematologic malignancy. (B) Comutations between genes in patients with BPDCN (n = 14).
Treatment response and survival outcomes
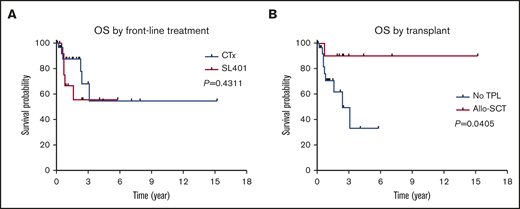
Among 42 patients who received treatment, a total of 23 (55%), 13 (31%), and 6 (14%) patients achieved a CR, PR, and progressive disease, respectively. Of note, skin disease response was assessed by mSWAT scoring in 10 patients who were treated with SL-401 and by conventional methods in 17 patients treated with CHOP-based regimens or hyper-CVAD. CR rate was higher in patients who were treated with hyper-CVAD compared with others; however, it was not statistically significant (50% in CHOP-based regimens vs 91% in hyper-CVAD, P = .064; 50% in SL-401 vs 91% in hyper-CVAD, P = .069). Other responses are summarized in Table 2. The median PFS was 9.9 months, and the median OS was not reached when all patients were included for the analyses. In the subgroup analyses, patients who were treated with hyper-CVAD had the longest PFS compared with patients treated with CHOP-based regimens (hazard ratio [HR] = 0.217; 95% CI, 0.050-0.933; P = .003) or SL-401 (HR = 0.385; 95% CI, 0.126-1.179; P = .075), although the PFS difference between hyper-CVAD and SL-401 did not reach the statistical significance. There was no PFS difference between SL-401 and CHOP-based regimen treated groups (HR = 0.931; 95% CI, 0.321-2.70; P = .894) (supplemental Figure 2A). Additional PFS analyses with censoring at the time of allo-SCT showed no statistical PFS difference between hyper-CVAD and SL-401 (HR = 0.537; 95% CI, 0.172-1.679; P = .285) or CHOP-based regimens and SL-401 (HR = 0.982; 95% CI, 0.335-2.880; P = .974) (supplemental Figure 2B). In the OS analysis based on first-line therapy, there was no difference between patients treated with SL-401 compared with patients treated with chemotherapy regimens (HR = 1.597; 95% CI, 0.460-5.548; P = .431) (Figure 2A). Further, there was no OS difference between individual types of front-line therapies (P = .678). In contrast, patients who received allo- or auto-SCT had longer OS compared with patients who did not receive transplant (HR = 0.225; 95% CI, 0.069-0.735; P = .052), although the difference did not achieve statistical significance. Additional analysis showed significantly longer OS outcomes in patients who received allo-SCT compared with no transplant (HR = 0.160; 95% CI, 0.0453-0.56; P = .041) (Figure 2B). Post-allo-SCT OS outcome was not statistically different between patients who received CHOP-based regimen or hyper-CVAD vs SL-401 as their first-line therapy (HR = 0.204; 95% CI, 0.005-8.203; P = .210) (supplemental Figure 3). We also investigated the impact of the extent of disease on OS outcome. Patients with only BM involvement showed the longest OS; however, the difference was not statistically significant (P = .588) (supplemental Figure 4A). A recent study demonstrated that age <60 years was associated with improved survival. Similar to this study, age <60 was associated with longer OS (HR = 0.481; 95% CI, 0.146-1.582; P = .258), but this was not statistically significant (supplemental Figure 4B). Additional analyses based on ASXL1 mutation (HR = 1.705; 95% CI, 0.2773-10.48; P = .5649) and presence of previous or concurrent hematologic malignancy (HR = 2.97; 95% CI, 0.65-13.57; P = .1602) did not show any difference in OS (supplemental Figure 4C-D). In a multivariate Cox model (adjusting for age, front-line therapy, sex, and transplant), stem cell transplant was significant factor for OS (HR = 0.137; 95% CI, 0.020-0.959; P = .045).
OS outcomes in BPDCN patients. OS outcomes according to front-line treatment (A) and allogeneic stem cell transplant (B).
OS outcomes in BPDCN patients. OS outcomes according to front-line treatment (A) and allogeneic stem cell transplant (B).
Discussion
Conventional and intensive chemotherapies including CHOP and hyper-CVAD have been commonly used for BPDCN treatment.10 Recent studies have shown that SL-401, a CD123-directed cytotoxin consisting of recombinant human interleukin-3 fused to a truncated diphtheria toxin, has a promising antitumor activity in BPDCN13-16 ; however, there is a lack of data regarding the optimal first-line therapy and sequence of treatments.
In our single-institution retrospective study involving 49 consecutive patients with BPDCN, we observed that hyper-CVAD is associated with a higher CR rate and longer PFS compared with CHOP-based regimens or SL-401, although there was no statistically significant PFS or OS difference between the SL-401- and hyper-CVAD-treated groups. Collectively, these observations suggest that both SL-401 and hyper-CVAD could be a reasonable option as the first-line treatment with the understanding that associate toxicities differ among these therapies.
Allo- and auto-SCT were shown to be associated with significantly improved survival outcome in the previous studies with BPDCN patients.10 Our study also showed significantly longer OS in patients who received allo-SCT compared with patients with no transplant although the survival benefit of auto-SCT and outcomes based on pretransplant disease status (CR vs non-CR) could not be concluded because of a limited number of patients. Of note, the percentage of transplanted patients was higher in the chemotherapy-treated group compared with the SL-401-treated group; this was mainly because of younger age and higher CR rates observed in the hyper-CVAD-treated patients. Although BPDCN is a disease of elderly patients with a median age of 70 years at time of diagnosis, our data along with other studies clearly suggest that allo-SCT should be strongly considered in eligible patients regardless of the initial treatment.10 The auto-SCT should be considered on case-by-case basis, considering the contradicting data based on 2 observational studies.12,19
A recent study showed that younger age (<60 years) was associated with superior survival outcomes.10 In our cohort, we also observed longer OS in younger patients (<60 years), although the difference did not achieve statistical significance. Of note, 53% (n = 8 of 15) of patients with age <60 underwent transplant, whereas only 18% (n = 6 of 34) of patients with age ≥60 received transplant. This suggests that longer OS observed in younger patients could be secondary to higher rate of transplant in this subgroup.
Previous genomic studies demonstrated that TET2, ASXL1, NRAS, KRAS, NPM1, IDH1, IDH2, and splicing factor mutations are commonly identified in BPDCN patients.20 Importantly, ZRSR2 loss-of-function mutation was identified as 1 of the most common somatic mutations (57.1%) in BPDCN patients and thought to be associated with male dominance based on its location on the X chromosome.21 Although NGS was performed in only 14 patients at the time of diagnosis in our study cohort, the somatic mutational profile was similar to other studies in general.20 However, only 2 of 14 (14%) patients were found to have ZRSR2 somatic mutations in our cohort; these results could have been confounded by higher rate (43%) of concurrent myeloid disease observed in patients who had NGS assessment. Future study is warranted to identify mutations with prognostic and/or predictive values and correlate their mutational trajectory with clinical outcomes based on serial NGS assessment.
We are aware of limitations of the present study resulting from the retrospective nature of collected data. Notably, the response criteria for the cutaneous disease have evolved over time and we were unable to apply consistent response criteria in some of the patients, which could have an impact on the treatment response outcomes. Further, we were unable to perform additional statistical analyses such as propensity analysis to properly account for the potential non-independent factors for the survival outcomes because of small number of patients and events. Therefore, our findings require cautious interpretation and need to be validated in the larger prospective studies. In addition, optimal sequence of treatments and combination strategies remain to be investigated in future studies. Meanwhile, our study supports current recommendations of using SL-401 or hyper-CVAD as the first-line treatments followed by consolidation with allo-SCT in the eligible responders to induction therapy to further improve survival outcomes in BPDCN patients.
Acknowledgments
The authors thank Lynn Moscinski, Mojdeh Naghashpour, and Raza Setoodeh for their contribution to the initial pathologic diagnosis of the cases.
This work was supported in part by a research grant from the Graduate Medical Education at the University of South Florida (S.Y. and L.Z.), a Research Training Award for Fellow from the American Society of Hematology (S.Y.), a Scholar Award from the American Society of Hematology (S.Y.), and by National Institutes of Health, National Cancer Institute K08 Grants CA237627 (S.Y.) and Moffitt Cancer Center Support Grant P30CA076292 (Q.M.).
Authorship
Contribution: S.Y., L.Z., and L.S. conceived and designed the study; S.Y., O.C., D.K., N.D.V., A.I., K.S., J.E.L., M.A.K.-D., L.Z., and L.S. collected and assembled the data; S.Y., O.C., D.K., Q.M., J.E.L., L.Z., and L.S. undertook data analysis and interpretation; and all authors participated in manuscript writing.
Conflict-of-interest disclosure: M.A.K.-D. reports a consultancy for Daiichi Sankyo. The remaining authors declare no competing financial interests.
Correspondence: Ling Zhang, Department of Hematopathology and Laboratory Medicine, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: ling.zhang@moffitt.org; and Lubomir Sokol, Department of Malignant Hematology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: lubomir.sokol@moffitt.org.
References
Author notes
L.Z. and L.S. contributed equally to this study.
For data sharing requests, send e-mail requests to the corresponding author, Lubomir Sokol, at lubomir.sokol@moffitt.org.
The full-text version of this article contains a data supplement.