Key Points
Baseline MTV on 18F fluorodeoxyglucose positron emission tomography is associated with PFS and OS in LBCL patients receiving axi-cel.
Strategies to improve outcomes in patients with high MTV should be considered.
Abstract
High metabolic tumor volume (MTV) predicts worse outcomes in lymphoma treated with chemotherapy. However, it is unknown if this holds for patients treated with axicabtagene ciloleucel (axi-cel), an anti-CD19 targeted chimeric antigen receptor T-cell therapy. The primary objective of this retrospective study was to investigate the relationship between MTV and survival (overall survival [OS] and progression-free survival [PFS]) in patients with relapsed/refractory large B-cell lymphoma (LBCL) treated with axi-cel. Secondary objectives included finding the association of MTV with response rates and toxicity. The MTV values on baseline positron emission tomography of 96 patients were calculated via manual methodology using commercial software. Based on a median MTV cutoff value of 147.5 mL in the first cohort (n = 48), patients were divided into high and low MTV groups. Median follow-up for survivors was 24.98 months (range, 10.59-51.02 months). Patients with low MTV had significantly superior OS (hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.10-0.66) and PFS (HR, 0.40; 95% CI, 0.18-0.89). Results were successfully validated in a second cohort of 48 patients with a median follow-up for survivors of 12.03 months (range, 0.89-25.74 months). Patients with low MTV were found to have superior OS (HR, 0.14; 95% CI, 0.05-0.42) and PFS (HR, 0.29; 95% CI, 0.12-0.69). In conclusion, baseline MTV is associated with OS and PFS in axi-cel recipients with LBCL.
Introduction
Large B-cell lymphoma (LBCL) patients may be cured with first-line therapy; however, up to 30% to 40% of patients may become refractory or relapse.1 Axicabtagene ciloleucel (axi-cel), a CD19 targeted chimeric antigen receptor (CAR) T-cell therapy, can successfully induce durable remissions after ≥2 lines of therapy.2,3 In the pivotal ZUMA-1 trial, high tumor burden estimated by the sum of the product of diameters (SPD) was associated with lower durable response rates at 1 year.4 Given the limitations of SPD as a measure of tumor burden, further evaluation is needed.
There is currently no standard for calculating tumor burden in lymphoma. For ZUMA-1, tumor burden was evaluated by the SPD of bidirectional measurements in ≤6 reference lesions on computed tomography (CT).5,6 Alternative methods include calculating maximum standardized uptake value (SUVmax) or baseline metabolic tumor volume (MTV) on 18F fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT.7,8 SUVmax is a semiquantitative measurement of glucose metabolism that has been associated with clinical outcomes in malignant lymphomas.7 It is based on a single pixel on the scan, which represents the maximum intensity of 18F-FDG activity in the tumor.8 Similar to SPD, SUVmax is not a direct 3-dimensional measure of tumor and only serves as an approximation of tumor burden.
MTV is of special interest, since it has the potential to capture all metabolically active, and therefore presumably malignant, areas within a tumor mass and across the body for an accurate tumor burden determination.8 It has been shown to be prognostic in non-Hodgkin’s lymphoma,9,10 with high MTV associated with worse outcomes following immunotherapy, chemotherapy, or radiation therapy.11,12 The clinical significance of baseline tumor burden as determined by MTV in CAR T-cell treated lymphoma patients, however, remains unclear. Therefore, this study aimed at assessing the relationship between baseline MTV and survival, including overall survival (OS) and progression-free survival (PFS), among axi-cel–treated patients. Evaluating the relationship between baseline MTV and response rates and toxicity was a secondary objective. Optimizing MTV calculation and comparing different tumor burden estimates were additionally investigated.
Patients and methods
Patient population
Approval for retrospective review of patient records was obtained from the institutional review board. Ninety-six patients with relapsed or refractory LBCL who received first axi-cel treatment from May 2015 to June 2019 were included. All patients had 18F-FDG PET/CT scans and clinical data. An initial cohort (cohort 1) of 48 patients was used to create the index association model constructed in 2018. A second test cohort (cohort 2) was created in 2019. Patients previously treated with CAR T-cell therapy, without measurable lesions on imaging, or without baseline PET were excluded. Elevated lactate dehydrogenase (LDH) before lymphodepleting chemotherapy was defined as LDH >2× upper limit of normal (ULN). Bridging therapy was defined as any lymphoma-specific therapy given after apheresis but prior to the start of fludarabine cyclophosphamide chemotherapy for lymphodepletion before CAR T-cell infusion. Patient characteristics data were compiled on 19 April 2020.
Tumor burden calculations
Baseline skull to midthigh with or without leg/whole-body 18F-FDG PET/CT scans obtained prior to axi-cel were evaluated for MTV using a custom tool implemented on MIM PACS version 6.8.4 (MIM Software, Cleveland, OH). Briefly, lesions with PET SUV greater than the user-selected liver reference were automatically identified, such that voxels ≥41% of SUVmax of the lesion were selected to create a metabolically active region.13 Voxels >41% SUVmax were included for calculation of lesion MTV (mL), as this was demonstrated most accurate for lymphoma.13,14 Additional details on the MTV process for automatic lesion selection are provided in supplemental Methods.
Following 18F-FDG PET/CT scan automatic marking, lesions were edited by a physician (E.A.D.) and confirmed by a radiologist (H.L. or M.S.M.). First, lesions due to physiologic FDG uptake (eg, brain or bladder) and nonmalignant lesions (eg, degenerative disk disease) were removed (MTV semiautomated). Malignant lesions erroneously omitted were then added, and individual lesion contours were adjusted via the paintbrush tools to match tumor boundary precisely (MTV manual). Bone marrow tumor infiltration was included in the calculations. Reactive marrow due to chemical or physical stimulation was excluded. Figure 1 shows an example of tumor lesion manipulation on an 18F-FDG PET/CT scan.
Example of a case of MTV calculations in a patient via the 2 investigated MTV estimation methods. PET images in the MTV semiautomated method (A) and MTV manual method (B), with tumor outlined in blue.
Example of a case of MTV calculations in a patient via the 2 investigated MTV estimation methods. PET images in the MTV semiautomated method (A) and MTV manual method (B), with tumor outlined in blue.
Available SPD (cm2) values and all SUVmax values were obtained from the clinical radiologist’s report.
Statistical analyses
MTV manual–generated values were used for analysis, given that we, a priori, believed this method would best quantify the tumor. In cohort 1, Kendall’s τ correlation coefficient was used to assess agreement in the ranked tumor burden values between tumor burden calculation techniques (MTV manual, MTV semiautomated, SPD, and SUVmax).
Clinical outcomes were determined for subjects in each patient cohort (n = 48) and compared between the high and low MTV groups. The high and low tumor volume groups were selected based on the median MTV value in cohort 1. Once the MTV cutoff was validated on cohort 2 for OS and PFS, the 2 cohorts were combined for further comparison. Differences in patient attributes were investigated using χ2 and Fisher's exact test and nonparametric test of median.
OS and PFS were calculated from the time of axi-cel infusion until death, progression, or the last date patient was known to be alive. Kaplan-Meier and log-rank test were used to assess differences in OS and PFS time estimates. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. Univariate Cox regression analysis (UA) and multivariate Cox regression analysis (MVA), adjusting for variables that showed significant association with OS and PFS in UA and variables that were clinically relevant, were used to assess the differences in OS and PFS estimates between patients with high vs low volume tumors. These variables included bridging therapy (patients who received therapy vs those who did not) and LDH status before lymphodepleting chemotherapy (LDH >2×ULN vs <2×ULN). We also conducted UA and MVA for both OS and PFS for the entire patient population.
Overall response rate (ORR) included patients with a partial response and a complete response to therapy. Complete response rate (CR) was reported if achieved by last follow-up for each cohort. For the 2 patient cohorts combined, the association of ORR and CR with MTV group (low vs high) was evaluated and odds ratio (OR) and 95% CI, were reported.
Maximum cytokine release syndrome (CRS) was graded by Lee criteria.15 Maximum neurotoxicity (NT) was graded by Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.16 Any or grade 3-4 (G3-4) CRS and any or G3-4 NT were reported in each cohort. For the cohorts combined, the incidence of toxicities among patients across MTV groups was also evaluated, and ORs with 95% CI were reported.
In all analyses, a value of P < .05 was defined as statistically significant. All statistical calculations were conducted using MedCalc Statistical Software version 19.1.5 (MedCalc Software bv, Ostend, Belgium).
Results
Patient characteristics
For all 96 subjects in this study, patient and disease characteristics at the time of axi-cel treatment were obtained (Table 1). Bridging therapy was given to 31% of patients in cohort 1 and 65% of patients in cohort 2. Notably, only cohort 1 (46%) included patients enrolled on a prospective clinical trial, which prohibited the use of bridging therapy.
Tumor burden estimation methods comparison
On baseline 18F-FDG PET/CT scan, MTV was calculated for all 48 patients in cohort 1 and 48 patients in cohort 2 by both MTV semiautomated and MTV manual. Median time between baseline imaging and axi-cel infusion was 9 days (range, 6-46 days) for cohort 1 and 11 days (range, 0-91 days) for cohort 2. Tumor burden results are presented in Table 2.
In cohort 1, comparison of tumor burden estimation methods showed that MTV manual had a positive correlation with MTV semiautomated (correlation coefficient, 0.61; 95% CI, 0.39-0.74). However, there was no agreement between MTV manual or MTV semiautomated and SPD (correlation coefficient, 0.39; 95% CI, 0.09-0.61; and correlation coefficient, 0.32; 95% CI, 0.06-0.53, respectively) or SUVmax (correlation coefficient, 0.14; 95% CI, −0.06 to 0.33; and correlation coefficient, 0.18; 95% CI, −0.04 to 0.34, respectively).
High or low tumor burden, quantified by MTV, associates with PFS and OS
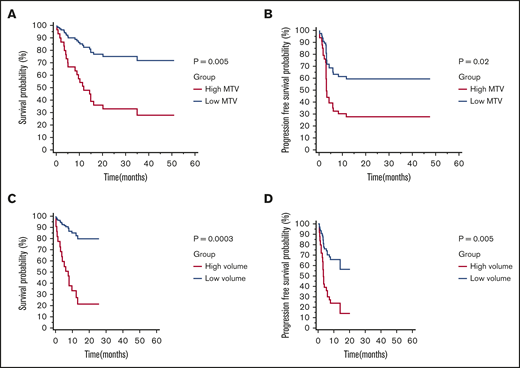
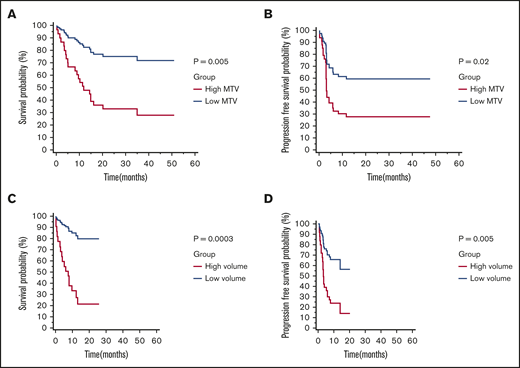
All end point analysis was performed using MTV manual values. In cohort 1, median OS was 34.98 months (95% CI, 14.33-34.98) and median PFS was 5.85 months (95% CI, 2.99-11.54). Median follow-up for survivors was 24.98 months (range, 10.59-51.02 months) (Table 1). Based on the median MTV value of 147.5 mL, patients were divided into a low (n = 24) or high (n = 24) MTV group. Patients with low MTV had superior OS (HR, 0.25; 95% CI, 0.10-0.66) and PFS (HR, 0.40; 95% CI, 0.18-0.89) (Figure 2A-B). Bridging therapy did not associate with OS (HR, 1.00; 95% CI, 0.38-2.58) or PFS (HR, 0.72; 95% CI, 0.30-1.70). Similarly elevated LDH was not associated with OS (HR, 1.45; 95% CI, 0.56-3.73) or PFS (HR, 1.04; 95% CI, 0.42-2.60).
Kaplan-Meier survival curves and log-rank Pvalues by low vs high MTV manual (cutoff 147.5 mL). OS (A) and PFS (B) for patients in cohort 1 (n = 48). OS (C) and PFS (D) for patients in cohort 2 (n = 48).
Kaplan-Meier survival curves and log-rank Pvalues by low vs high MTV manual (cutoff 147.5 mL). OS (A) and PFS (B) for patients in cohort 1 (n = 48). OS (C) and PFS (D) for patients in cohort 2 (n = 48).
However, given the potential for bridging therapy impacting tumor burden and other potential confounding variables, we performed MVA considering receipt of bridging therapy, raised LDH > 2×ULN before receiving conditioning chemotherapy, and MTV. On MVA, high MTV remained statistically significant for inferior OS (HR, 0.20; 95% CI, 0.07-0.57; P = .002) and PFS (HR, 0.30; 95% CI, 0.12-0.72; P = .007). Bridging therapy was not associated with OS (HR, 1.02; 95% CI, 0.39-2.69; P = .95) or PFS (HR, 0.78; 95% CI, 0.32-1.87; P = .58). Similarly LDH status was not associated with OS (HR, 0.60; 95% CI, 0.21-1.68; P = .33) or PFS (HR, 0.51; 95% CI, 0.18-1.43; P = .20) (Table 3).
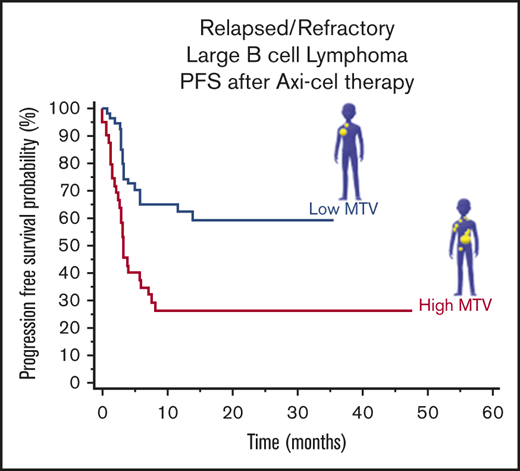
The model was then tested in cohort 2. In cohort 2, median OS was not reached, and median PFS was 13.84 months (95% CI, 3.94-13.84). Median follow-up for survivors was 12.03 months (range, 0.89-25.74 months). One patient was lost to follow-up past 1 month (Table 1). The majority of the patients (n = 30) were in the low MTV group, compared with the high MTV group (n = 18), by the previously defined cutoff (147.5 mL). Patients with low MTV values were found to have superior OS (HR, 0.14; 95% CI, 0.05-0.42) and PFS (HR, 0.29; 95% CI, 0.12-0.69) (Figure 2C-D). Bridging therapy use was not statistically significant for inferior OS (HR, 2.99; 95% CI, 0.85-10.43) or PFS (HR, 2.00; 95% CI, 0.78-5.14). However, LDH status was associated with OS (HR, 5.86; 95% CI, 2.18-15.71) and PFS (HR, 4.22; 95% CI, 1.59-11.22).
On MVA after adjusting for bridging therapy use and LDH status, high MTV remained statistically significant for inferior OS (HR, 0.18; 95% CI, 0.04-0.69; P = .01) and PFS (HR, 0.34; 95% CI, 0.12-0.96; P = .04); bridging therapy use was not statistically significant for inferior OS (HR, 0.77; 95% CI, 0.15-3.84; P = .75) or PFS (HR, 0.93; 95% CI, 0.29-2.93; P = .93). LDH status was associated with OS (HR, 3.17; 95% CI, 1.07-9.40; P = .03) and PFS (HR, 3.18; 95% CI, 1.09-9.31; P = .03) (Table 3).
We applied our model to both cohorts (n = 96) and confirmed our results (supplemental Figure 1 and supplemental Table 1). To determine if there was a group with a low likelihood of benefitting from axi-cel, we evaluated MTV by quartiles. The 2 highest quartiles had similar OS and PFS (supplemental Figure 2 and supplemental Table 2). Finally, we tested our model on a limited group of patients (n = 72) that had a more stringently defined baseline PET, and results were similar to our main analysis (supplemental Table 3).
Response rates in low vs high tumor burden patients
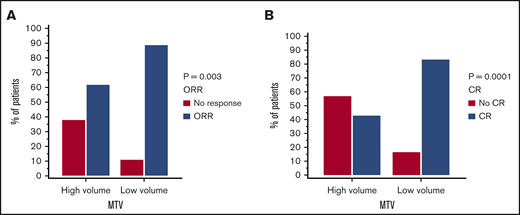
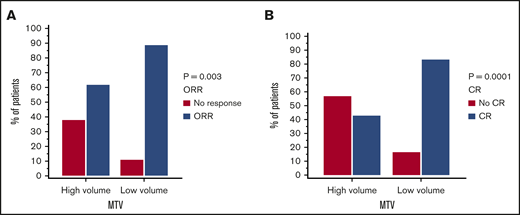
Response rates per cohort are summarized in Table 1. In the 2 cohorts combined 74 out of 96 patients had a response (77% ORR) and 63 out of 96 patients had CR (65.6%) to axi-cel. Utilizing our model in the combined cohorts, low MTV was shown to be associated with superior ORR (OR, 4.92; 95% CI, 1.71-14.10; P = .003) and CR (OR, 6.66; 95% CI, 2.60-17.08; P < .0001) (Figure 3A-B).
Response rates to axi-cel by low vs high MTV manual (cutoff 147.5 mL). ORR (A) and CR (B) for both cohorts combined (n = 96).
Response rates to axi-cel by low vs high MTV manual (cutoff 147.5 mL). ORR (A) and CR (B) for both cohorts combined (n = 96).
Toxicity in low vs high tumor burden patients
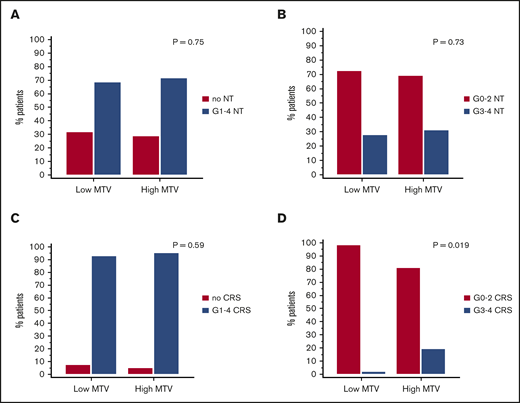
Toxicity rates per cohort are summarized in Table 1. In the 2 cohorts combined, 67 out of 96 patients (69.7%) had any-grade NT, 28 out of 96 patients (29.1%) had G3-4 NT, 90 out of 96 patients (93.7%) had any-grade CRS, and 9 out of 96 patients (9.3%) had G3-4 CRS. No G5 NT or CRS were observed in any patients. Applying our model to the combined cohorts, high MTV was not associated with any-grade NT or G3-4 NT (OR, 1.14; 95% CI, 0.47-2.77; P = .75; and OR, 1.16; 95% CI, 0.48-2.82, P = .73, respectively) (Figure 4A-B), and it was not associated with any-grade CRS (OR, 1.6; 95% CI, 0.27-9.18; P = .59). However, high-grade tumor burden by MTV was associated with G3-4 CRS (OR, 12.4; 95% CI, 1.49-104.2; P = .019) (Figure 4C-D).
Toxicities to axi-cel by low vs high MTV manual (cutoff 147.5 mL). Any NT (A) or G3-4 NT (B) and any CRS (C) or G3-4 CRS (D) for both cohorts combined (n = 96).
Toxicities to axi-cel by low vs high MTV manual (cutoff 147.5 mL). Any NT (A) or G3-4 NT (B) and any CRS (C) or G3-4 CRS (D) for both cohorts combined (n = 96).
Discussion
This study establishes the association of MTV on baseline 18F-FDG PET/CT with OS and PFS in patients with refractory/relapsed LBCL treated with axi-cel using 2 separate cohorts of patients. There was lack of agreement between MTV derived by either of 2 different MTV calculation methods, MTV semiautomated and MTV manual, and SPD or SUVmax, which was expected considering the difference in extent and type of measurement. The manually and semiautomated MTV values correlated; however, there was considerable variation in the estimated MTV depending on method. We, a priori, considered the MTV manual method to be superior, as it clearly abrogates the exclusion of some tumor areas using the MTV semiautomated method (Figure 1). Therefore, our model was constructed using MTV manual values.
Due to the relatively limited number of patients in cohort 1, median MTV was chosen as the cutoff. Although this value was lower than the reported general range for diffuse LBCL and close to those for LBCL patients receiving CAR T-cell therapy, it is difficult to compare MTV values calculated using different methods (Table 2). For example, pretisagenlecleucel MTV in JULIET, the cutoff was 100 mL, determined by median MTV via MIM automatic methodology.17 Alternatively, Wang et al selected a cutoff of 72 cm3, as determined by median MTV, in their CAR T-cell study.18
Given the more frequent use of bridging therapy in the second cohort, we performed MVA and included receipt of bridging. Importantly, the relationship between MTV and OS and PFS did not change based on bridging therapy use and status of LDH before conditioning. Combining both cohorts, the low MTV group had significantly higher ORR and CR. Additionally, high baseline MTV was prognostic of G3-4 CRS by Lee criteria, but not of any CRS or any NT or G3-4 NT by CTCAE criteria. We validated the efficacy findings in a subgroup of the patients that had scans within 28 days of starting conditioning chemotherapy (1 patient had a baseline scan the day of CAR T-cell infusion) and excluding those without postbridging scans.
We found upon analysis of PFS and OS by MTV quartile that the 2 highest MTV quartiles had similarly worse outcomes. It is unclear why tumor burden appears to impact efficacy when it reaches a certain threshold rather than in a continuous manner. Despite the worse outcomes, a subset of patients with high tumor burden have ongoing remissions, suggesting that high tumor burden alone should not preclude a patient from receiving axi-cel.
Since CAR T-cell therapy is relatively new, a very limited number of investigations exist on the subject of tumor burden, especially calculated by MTV. In the JULIET19 and TRANSCEND20 studies regarding 2 different types of CAR T-cell therapy (CTL019 and JCAR017, respectively), higher tumor burden was associated with greater toxicity. In the JULIET post hoc analysis including 95 treated relapsed/refractory diffuse LBCL patients, high MTV was associated with G3-4 CRS by Lee criteria, but not any NT or G3-4 NT by CTCAE. 17 In a smaller study of 19 patients with refractory/relapsed non-Hodgkin lymphoma treated with CAR T-cell therapy, after a median follow up of 5 months, calculated baseline MTV did not differ significantly between patients who had responded and those who had not (P = .62), and MTV was not significantly associated with OS (P = .67). Lower MTV was significantly associated with mild and moderate G0-2 CRS compared with G3-4 CRS (P = .008).18 In agreement with these studies, our results showed an association of MTV with G3-4 CRS, but not NT by CTCAE. However, we found MTV to be associated with survival and response to therapy.
The main strengths of our study are the methodically derived manual baseline MTV values and the successful validation in a separate cohort, both of which increase validity of our results demonstrating that tumor burden impacts efficacy outcomes. The reproducibility of MTV manual values can be achieved using MTV calculating software by both nonradiology and radiology physicians; however, we encourage manual resizing of lesions. We did not capture processing time per scan; however, we believe MTV manual takes longer than MTV semiautomated due to the additional processing steps. Anecdotally, we found the time per manual scan was highly variable depending on the number, size, and complexity of lesions. Further optimization to improve the efficiency and accuracy of automated tumor contouring may reduce the time for, or entirely eliminate the need for, manual processing.
This study is limited by its retrospective nature and the low number of subjects available due to the novelty of the therapy. Notably, there were differences in the 2 patient cohorts, as they varied in terms of follow-up time, secondary revised International Prognostic Index scores, and use of bridging therapy, which itself could have had a confounding effect on both outcomes and on tumor burden in the case where PET scans were done before bridging. Additionally, we did not capture American Society for Transplantation and Cellular Therapy grading in a majority of our patients,21 although we and others reported the high concordance of NT grade 3-4 by CTCAE and American Society for Transplantation and Cellular Therapy.22,23
In summary, this study showed that tumor burden, measured by MTV on baseline 18F-FDG PET/CT scan, is associated with PFS and OS in axi-cel–treated LBCL patients. Development of a comprehensive MTV-based prognostic model for clinical practice likely requires additional investigation in a larger number of patients with long-term survival data. Since patients with lower tumor burden had superior survival and response rates, future research should focus on investigating tumor debulking prior to CAR T-cell therapy or earlier referral for therapy in order to improve clinical outcomes in this group of patients.
For data sharing, e-mail the corresponding author, Frederick L. Locke (frederick.locke@moffitt.org).
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute grants K23-CA201594 and P30 CA076292-18S4.
Authorship
Contribution: F.L.L., Y.B., and E.A.D. contributed to study conception and design; E.A.D., H.L., M.S.M., and G.S.K. collected and assembled data; E.A.D. and R.S.M. contributed to data analysis and interpretation; E.A.D. wrote the manuscript; H.L., M.S.M., G.S.K., R.S.M., A.L., J.C.C., T.N., F.K., M.D.J., Y.B., and F.L.L. contributed to manuscript revision; and all authors reviewed and approved of the submitted manuscript.
Conflict-of-interest disclosure: C.A.B. is a scientific advisor to Kite/Gilead and on the Novartis speaker bureau. J.C.C. is on the Kite/Gilead speaker bureau. T.N. provides research support to Novartis (to Moffitt Cancer Center on clinical trial support) and Karyopharm (to Moffitt Cancer Center on clinical trial support). M.L.D. has stock options in Adaptiv Biotechnologies and Certainty Therapeutics; personal fees in Servier, Glaxo Smith Kline, Celyad, and Kite; personal fees and stock in Precision BioScience and Bellicium Pharmaceuticals; and a CAR design patent with Atara Biotherapeutics. J.P.-I. is a consultant for Janssen, Pharmacyclics, AbbVie, AstraZeneca, Takeda, and TEVA and speaker for Janssen, AbbVie, and Takeda. B.D.S. receives personal fees from Celgene/Juno/BMS, Adaptive, Novartis, Pharmacyclics, Spectrum/Acrotech, and AstraZeneca for consultancy (<$5000/year); received grants from Jazz for an investigator-initiated trial and Incyte for salary support for research (<$5000/year); and received a grant and personal fees from Kite/Gilead for consultancy (<$5000/year) and salary support for research (<$5000/year). M.D.J. has consultancy/advisory roles with Kite/Gilead and Novartis. F.L.L. is a compensated scientific advisor to Kite/Gilead, Novartis, BMS/Celgene, Calibr, Wugen, GammaDelta Therapeutics, and Allogene and a consultant for Cellular Biomedicine Group (with grant options), and his institution holds the following patents: double-mutant survivin vaccine, CAR T cells with enhanced metabolic fitness, methods of enhancing CAR T-cell therapies, and mathematical model of CAR T evolution. The remaining authors declare no competing financial interests.
Correspondence: Frederick L. Locke, Moffitt Cancer Center, 12902 USF Magnolia Dr, Tampa, FL 33612; e-mail: frederick.locke@moffitt.org.
References
Author notes
The full-text version of this article contains a data supplement.