Key Points
Vancomycin exposure in the pre-engraftment period was associated with an increased risk for CMV reactivation after allogeneic HCT.
Some gram-positive bacteria may protect against CMV reactivation.
Introduction
The commensal bacteria modulate host susceptibility to viral infections.1 A growing body of evidence has established associations between microbiota changes during allogeneic hematopoietic cell transplantation (HCT) and transplant outcomes, including graft-versus-host disease (GVHD),2 mortality,3 and relapse.4 Less is known about the association between the microbiota and viral reactivations. A lower relative abundance of butyrate-producing bacteria in the fecal microbiota was associated with a higher risk for progression from upper to lower respiratory tract infection in a previous study of allogeneic HCT patients.5 Cytomegalovirus (CMV) reactivation occurs in ∼25% of allogeneic HCT recipients and is associated with significant morbidity and mortality.6,7 Because antibiotics markedly alter the microbiota in HCT recipients, we hypothesized that antibacterial antibiotic exposures in the pre- and early posttransplant period may influence the risk of CMV reactivation.
Methods
We retrospectively analyzed data from adult CMV-seropositive recipients of umbilical cord blood (UCB; n = 146) or CMV-seronegative HLA-matched sibling donors (MSDs; n = 67) between July 2011 and September 2019. We focused on CMV-seronegative to CMV-seropositive transplants to exclude the effect of anti-CMV immunity transferred with the graft. Patients who died before day 14 after HCT (day +14) were excluded. All patients underwent weekly surveillance for CMV viremia by quantitative polymerase chain reaction of plasma until day +100, with a positive result defined as >137 copies per milliliter. We collected exposure data for the following antibacterial antibiotic classes between day −7 and day +100 or until CMV reactivation, whichever occurred first: fluoroquinolones (ciprofloxacin, levofloxacin), third-generation or higher cephalosporins, vancomycin, piperacillin-tazobactam, carbapenems (imipenem, meropenem, ertapenem), metronidazole, and clindamycin. Our standard algorithm for antibacterial prophylaxis is levofloxacin 250 mg daily orally starting at day −1 and ending at the onset of neutropenic fever or when the absolute neutrophil count recovers to >1.5 × 109/L, whichever occurs first. We generally use ceftazidime (until July 2013) or cefepime (after July 2013) as frontline empiric treatment of neutropenic fever. We use empiric IV vancomycin for neutropenic fever when central line–associated infection or cellulitis is suspected and, occasionally, for persistent unexplained fever. We use empiric metronidazole or clindamycin when there is clinical evidence of enterocolitis or significant oral mucositis. For transplants with a CMV-seropositive donor or recipient (all patients in this study), we use oral acyclovir, 800 mg 5 times daily, starting at day −4 for prophylaxis. We use anti-thymocyte globulin (ATG) during conditioning for UCB transplants if the last intensive chemotherapy was >6 weeks before HCT.
We used R 3.4 (R Foundation for Statistical Computing, Vienna, Austria) for time-to-event regression analysis of CMV reactivation (by day +100), with a competing risk for death without CMV reactivation. Exposure to each antibiotic class, the main predictor, was coded as 1 if the patient received ≥1 dose of the antibiotic, otherwise it was coded as 0. Prespecified covariates in multivariable models included donor type (UCB vs MSD), ATG use in conditioning, grade 2-4 acute GVHD before CMV reactivation, and exposure to ganciclovir/valganciclovir (predominantly to treat human herpes virus-6 reactivation after UCB transplants) before CMV reactivation. We modeled grade 2-4 aGVHD and ganciclovir/valganciclovir exposure as time-varying variables. We also included a variable for the interaction of donor type and time, because the hazard functions by donor type were nonproportional with time. We built 2 prespecified models: 1 using antibiotic exposures by day +14 as a binary variable and the other using antibiotic exposure by day +100 as a time-varying variable. Time-varying variables are those whose value depends on time. If a patient was first exposed to a specific antibiotic at time t, we changed the exposure variable at that time from 0 to 1. The patient belonged to the unexposed group until time t and then moved to the exposed group.
Results and discussion
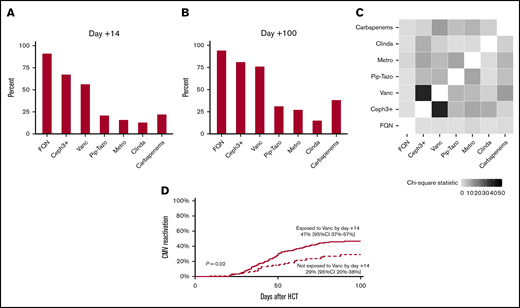
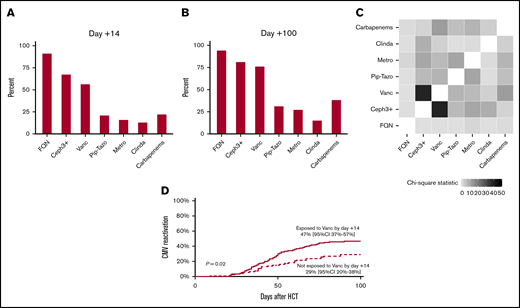
Supplemental Table 1 summarizes the baseline characteristics of the patients, and supplemental Table 2 provides the results of univariate analysis. All patients were CMV-seropositive recipients of seronegative donor allografts. The cumulative incidence of CMV reactivation and grade 2-4 acute GVHD by day +100 was 39% and 42%, respectively. Forty (19%) patients received ganciclovir/valganciclovir before CMV reactivation. Thirty-six (17%) patients received ATG during conditioning. Figure 1A-C summarize antibiotic exposures. Because only 7 (3%) patients received oral (not IV) vancomycin, we did not consider oral vancomycin in the analysis.
Antibiotic exposures and CMV reactivation. Antibiotic exposures by day +14 (A) and day +100 (B). (C) Heat map showing antibiotic coexposures, with the color gradient representing the χ2 statistic. (D) Patients exposed to vancomycin experienced a higher rate of CMV reactivation. Ceph3+, third-generation or higher cephalosporins; Clinda, clindamycin; FQN, fluoroquinolones; Metro, metronidazole; Pip-Tazo: piperacillin-tazobactam; Vanc, vancomycin.
Antibiotic exposures and CMV reactivation. Antibiotic exposures by day +14 (A) and day +100 (B). (C) Heat map showing antibiotic coexposures, with the color gradient representing the χ2 statistic. (D) Patients exposed to vancomycin experienced a higher rate of CMV reactivation. Ceph3+, third-generation or higher cephalosporins; Clinda, clindamycin; FQN, fluoroquinolones; Metro, metronidazole; Pip-Tazo: piperacillin-tazobactam; Vanc, vancomycin.
In multivariable analysis (Table 1), the only antibiotic exposure by day +14 associated with CMV reactivation was vancomycin (P = .02), with a hazard ratio (HR) of 1.97 and 95% confidence interval (CI) of 1.11 to 3.51. The exposed group experienced an 18% higher cumulative incidence of CMV reactivation by day +100 compared with the unexposed group (47%; 95% CI, 37-57 vs 29%, 95% CI, 20-38), respectively (Figure 1D). Grade 2-4 acute GVHD and ganciclovir/valganciclovir exposure were associated with an increased risk (HR, 1.69; 95% CI, 1.05-2.73; P = .03) and a decreased risk (HR, 0.45; 95% CI, 0.22-0.93; P = .03) for CMV reactivation, respectively. The association between graft source and CMV reactivation was time dependent, with a lower risk with UCB by day +30 and a higher risk after day +30. In multivariable analysis with antibiotic exposures considered time-varying variables, we again found an association between vancomycin exposure and CMV reactivation (HR, 1.87; 95% CI, 0.99-3.54; P = .05). We found similar associations for GVHD, ganciclovir/valganciclovir, and graft source as in the analysis with antibiotic exposures by day +14.
For the first time, we report an association between vancomycin exposure and increased risk for CMV reactivation after allogeneic HCT. Although vancomycin exposure may be a surrogate for a risk factor for CMV reactivation not considered in this analysis, an alternative explanation is that specific gram-positive bacteria may protect against CMV reactivation. The specific bacteria and their location cannot be determined by the present study. Although the lower gut is the most extensively evaluated microbiota compartment in HCT studies, it is possible that the putative bacteria in our study reside in the upper or lower gut, respiratory tract, skin, or elsewhere. Our finding of a stronger association for day +14 analysis than for day +100 analysis is consistent with the reported importance of dysbiosis in the peri-engraftment period for other outcomes.3 As a possible mechanism for our main finding, some gram-positive bacteria may stimulate the emerging immune system after HCT, conferring protection against CMV reactivation. Exposure to vancomycin could eliminate this beneficial stimulation in the critical peri-engraftment period and promote subsequent CMV reactivation.
Previous studies have elucidated some of the mechanisms involved in the association between the commensal microbiota and viral infections. Examples include bacterial products binding to viruses and decreasing their infectivity (nasal Staphylococcus epidermidis against the influenza virus8 and vaginal lactobacilli against herpes simplex virus-29 ), gut Clostridium orbiscindens metabolite augmenting type 1 interferon signaling and protecting against influenza virus–related lung damage,10 and Toll-like receptor signaling stimulated by commensal bacterial lipoteichoic acid, thereby increasing mast cell recruitment and enhancing their antiviral cathelicidin expression.11 Microbiota-mediated beneficial effects can be therapeutic targets. Fecal microbiota transplantation protected germ-free mice against influenza virus lethality via interleukin-10 and interleukin-13 and suppression of detrimental inflammation.12 In a pilot study, fecal microbiota transplantation enhanced HBe antigen clearance in patients with chronic hepatitis B infection.13
Concurrent antibiotic exposures (common in HCT recipients) make it difficult to attribute an apparent effect to a specific antibiotic with certainty. Exposure to 1 antibiotic may be a marker for other antibiotic exposures. A synergistic effect by ≥2 antibiotics is also possible. However, comparing the relative effect sizes for different antibiotics in multivariable analysis suggests an independent association for vancomycin. Other possibilities include an effect by exposure duration rather than exposure as a binary variable. Finally, exposure to multiple antibiotics may reflect a defect in immune reconstitution influencing CMV reactivation, rather than being a cause via the microbiota. If validated in other cohorts, our results could inform antibiotic stewardship programs to limit vancomycin use in allogeneic HCT recipients. In addition, if the putative vancomycin-sensitive bacteria are characterized in mechanistic studies, selective restoration by microbiota transfer may be tested.
Data sharing requests should be sent to Armin Rashidi (arashidi@umn.edu).
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (P30 CA77598), utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, and by the National Institutes of Health, National Center for Advancing Translational Sciences (UL1TR002494 and KL2TR002492).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.Z. and A.R. collected data and wrote the manuscript; R.S. analyzed data; and D.J.W. critically reviewed the results and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Armin Rashidi, Division of Hematology, Oncology, and Transplantation, University of Minnesota, 14-100 PWB, MMC 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: arashidi@umn.edu.
References
Author notes
The full-text version of this article contains a data supplement.