TO THE EDITOR:
Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) are major limitations of chimeric antigen receptor (CAR) T-cell therapy.1 Although tocilizumab, an anti–interleukin-6R (IL-6R) antibody, can ameliorate CRS, it has limited effectiveness in ICANS.1,2 Systemic high-dose corticosteroids and supportive care are the current mainstay therapies for ICANS. Because infiltration of immune effector cells (IECs) into the central nervous system (CNS)3 is implicated in ICANS, we administered intrathecal (IT) chemotherapy for steroid-refractory ICANS with rapid resolution of toxicities. We describe the clinical course and outcomes of 2 patients with steroid-refractory ICANS treated with IT chemotherapy. Institutional Review Board submission was deferred per institutional policy for case series that include <3 patients.
A 71-year-old man with chemorefractory diffuse large B-cell lymphoma (DLBCL) received second-generation 41BB CD3ζ anti-CD19 anti-CD20 (LV20.19) bispecific CAR T cells on a phase 1 clinical trial (NCT03019055). On day +5 post-LV20.19 CAR infusion, the patient developed grade 2 CRS that resolved with tocilizumab. On day +7, he developed grade 2 neurotoxicity by Common Terminology Criteria for Adverse Events v5. Treatment with dexamethasone, 10 mg every 6 hours, was initiated. With rapid improvement, his dexamethasone was tapered. Unfortunately, he progressed with grade 3 neurotoxicity on day +13 that was manifested by tremor, mutism, and nonresponsiveness. Lumbar puncture (LP) was performed, and cerebrospinal fluid (CSF) analysis revealed a white blood cell (WBC) count of 18 cells per microliter (Figure 1A), with a predominantly non–CAR CD4+ T-cell population (Figure 1B). Pulse IV SOLU-MEDROL, 500 mg × 3 days, was initiated, followed by dexamethasone, 10 mg every 6 hours, without any clinical improvement. Brain magnetic resonance imaging did not reveal any significant intracranial abnormality. An electroencephalogram was negative for seizures, and the patient was diagnosed with steroid-refractory ICANS.
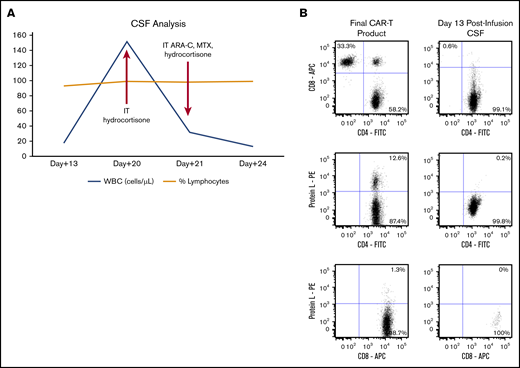
CSF analysis for patient 1. (A) Trends in WBCs in the CSF over time after CAR T-cell infusion and time points of intervention. (B) Flow cytometry plots of final CAR T-cell product and CSF. T cells in the CSF at day 13 postinfusion were predominantly CD4+ non-CAR T cells. Preinfusion final CAR T-cell product phenotype after staining cells with a combination of markers for CD3, CD4, CD8, and protein L (to detect the CAR) and analyzing the stained cells by flow cytometry (left panels). The same panel of markers was used to analyze CSF collected at day 13 postinfusion (right panels). The plots were based on gated CD3+ T cells. ARA-C, cytarabine; MTX, methotrexate.
CSF analysis for patient 1. (A) Trends in WBCs in the CSF over time after CAR T-cell infusion and time points of intervention. (B) Flow cytometry plots of final CAR T-cell product and CSF. T cells in the CSF at day 13 postinfusion were predominantly CD4+ non-CAR T cells. Preinfusion final CAR T-cell product phenotype after staining cells with a combination of markers for CD3, CD4, CD8, and protein L (to detect the CAR) and analyzing the stained cells by flow cytometry (left panels). The same panel of markers was used to analyze CSF collected at day 13 postinfusion (right panels). The plots were based on gated CD3+ T cells. ARA-C, cytarabine; MTX, methotrexate.
On day 20, repeat CSF analysis revealed a WBC count of 152 cells per microliter (Figure 1A). IT hydrocortisone, 100 mg, was administered for steroid-refractory ICANS. He experienced transient improvement in alertness with no adverse toxicity. On day +21, repeat LP was performed; WBC count in CSF had decreased to 32 cells per microliter (Figure 1A) and an opening pressure of 16.5 cm H2O. A combination of IT cytarabine (50 mg) + methotrexate (12 mg) + hydrocortisone (50 mg) was administered. On day 22, the patient’s neurologic symptoms improved, with dramatic recovery of alertness, verbalization, and orientation. Subsequently, his dexamethasone was tapered over the next 10 days. By day +28, a complete response of the DLBCL was documented by positron emission tomography/computed tomography, and neurological recovery was established. He remains well, in complete remission, and fully functional as of day +180.
A second patient, a 69-year-old female with chemorefractory DLBCL, received CD19 CAR T-cell therapy with tisagenlecleucel. She developed grade 1 CRS on day +1, which was treated with antipyretics. On day +4 she developed a depressed level of consciousness consistent with grade 1 neurotoxicity (Common Terminology Criteria for Adverse Events v5). On day +5, the patient had progressive neurotoxicity with mutism and was started on dexamethasone, 10 mg every 6 hours. An electroencephalogram demonstrated diffuse slowing with frequent triphasic waves that were sharply demarcated and rhythmic, concerning for possible seizure activity; consequently, the patient was intubated for airway protection (grade 4 neurotoxicity). She was started on lorazepam, and corticosteroids were escalated to SOLU-MEDROL, 500 mg every 12 hours. Brain magnetic resonance imaging showed symmetric flair hyperintensity within the sulci of both cerebral hemispheres. She then developed hypotension requiring vasopressors and received 1 dose of tocilizumab for systemic CRS. After 4 days of high-dose corticosteroids, the patient remained intubated and unresponsive, despite being off all sedation, and was diagnosed with steroid-refractory ICANS.
An LP on day +8 revealed a WBC count of 2 cells per microliter, with opening pressure of 23 cm H2O. Flow cytometry demonstrated a relatively small T-cell infiltrate that primarily consisted of CD4+ T lymphocytes (Figure 2), similar to the first patient. The patient was administered 50 mg of IT hydrocortisone and 12 mg of methotrexate while remaining on high-dose SOLU-MEDROL. She awoke 36 hours post-IT chemotherapy and responded to simple commands. She was extubated on day +10 and had complete resolution of neurotoxicity by day +14. SOLU-MEDROL was continued until day +14 and subsequently tapered. Evaluation on day +28 revealed a partial response of the DLBCL.
Flow cytometry plot for patient 2. T cells from CSF of patient 2 at day 8 postinfusion were predominantly CD4+. Cells isolated from CSF were stained with a combination of CD3, CD4, CD8, and CD45 and analyzed by flow cytometry. The cells were gated on lymphocytes, the lymphocytes were analyzed for CD3 expression (left panel), and the gated CD3 cells were analyzed for the presence of CD4 and CD8 (right panel).
Flow cytometry plot for patient 2. T cells from CSF of patient 2 at day 8 postinfusion were predominantly CD4+. Cells isolated from CSF were stained with a combination of CD3, CD4, CD8, and CD45 and analyzed by flow cytometry. The cells were gated on lymphocytes, the lymphocytes were analyzed for CD3 expression (left panel), and the gated CD3 cells were analyzed for the presence of CD4 and CD8 (right panel).
Neurologic toxicities are a major limitation of CAR T-cell–based immunotherapies. Although most cases of ICANS are mild and self-limited, it is a potentially life-threatening complication in select patients. Several variables impact the development of ICANS, including the type of malignancy, disease burden, depth and form of lymphodepletion,4 presence of concurrent CRS, and age and performance status of the patient.5,6 In addition, the CAR T-cell construct likely impacts the toxicity profile; CAR T cells with a CD28 costimulatory domain are associated with higher rates of ICANS than are 41BB constructs, with several reported deaths due to cerebral edema.7,8 However, these associations are clearly not absolute, because both patients, who experienced severe neurotoxicity, received a CAR T-cell product with a CD3ζ 4-1BB construct.
The signs and symptoms of ICANS are variable, as described in these 2 cases, with tremor, mutism, seizure activity, and loss of consciousness. Clinical trials have demonstrated the onset of neurologic toxicity at a median of 4 to 5 days after administration of CAR T cells, with most patients having resolution within 3 to 4 weeks.9 Although the exact pathophysiology of ICANS remains an unresolved question, blood-brain barrier disruption from systemic inflammation, in association with rapid proliferation of IECs and markedly increased levels of cytokines, likely plays a role.6
The optimal management of ICANS remains a clinical question, with various guidelines in place for the 2 CAR T-cell therapies that are approved by the US Food and Drug Administration. Treatment with dexamethasone and supportive care measures (eg, antiseizure medications) remains the frontline option, despite clinical concerns that administration of systemic steroids may limit CAR T-cell efficacy.10 Targeting IL-6R with tocilizumab has had little efficacy in the prevention or treatment of neurotoxicity.11 Preclinical models have demonstrated that targeting the IL-1 pathway with anakinra may be superior to tocilizumab in mitigating neurotoxicity. This may be due, in part, to the ability of anakinra to cross the blood-brain barrier, unlike tocilizumab.12 A clinical trial to test the efficacy of prophylactic anakinra in preventing CAR T-cell–associated neurotoxicity is under development (NCT04150913). We present a novel approach to the management of steroid-refractory ICANS by directly targeting the CNS with IT hydrocortisone to reduce inflammation, in combination with a cytotoxic agent to eliminate the IECs that may be the drivers of neurotoxicity. A targeted approach may be ideal for patients treated with CAR T cells to minimize the impact of systemic steroids on CAR T-cell activity, which is needed for disease control.
Steroid-refractory ICANS is a potentially life-threatening complication, with no established second-line therapy. We administered IT chemotherapy in 2 cases, with rapid and sustained resolution of ICANS and no long-term complications to date, suggesting the safety of this approach. Earlier use of IT chemotherapy may result in a faster recovery, decreasing the potential morbidity associated with prolonged use of a high-dose systemic steroid. Future studies with a larger number of patients will be important to determine the role of IT chemotherapy in the management of ICANS and to understand whether its administration could negatively impact clinical outcomes after CAR T-cell therapy by increasing the probability of CNS relapse.
Contribution: All authors collected, analyzed, and interpreted data, as well as wrote the manuscript and approved the final version.
Conflict-of-interest disclosure: N.N.S. has received honoraria and/or travel support from Incyte, Celgene, and Miltenyi Biotec; has served on scientific advisory boards for Kite Pharma, Celgene, and Cellectar Biosciences; and has received institutional research support for clinical trials from Bristol-Myers Squibb and Miltenyi Biotec. B.D.J. has received travel support and institutional research support for clinical trials from Miltenyi Biotec. T.S.F. has received research funding from Millennium Pharmaceuticals, Kyowa, TG Therapeutics, Portola Pharmaceuticals, and Curis; has received consulting honoraria from Genentech, Adaptive Biotechnologies, AbbVie, Verastem Oncology, Kite Pharma, and MorphoSys; and has received speaking honoraria from Genentech, Sanofi, Seattle Genetics, AstraZeneca, Celgene, and Adaptive Biotechnologies. R.V.R. has received honoraria and travel support from Celgene, Amgen, and Jazz Pharmaceuticals. P.H. has received honoraria and travel support from Celgene, Takeda, Amgen, Incyte, Bristol-Myers Squibb, Janssen Pharmaceuticals, Karyopharm, Pharmacyclics, and AbbVie.
Correspondence: Nirav N. Shah, Medical College of Wisconsin, 9200 W Wisconsin Ave, Milwaukee, WI 53226; e-mail: nishah@mcw.edu.