Key Points
The combination of lenalidomide and rituximab is highly effective in patients with CLL and low baseline β2-microglobulin levels.
Mutations in the NOTCH signaling pathway are associated with worse outcome in patients with CLL treated with lenalidomide and rituximab.
Abstract
This phase 2 study was conducted to prospectively evaluate how clinical and biological factors correlate with outcome in patients with treatment-naive (TN) and relapsed (R) chronic lymphocytic leukemia (CLL) treated with lenalidomide and rituximab. Oral lenalidomide 10 mg was administered daily starting on day 9 of cycle 1. IV rituximab 375 mg/m2 was administered weekly during cycle 1 and every 4 weeks for cycles 3 to 12. Sequencing of a custom panel of 295 genes was performed in pretreatment bone marrow samples. The study included 61 patients with TN CLL and 59 with R CLL; the overall response rate (ORR) was 73% and 64%, respectively. A baseline β2-microglobulin level <4 mg/L was associated with higher ORR in both groups (both, P = .03), and absence of mutations in the NOTCH signaling pathway showed a trend for association with higher ORR in R CLL (P = .10). Median PFS was 50 months in TN patients and 28 months in R patients. On multivariate analysis, age ≥65 years (P = .02) was associated with shorter PFS in TN patients, whereas according to univariate analysis, >2 previous therapies (P = .02) was the only factor associated with shorter PFS in R patients. A trend for association between mutations in the NOTCH pathway and shorter PFS was observed in TN CLL (P = .15). Further exploration of the NOTCH pathway may help optimize the efficacy of this combination in patients with CLL. This study protocol was approved by the University of Texas MD Anderson Cancer Center institutional review board and registered at clinicaltrials.gov (#NCT01446133).
Introduction
Lenalidomide is an immunomodulatory agent with both direct and indirect antineoplastic activity in several hematologic malignancies; the indirect effects are mediated through modulation of several components of the tumor microenvironment.1 Lenalidomide is approved for the treatment of multiple myeloma, low-risk myelodysplastic syndrome with cytogenetic 5q abnormality, and relapsed mantle cell lymphoma; it has also shown clinical activity in follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia (CLL).2 Lenalidomide as a single agent in patients with CLL induces an overall response rate (ORR) of 56% to 65% in treatment-naive (TN) patients3,4 and 32% to 47% in relapsed (R) patients,5,6 and durable responses have been observed.7 Because lenalidomide and rituximab exhibit synergistic activity in in vitro models of CLL,8 their combination was tested in clinical trials, and ORRs of 78% to 95% in TN patients9 and 61% to 66% in R patients with CLL10,11 were reported. However, the use of lenalidomide is frequently associated with toxic effects, including neutropenia, recurrent infections, neuropathy, and diarrhea; these toxic effects lead to treatment discontinuation, despite ongoing responses, in up to 20% of patients with CLL.12 We therefore conducted a phase 2 study to determine the activity and tolerability of this combination in patients with TN CLL and R CLL and to prospectively evaluate how clinical characteristics, gene mutations, and other prognostic factors correlated with ORR and survival.
Methods
Patient eligibility
Patients with TN CLL or R CLL were enrolled into a single-center, open-label, phase 2 study of lenalidomide and rituximab at The University of Texas MD Anderson Cancer Center from January 2012 through November 2014. All patients had a diagnosis of CLL and active disease with an indication for therapy in accordance with the guidelines of the 2008 International Workshop on Chronic Lymphocytic Leukemia.13 TN patients were not candidates (based on age and/or comorbidities) for or were unwilling to receive chemoimmunotherapy, whereas R patients had received previous treatment with purine analogue–based chemotherapy or chemoimmunotherapy. Fludarabine refractoriness was defined as no response or progression within 6 months of the most recent fludarabine-containing regimen. Patients were required to have an Eastern Cooperative Oncology Group performance status ≤2 and adequate renal (serum creatinine level, <2 mg/dL) and hepatic (serum bilirubin level, <2 mg/dL) function. Patients with other malignancies diagnosed within 3 years of study entry were excluded, with the exception of patients with localized skin, breast, or prostate cancer who had received a curative treatment modality. Patients with active hepatitis B or C virus, HIV positivity, or a history of tuberculosis, as well as patients with a history of deep vein thrombosis or pulmonary embolism within 6 months of study entry, were excluded. This study protocol was approved by the University of Texas MD Anderson Cancer Center institutional review board and registered at clinicaltrials.gov (#NCT01446133). Informed consent was obtained in accordance with institutional guidelines and the Declaration of Helsinki.
Pretreatment evaluation
Before initiation of therapy, all patients were assessed by history taking (including history of cancers other than CLL), physical examination, and peripheral blood studies, including blood counts, serum chemistry, and β2-microglobulin (B2M) level. Bone marrow aspiration and biopsy were performed before therapy and evaluated by using flow cytometry for CD38 and ZAP-70 expression and by a direct sequencing method for immunoglobulin heavy chain variable region gene mutation. Karyotype abnormalities were detected by using fluorescent in situ hybridization (FISH) with standard CLL probes on bone marrow samples (Vysis CLL FISH Probe Kit; Abbott Laboratories, Abbott Park, IL).
Study treatment
IV rituximab (375 mg/m2) was administered on days 1, 8, 15, and 22 during cycle 1 and once every 4 weeks on day 1 for cycles 3 to 12. Lenalidomide, given orally, was started on day 9 of cycle 1 at 10 mg/d and administered daily thereafter. Each cycle of treatment was 28 days. Treatment duration was planned for 12 cycles, although patients could continue lenalidomide beyond 12 cycles if there was a significant clinical benefit, such as an ongoing partial or complete response. Local patients received all their treatment at MD Anderson, and the remaining patients received their treatment from a local oncologist. Allopurinol as tumor lysis prophylaxis was administered for the first 14 days of cycle 1. Growth factor support according to the American Society of Clinical Oncology guidelines was permissible. No antibacterial, antiviral, deep vein thrombosis, or tumor flare prophylaxis was mandated. Lenalidomide dosing could be adjusted for sustained (>7 days) grade 3/4 neutropenia or thrombocytopenia. Dose reductions were recommended for grade 3 rash, allergic reaction, or neuropathy. Lenalidomide was discontinued for grade 4 nonhematologic toxic effects.
Response and toxicity assessment
Response was evaluated after 3, 6, and 12 cycles and every 6 cycles thereafter. Clinical response was defined as best response obtained with therapy, assessed according to the 2008 International Workshop on Chronic Lymphocytic Leukemia criteria.13 Bone marrow aspirate and biopsy evaluation with flow cytometry were performed (sensitivity <0.01) at each assessment. Computed tomography scans were not required for response assessment but were obtained if clinically indicated. Treatment-related toxicity was assessed by using Common Terminology Criteria for Adverse Events (version 4.0).
Study end points and statistical analysis
The primary end point of this study was ORR. The secondary end points were treatment safety and association of baseline characteristics with response and survival. Logistic regression analysis was performed to assess the associations between patient characteristics and response. Progression-free survival (PFS) was defined as the time from the start of therapy to progression of disease, death, or last follow-up (whichever occurred first), and overall survival (OS) was defined as the time from the start of therapy to death or last follow-up. PFS and OS were calculated for all patients in the study and for subgroups of patients by using Kaplan-Meier estimates and were compared between subgroups by using the log-rank test. Multivariable Cox regression analyses were performed to assess the associations between patient characteristics and PFS or OS. To calculate sample size, a Bayesian design was applied. Given that β(1,1) is the prior distribution for each group and given a response rate of 60% in TN patients and 40% in R patients, a 95% credible interval of response rate was 0.47 to 0.72 in TN patients and 0.29 to 0.53 in R patients. Sixty patients were needed per group. All analyses were performed by using SPSS version 23.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and considered significant if P ≤ .05.
Correlative studies
Sequencing of a SureSelect custom panel of 295 genes (Agilent Technologies, Santa Clara, CA) had been previously performed in pretreatment bone marrow samples as part of another project.14 Extracted genomic DNA was fragmented and bait-captured according to the manufacturer’s protocols. Captured DNA libraries were then sequenced by using a HiSeq 2000 sequencer (Illumina, San Diego, CA) with 76-bp paired-end reads. Modified MuTect and Pindel algorithms against a pooled common normal reference were used to call high-confidence cancer gene mutations in the samples. Observed gene mutations were subsequently categorized into 6 pathway groups, as previously defined by Landau et al15 : NOTCH signaling, inflammatory/B-cell receptor signaling, WNT signaling, DNA damage and cell cycle control, chromatin modification, and RNA and ribosomal.
Results
Patient characteristics
One hundred twenty patients were enrolled in this study: 61 with TN CLL and 59 with R CLL. The baseline characteristics of the 2 groups are summarized in Table 1. Therapy was administered by a local provider in 40 (66%) TN patients and 43 (73%) R patients.
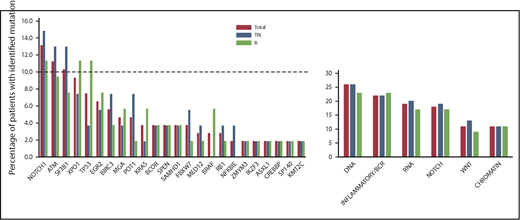
The landscape of baseline gene mutations (available for 54 TN patients and 53 R patients) is depicted in Figure 1. Forty-two (78%) TN patients and 39 (74%) R patients carried at least 1 mutation, and 21 (39%) TN patients and 18 (34%) R patients had >1 mutation. The frequency of identified single-gene mutations and mutated pathway groups did not differ significantly between the TN and R groups.
Genemutation distribution according to treatment arm. No difference in the frequency of gene mutations and mutated pathway groups before initiation of the study treatment was observed when comparing TN patients vs R patients. NOTCH signaling pathway: NOTCH1, SPEN, FBXW7; inflammatory/B-cell receptor pathway: BIRC3, EGR2, NFKBIE, KRAS, SAMHD1, BRAF; WNT signaling pathway: MGA, MED12, FBXW7; DNA damage and cell cycle control pathway: TP53, ATM, POT1, BRCC3, RB1; chromatin modification pathway: ZMYM3, IKZF3, ASXL1, CREBBP, SP140, KM2TC; and RNA and ribosomal processing pathway: SF3B1, XPO1.
Genemutation distribution according to treatment arm. No difference in the frequency of gene mutations and mutated pathway groups before initiation of the study treatment was observed when comparing TN patients vs R patients. NOTCH signaling pathway: NOTCH1, SPEN, FBXW7; inflammatory/B-cell receptor pathway: BIRC3, EGR2, NFKBIE, KRAS, SAMHD1, BRAF; WNT signaling pathway: MGA, MED12, FBXW7; DNA damage and cell cycle control pathway: TP53, ATM, POT1, BRCC3, RB1; chromatin modification pathway: ZMYM3, IKZF3, ASXL1, CREBBP, SP140, KM2TC; and RNA and ribosomal processing pathway: SF3B1, XPO1.
Efficacy
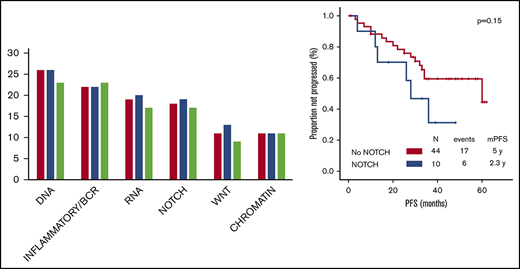
Fifty-five TN patients and 53 R patients were evaluable for response. Twelve patients discontinued therapy before the first response assessment (because of toxicity in 8 patients, loss to follow-up in 3 patients, and death in 1 patient). The median number of cycles provided was 33 (range, 1-62) for TN patients and 25 (range, 1-62) for R patients. ORR was 73% for TN patients and 64% for R patients; complete response was achieved in 35% of TN patients and 28% of R patients, with bone marrow minimal residual disease eradication in 16% of TN patients and 2% of R patients. Responses to treatment are summarized in Table 2. The associations between each baseline characteristic and achievement of response were evaluated. Among TN patients, a baseline B2M level <4 mg/L was associated with higher ORR on univariate analysis (85% vs 55%; P = .03). Among R patients, age <65 years (78% vs 50%; P = .05), Rai stage of 0 to II (79% vs 48%; P = .03), B2M level <4 mg/L (84% vs 46%), and estimated glomerular filtration rate ≥60 mL/min (76% vs 38%; P = .01) were associated with higher ORR on univariate analysis. A trend for association between absence of mutations in the NOTCH signaling pathway and higher ORR was also observed (73% vs 38%, P = .10; P > .15 for the remaining 5 pathways). On multivariate analysis, only B2M level maintained its association with ORR (odds ratio, 0.2; 95% confidence interval [CI], 0.1-0.9; P = .03).
Toxicity
Grade 3/4 hematologic toxic effects were observed in 33 (54%) TN patients and 36 (61%) R patients. Neutropenia was the most common hematologic toxic effect in both groups (47% and 59% of patients, respectively); 5% of cycles in TN patients and 17% of cycles in R patients were complicated by grade 3/4 neutropenia. The median duration of grade 3/4 neutropenia was 7 days (range, 7-14 days) in TN patients and 10 days (range, 7-14 days) in R patients. Grade 3/4 nonhematologic toxic effects were reported in 14 (23%) TN patients and 15 (25%) R patients, and infections were the most common nonhematologic toxic effect in both groups (10% and 22%). The remaining grade 3/4 toxic effects are summarized in Table 3. Three (6%) TN patients experienced grade 1/2 tumor flare reaction, whereas no cases of tumor flare reaction were observed in R patients.
Treatment discontinuation and characteristics associated with PFS
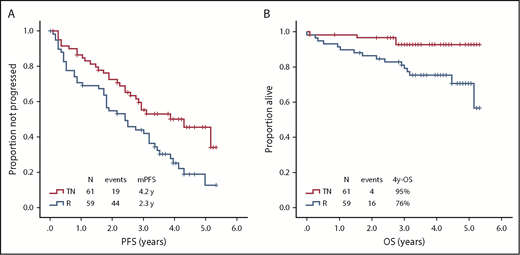
After a median follow-up of 38 months (range, 1-62 months), 45 (74%) TN patients had their treatment interrupted. Reasons for discontinuation were refractoriness or progression in 15 (33%) patients, toxic effects in 25 (56%), second primary neoplasms in 2 (4%), and patient’s choice in 3 (7%). Twenty-nine (48%) TN patients experienced progression, and the median PFS was 50 months (95% CI, 31-69) (Figure 2A).
Survival according to treatment arm. (A) PFS according to treatment arm. (B) OS according to treatment arm. mPFS, median PFS.
Survival according to treatment arm. (A) PFS according to treatment arm. (B) OS according to treatment arm. mPFS, median PFS.
The baseline characteristics associated with shorter PFS on univariate analysis in TN patients were age ≥65 years (28 vs 60 months; P = .002) and complex karyotype (25 vs 60 months; P = .01). A trend for association between mutations in the NOTCH signaling pathway and shorter PFS was also observed (28 vs 60 months, P = .15; P > .15 for the remaining 5 pathways). On multivariate analysis including factors significant on univariate analysis, age ≥65 years (hazard ratio, 3.2; 95% CI, 1.2-8.3; P = .02) maintained its associations with shorter PFS.
After a median follow-up of 46 months (range, 1-62 months), 52 (88%) R patients had their treatment interrupted. Reasons for discontinuation were refractoriness or progression in 27 (52%) patients, toxicity in 20 (38%), second primary neoplasms in 1 (2%), and patient’s choice in 4 (8%). Forty-four (75%) R patients experienced progression, and the median PFS was 28 months (95% CI, 17-39) (Figure 2A).
The only baseline characteristic associated with shorter PFS on univariate analysis in R patients was >2 previous therapies (18 vs 37 months; P = .02).
Causes of death and characteristics associated with OS
After a median follow-up of 38 months (range, 1-62 months), 4 (7%) TN patients had died, including 2 who died while on study (1 who died of infection after 18 cycles, and 1 who died of a second primary neoplasm, a metastatic pancreatic adenocarcinoma, after 1 cycle); median OS was not reached (Figure 2B). Causes of death after study completion were infection (1 patient) and intracranial hemorrhage (1 patient), both during subsequent therapy.
The only baseline characteristics associated with shorter OS on univariate analysis in TN patients was Rai stage III to IV (4-year OS, 80% vs 100%; P = .01). A trend for association between mutations in the NOTCH signaling pathway and shorter OS was observed (4-year OS, 80% vs 97%, P = .13; P > .15 for the remaining 5 pathways).
After a median follow-up of 46 months (range, 1-62 months), 16 (27%) R patients had died, including 5 who died while on study (3 who died of infections, after 3, 20, and 25 months; 1 who died of a second primary neoplasm, metastatic lung squamous cell carcinoma, after 12 cycles; and 1 sudden death of unknown etiology after 1 cycle); median OS was not reached (Figure 2B). After study completion, 11 patients died; death was due to infection in 1 patient, progressive CLL in 8 patients, second primary neoplasm in 1 patient, and myocardial infarction in 1 patient.
The baseline characteristics associated with shorter OS on univariate analysis in R patients were Rai stage III to IV (12 vs 4 deaths; P = .01), B2M level ≥4 mg/dL (13 vs 3 deaths; P = .008), FISH positivity for del17p or del11q (10 vs 4 deaths; P = .001), and >2 previous therapies (10 vs 6 deaths; P = .02). On multivariate analysis, FISH positivity for del17p or del11q (hazard ratio, 5.1; 95% CI, 1.3-20; P = .02) maintained its association with shorter OS.
Second primary neoplasms
Two TN patients (3%) developed a second primary neoplasm while receiving lenalidomide: 1 case of breast cancer (cycle 18) and 1 case of pancreatic cancer (cycle 1). Four R patients (8%) developed a second primary neoplasm while on study: 1 case of lung cancer (at cycle 12), 1 case of thyroid cancer, 1 case of oral squamous cell carcinoma (both at cycle 6), and 1 case of renal cell carcinoma (at cycle 12). A history of a cancer other than CLL was present in 14 (23%) TN patients and 23 (39%) R patients.
Discussion
This phase 2 trial found that the combination of lenalidomide and rituximab is an effective and safe regimen for the treatment of patients with CLL, both as frontline therapy and as salvage therapy.
Lenalidomide has a pleiotropic effect on the CLL microenvironment, favoring restoration of immunologic synapsis formation by T cells,16 antibody production by B cells,17 and an increased number and function of natural killer cells and tumor-associated macrophages.18,19 Despite its promising clinical efficacy as a single agent both as frontline and as salvage therapy for patients with CLL,4,6 its use in patients with CLL is associated with hematologic and nonhematologic toxic effects that often require dose reduction and careful patient monitoring. Lenalidomide has also been shown to be an effective consolidation strategy after frontline or salvage chemoimmunotherapy, associated with response improvement20 and prolonged PFS compared with placebo, when used as a maintenance strategy.21,22 In addition, response to lenalidomide can be long-lasting, persisting even beyond treatment discontinuation, likely owing to its immunologic effects.7,12
In vitro studies have shown that lenalidomide can enhance natural killer cell function and monocyte-dependent cellular cytotoxicity of rituximab-treated CD20-positive CLL cells.8 This finding has led to clinical trials investigating the efficacy of the combination of lenalidomide and anti-CD20 monoclonal antibodies in patients with either R CLL or TN CLL; the combination showed an ORR (78%-95% in TN patients and 61%-71% in R patients) superior to that observed with lenalidomide as monotherapy.9-11,23
We evaluated clinical and laboratory characteristics predictive of response to treatment with lenalidomide and rituximab and found that a low baseline B2M level was associated with increased ORR, in both TN and R patients, which was not previously described. In a phase 2 study of the combination of lenalidomide and rituximab for the treatment of patients with TN CLL, James et al9 reported a significantly increased ORR for patients receiving ≥5 mg of lenalidomide (100%) compared with <5 mg (50%; P = .05). However, this difference was not observed for patients aged >65 years in the same study. The TN population in the current study was older (median age of 66 years in our study vs 57 years in the study by James et al), likely explaining the lack of association between lenalidomide dose and ORR in our analysis. In a phase 2 study of the combination of lenalidomide and rituximab for the treatment of patients with R CLL previously reported by our group, the response to this regimen was associated with previous response to fludarabine: the ORR was 70% in patients whose disease was not refractory to their last fludarabine-containing regimen but only 33% in patients whose disease was refractory to fludarabine (P = .04).10 In the current study, only 7 fludarabine-refractory patients were evaluable for response, hampering any significant statistical comparison. Our group previously found that the response to single-agent lenalidomide can improve over time, with a median time to best response of 25 months, and that low baseline B2M level was associated with long-term response.7 Median follow-up in the previous studies investigating this combination was too short (<3 years) to detect any association between low baseline B2M level and increased best ORR, likely explaining conflicting results with our current study.
In the current study, mutations in the NOTCH signaling pathway, including NOTCH1, SPEN, and FBXW7, revealed a trend for association with inferior clinical outcomes, particularly in TN patients. SPEN (previously known as MINT or SHARP) forms a complex with a DNA-binding protein, RBPJ, and represses the transactivation activity of NOTCH1.24,25 The mutations in SPEN typically observed in CLL are truncating mutations clustered on exon 11, disrupting the SPOC C terminus domain, which is essential for SPEN heterodimerization and its inhibitory role in NOTCH signaling.26 Loss-of-function mutations may therefore increase NOTCH signaling, favoring resistance to lenalidomide and disease progression, as previously described in multiple myeloma.27 In our study, mutations in FBXW7, which leads to activation of the NOTCH pathway,28 were also associated with a trend for shorter PFS and OS in patients with TN CLL. Activation of the NOTCH pathway may thus contribute to resistance to treatment, as suggested by a report of promotion of apoptosis and decreased proliferation of CLL cells in vitro after NOTCH1 inhibition with PF-03084014.29
We acknowledge some limitations of our gene mutation analysis: this was an exploratory analysis, and the initial study design was not powered to detect differences in outcomes based on pretreatment gene mutations. In addition, the absolute number of single gene mutations per arm was small (<5) and the number of considered gene mutations very large. Although this was partially corrected by clustering them in pathway groups (each with an absolute number of mutations ≥5 per arm), the observed association did not achieve statistical significance, and larger datasets are needed to confirm these findings. Finally, given the exploratory nature of the correlative studies, no functional studies were performed to explain the biological mechanism of observed associations.
Deletion of 17p and/or 11q as detected by FISH was associated with shorter survival in our study, a finding also observed in a previous report, likely causing poor response to subsequent lines of therapy in patients with these abnormalities.14
Finally, no differences in ORR, PFS, OS, or toxic effects were observed between patients who received all components of their treatment at our center and patients who received treatment under the care of their local provider. This outcome shows that this treatment can be effectively and safely performed through collaboration between academic centers and local hematologists and oncologists.
In conclusion, the combination of lenalidomide and rituximab is an effective and safe regimen for the treatment of patients with TN CLL or R CLL. B2M level predicted response to this regimen in both TN and R patients, whereas gene mutations inducing increased NOTCH signaling, such as NOTCH1, SPEN, and FBXW7 mutations, predicted shorter PFS after this treatment. These findings indicate that further exploration of the NOTCH pathway may help optimize the efficacy of this combination in patients with CLL and potentially in those with other low-grade lymphomas.
Acknowledgments
The authors thank Christina Hinojosa and Diana L. Rodriguez for data collection and Sarah J. Bronson for manuscript revision.
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health, National Cancer Institute through a Cancer Center Support Grant (P30CA016672). C.B.P. was partially supported by National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30CA016672 (Biostatistics shared resource) and by MD Anderson CLL Moon Shot 710499-80-111996-21.
Authorship
Contribution: A.F. designed, performed, and analyzed the trial, provided clinical care to patients, and wrote the paper; P.S. analyzed data, performed statistical analysis, and wrote the paper; C.B.P. reviewed the statistical analysis and coauthored the paper; P.A.T., M.J.K., N.G.D., N.J., J.A.B., Z.E., S.M.O., H.M.K., and W.G.W. provided clinical care to patients and coauthored the paper; and K.T. and P.A.F. performed genomic analysis and coauthored the paper.
Conflict-of-interest disclosure: M.J.K. was a consultant for Celgene Corporation. W.G.W. was a consultant/advisory board member for Celgene Corporation. S.M.O. was a consultant for Celgene Corporation. A.F. received research support from Celgene Corporation. The remaining authors declare no competing financial interests.
Correspondence: Alessandra Ferrajoli, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: aferrajo@mdanderson.org.