Abstract
Although the hemostatic potential of adult platelets has been investigated extensively, regulation of platelet function during fetal life is less clear. Recent studies have provided increasing evidence for a developmental control of platelet function during fetal ontogeny. Fetal platelets feature distinct differences in reactive properties compared with adults. These differences very likely reflect a modified hemostatic and homeostatic environment in which platelet hyporeactivity contributes to prevent pathological clot formation on the one hand but still ensures sufficient hemostasis on the other hand. In this review, recent findings on the ontogeny of platelet function and reactivity are summarized, and implications for clinical practice are critically discussed. This includes current platelet-transfusion practice and its potential risk in premature infants and neonates.
Introduction
Platelets are the primary effectors of hemostasis. Their function and underlying activation patterns have been a matter of intense research for many years and, thus, are well described in adults.1 In contrast, limited knowledge exists concerning the in vivo functionality of fetal platelets and their regulation within the developing organism.
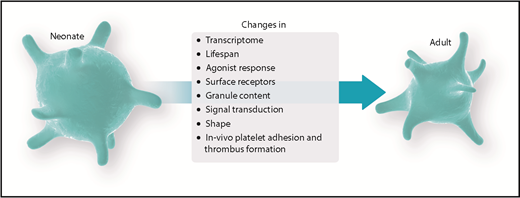
Fetal and neonatal platelets have been described to feature distinct activation responses compared with their adult counterparts, ultimately being designated as hyporeactive.2 Nonetheless, it has been speculated whether this hyporeactivity reflects a physiologically and/or clinically desirable state. Whereas the adult organism is routinely challenged with a variety of stressors and injuries, the fetal organism resides in a protected environment, aimed at optimization of growth and development, until birth. Only recently have new imaging modalities allowed in vivo examination of the fetal platelet hemostatic potential in addition to existing data from in vitro animal studies and/or human peripheral and umbilical cord blood samples.3-5 Combined, these findings led to the assumption of an age-dependent regulation of fetal platelet function, raising concerns about the possible adverse effects of platelet transfusions in the neonate.6 Furthermore, most research has focused primarily on in vitro tests and the hemostatic potential of fetal platelets, disregarding consequences of the immunological and/or angiogenic potential of platelets in the neonate. We will briefly address those additional functions but primarily focus on changes in hemostatic platelet function during the fetal and neonatal periods. Finally, we will critically put into context the recent experimental findings with platelet-transfusion practice.
Fetal vs adult platelet function: an age-dependent maturation process
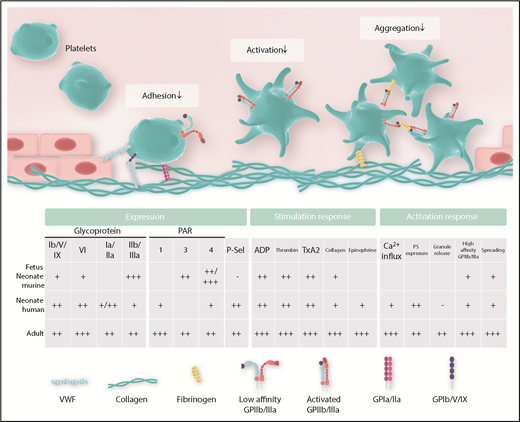
Thrombus formation occurs in a stepwise fashion and involves the concerted action of specific platelet receptors and their respective ligands. In a simplified model, these steps are adhesion, activation, aggregation, and firm clot formation (Figure 1).7 Later steps include clot retraction and consolidation, ultimately allowing the mature organism to offer adequate repair mechanisms upon vessel injury.8-10
Important platelet expression and activation responses in the neonate.
The platelet surface receptor complex GPIb/V/IX has a central role in mediating initial platelet adhesion directly via interaction with von Willebrand Factor (VWF) and indirectly with collagen, a component of the extracellular matrix exposed to platelets after disruption of vessel integrity. Additional collagen receptors on platelets include GPIa/IIa and GPVI. Platelet adhesion triggers platelet activation, which is also supported by ligand binding to multiple platelet-expressed receptors, including thrombin receptors (protease-activated receptor 1/4 [PAR1/4] in humans, PAR3/4 in mice), adenosine diphosphate (ADP) receptors (P2Y12, P2Y1), the thromboxane receptor, and α-adrenergic receptors. Activating these receptors, in turn, further consolidates platelet adhesion, aggregation, and cross-linking through activation of GPIIb/IIIa (αIIbβ3 integrin), which binds to VWF, fibronectin, vitronectin, and fibrinogen.11 Compared with adults, the fetal organism faces distinct challenges, such as the prevention of thrombus formation and immune activation and the uninhibited delivery of factors needed for organ growth and development. From this, it appears plausible that cells differ in their activation responses to match up with their local requirements (Figures 1 and 2).
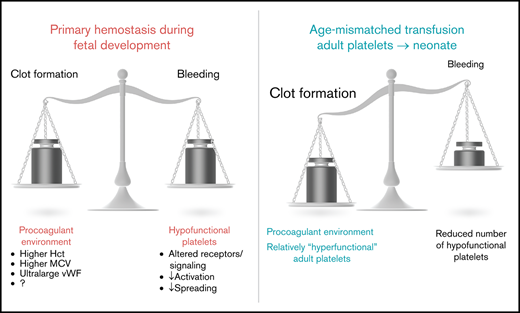
Ontogenetic regulation of platelet function. Experimental data suggest a balanced environment in which platelet function is tightly regulated to achieve an age-dependent modulation of platelet responses.
Ontogenetic regulation of platelet function. Experimental data suggest a balanced environment in which platelet function is tightly regulated to achieve an age-dependent modulation of platelet responses.
Surface expression of adhesion relevant molecules
The functional interaction of platelets with various binding partners relies on the adequate expression of relevant surface molecules. Differences in the expression pattern of surface receptors on fetal and neonatal platelets, as well as their respective ligands, have been reported for human, mouse, and rat samples. Figure 1 and Table 1 summarize the available data on expression of platelet receptors and ligands in the fetus and neonate, respectively. For platelet-expressed adhesion receptors, Schlagenhauf et al reported higher GPIbα levels in term newborn platelet lysates isolated from human cord blood.12 Earlier studies, including data from the same group, found similar receptor numbers for GPIb on platelets in human neonates and adults.13,14 More recently, surface levels of GPIbα and GPIbβ were measured by flow cytometry in mouse samples of different gestational ages, revealing reduced levels of GPIb in fetal platelets compared with adults.3 The reported differences in GPIb expression most likely stem from differences in methodology, age, and species analyzed. Levels for the collagen-binding integrin receptor GPIa/IIa were similar in human newborn and adult platelets in one study,5 whereas another study reported reduced levels of GPIa/IIa in the full-term neonate.15 For the collagen receptor GPVI, reduced values were reported in preterm and term human neonates.16 Most prominent for its function in platelet aggregation and platelet cross-linking, GPIIIa levels, as part of the GPIIb/IIIa receptor, have been reported to be reduced in platelets from preterm infants compared with term newborns.17 Similarly, the investigators of that study reported reduced levels of GPIIb/IIIa in neonatal vs adult platelets, whereas other investigators described equal amounts of GPIIb/IIIa in term newborns compared with adults.12,15,17 We investigated GPIIb/IIIa expression in mouse fetuses and found an overall increase in GPIIb expression at embryonic day (E)13.5 (of 21 days’ gestation) compared with later time points.3
Platelet adhesion
As outlined above, platelets can adhere to the endothelium or subendothelial tissue via their surface receptors GPIb/V/IX, GPIa/IIa, and GPVI (Figure 1). This allows direct binding to collagen or interaction with subendothelial and circulating VWF, resulting in slowing down and adhesion of the formerly free-flowing platelet. Also, GPIIb/IIIa is known for its ability to bind to VWF.
Using whole blood, Baker-Groberg et al showed that platelets from healthy full-term neonates adhered similarly on collagen and VWF under static and dynamic flow conditions compared with adults.18 Yet, previous data by Shenkman et al revealed that whole-blood samples from term neonates exhibited an increased adhesion, but similar aggregate formation, on subendothelial extracellular matrix, which was attributed to the hyperactive multimerized VWF in neonatal plasma.19 In another study, reduced deposition of platelets from premature infants was found on extracellular matrix under shear flow conditions, again tested with whole blood.20 Caparrós-Pérez and colleagues reported delayed adhesion of neonatal platelets in a static adhesion assay in which plasmatic components, such as VWF multimers, were excluded.21 These data were recently amended by in vivo observations of thrombus formation in the mouse fetus in which an age-dependent regulation of platelet adhesion was detected. We observed decreased in vivo platelet adhesion in early-gestation (E13.5) fetuses compared with older fetuses toward the end of gestation. Additionally, the time to platelet adhesion was prolonged in the very young fetuses.3 The aforementioned differences in the expression of relevant adhesion receptors, including GPIb and GPIa/IIa, are likely to impact the observed reduced adhesion capacity of fetal platelets; the higher levels of VWF multimers could explain the increased adhesion capacity in term neonates in whole blood, but the exact mechanisms have to be determined in more detail. The overall number of studies on neonatal platelet adhesion is rather limited, because the major readout of many studies focuses on platelet aggregation or agonist responses. Of interest, it becomes apparent that the developmental stage of platelets (preterm vs full term) has to be taken into consideration when interpreting experimental data on platelet adhesion, and one also has to clearly distinguish between whole-blood assays, involving the presence of VWF multimers and/or an increased hematocrit, and procedures performed on isolated platelets. These likely explain the differences between the observations by Shenkman et al (increased platelet adhesion in whole blood)19 and those by Caparrós-Pérez and colleagues (delayed adhesion and spreading in plasma-free medium),21 although other factors (static vs dynamic adhesion assays) are likely to contribute to the differences as well. The existence of a procoagulant environment will be discussed in more detail in “Whole-blood hemostasis.”
Platelet activation
Following platelet adhesion, activation of platelets is critical for concerted thrombus formation. Commonly used platelet agonists in studies with fetal/neonatal platelets include thrombin (binding to PAR3/4 [mouse] and PAR1/4 [human]), ADP (binding to P2Y receptors), thromboxane A2 (binding to thromboxane receptor), α2-adrenergic agonists, such as epinephrine (binding to α2-adrenergic receptors), and collagen (binding to GPVI and GPIa/IIa).22
Thrombin–PAR interaction.
Baker-Groberg et al reported that activation responses in human neonatal platelets were reduced following stimulation of thrombin receptors (G protein–coupled receptors).18 Also, older studies found impaired responses upon thrombin stimulation.2,17 Generally, in vitro studies of thrombin stimulation revealed reduced activation of neonatal platelets from preterm and term human neonates.23,24 In addition, an age-dependent lack of activation was demonstrated during mouse fetal ontogeny.3 Speculations on birth-induced preactivation of neonatal platelets as a cause for reduced thrombin-mediated activation were shown to not be plausible.25 Although the cause for the hyporeactivity remains to be elucidated in more detail, current data suggest that there are also species-specific differences. PAR1 and PAR4 levels were found to be reduced in human neonatal platelets, which may result in the observed hyporeactivity toward thrombin.26 This finding could not be reproduced in the mouse; PAR3 levels in fetuses were not different from adults, whereas PAR4 levels were even increased, especially at midgestation,3 pointing toward altered downstream signaling components instead.
ADP–P2Y receptor interaction.
In vitro studies of ADP-induced platelet activation revealed reduced activation of neonatal platelets from preterm and term human neonates.18,23,24 Reduced activation of fetal platelets has been reported in the mouse,3 yet detailed studies examining surface expression of P2Y receptors are missing, as are analyses of downstream signaling components.
Adrenergic and thromboxane receptors.
Stimulation with epinephrine and a thromboxane analog revealed platelet hyporeactivity in neonatal platelets.27,28 Corby and O’Barr reported reduced levels of α-adrenergic receptors on newborn platelets compared with adults as a possible cause of epinephrine-dependent hyporeactivity,27 whereas no difference was reported for thromboxane receptors among adults and neonates.29,30 Rather, reduced platelet activation following stimulation with thromboxane is thought to be the result of decreased activity of the GTP-binding protein Gq and PLCβ, leading to impaired calcium mobilization in neonatal platelets that is even more pronounced in premature platelets, suggesting a developmental regulation during fetal life.28
Collagen receptors GPVI and GPIa/IIa and podoplanin receptor CLEC-2.
Human neonatal platelets are hyporeactive to stimulation with collagen or collagen-related peptide.5,18 GPVI mediates intracellular signaling via an immunoreceptor tyrosine-based activation motif, resulting in platelet activation, secondary agonist release, and integrin activation.31 A recent study discovered reduced GPVI-dependent activation in preterm and term neonates, with reduced levels of GPVI described in that study.16 The same study also reported reduced levels of the α2 integrin subunit, as part of the GPIa/IIa receptor, which might contribute to the hyporeactivity of neonatal platelets. The other immunoreceptor tyrosine-based activation motif–bearing receptor on platelets, C-type lectin-like receptor 2 (CLEC-2), which is capable of binding to podoplanin, was also shown to be reduced on neonatal platelets and exhibited a decreased responsiveness to CLEC-2 agonist binding.16
In summary, solid evidence exists that neonatal platelets are hyporeactive toward most agonists. Interestingly, when using stimulants that bypass surface receptors, such as calcium ionophore, a stronger activation response could be elicited compared with receptor-dependent agonists, such as thrombin or ADP,3 yet changes remained below the level of adult platelets. This finding suggests that hyporeactivity of fetal/neonatal platelets is often multifactorial, including lower receptor levels and/or altered intracellular signaling pathways.
Activation responses of fetal/neonatal platelets
Stimulation of platelets by successful agonist–receptor interactions elicits a series of activation responses, including an increase in intracellular calcium levels, degranulation, a conformational change in the GPIIb/IIIa complex toward a high-affinity conformation, and cytoskeletal reorganization allowing platelet spreading.
Intracellular calcium signaling.
Calcium mobilization represents one of the primary end points of a multitude of intracellular signaling pathways that is used to assess platelet activation responses.32 Neonatal platelets exhibit impaired calcium signaling following thrombin or collagen stimulation, as shown by Gelman et al and reviewed by Varga-Szabo et al, possibly affecting multiple prominent platelet responses in the newborn.33,34 Of note, Gelman et al additionally showed that calcium content of the dense tubular system was unchanged in the neonatal platelets, pointing out that the observed phenotype is most likely due to a reduction in calcium mobilization.33 Similarly, Israels et al found that calcium mobilization was impaired following stimulation with the thromboxane analog U46619.28
Degranulation.
Platelet degranulation leads to the surface expression of stored molecules, such as P-selectin, as well as the exocytosis of secondary mediators, such as ADP.10 Recent work showed that platelets from healthy full-term neonates exhibit decreased degranulation due to downregulation of the Munc18b–syntaxin-11 complex.21 Interestingly, the total number of granules did not appear to be different in this study. One of the classical markers of degranulation is P-selectin, stored within α-granules, which is mobilized to the platelet surface upon stimulation. Interestingly, although human and murine data showed reduced platelet mobilization, we observed reduced total P-selectin content in mouse samples,3 whereas Caparrós-Pérez et al reported normal total P-selectin levels.21 Although, generally, differences between cord blood and peripheral blood samples have to be considered as well, Sitaru et al reported no difference in α-granule release in cord and peripheral blood samples in neonates, as measured by P-selectin+ platelets in the first 24 hours of life. Platelets from both sources showed significantly lower levels of P-selectin expression compared to adult platelets; however, Sitaru et al demonstrated that there was significant improvement in P-selectin expression levels following stimulation over the first 10 to 14 days of life.17 In contrast to reduced platelet degranulation, no difference in phosphatidylserine content or exposure was found between human neonatal and adult platelets.35
Conformational change of GPIIb/IIIa.
Following agonist stimulation, a central event in thrombus formation is the conformational change in the platelet integrin αIIbβ3 (GPIIb/IIIa) from a bent inactive form to a fully extended active form necessary for successful binding of ligands, including fibrinogen and VWF. The regulation of integrin activation and inside-out signaling responses critically rely on the integrin adaptor proteins kindlin-3, talin-1, and Rap-1 among others.36 Reduced levels of these integrin adaptor molecules have recently been demonstrated in murine fetal platelets and might contribute to decreased integrin function at midgestation.3 This finding corroborates previous data on platelet hyporeactivity with reduced PAC-1 (antibody directed against activated GPIIb/IIIa complex) binding in human neonates.17 In contrast, one clinical study in neonates reported a hyperreactive state of platelets during birth, resulting in increased PAC binding. However, this study has a number of methodological issues (platelet fixation protocol, negative control using CD61 single staining instead of isotype controls) that might be responsible for in vitro activation of platelets or overestimation of activation. Therefore, the results should be interpreted with caution.37 Of interest, Caparrós-Pérez et al performed a platelet transcriptome analysis and showed variations in the neonatal platelet transcriptome compared with adults.38 In fact, they reported that genes involved in cell signaling and calcium metabolism were among those most downregulated in neonatal platelets. Interestingly, this included reduced expression of ITGB3BP encoding for the integrin β3 binding protein, which has been associated with aggregation, platelet production, and regulation of integrin affinity.39,40 Future transcriptomic and proteomic studies should provide additional valuable insights to identify the molecular mechanisms underlying the observed peculiarities of fetal platelets and their functional changes during ontogeny.38
Platelet spreading.
Integrin activation is important for ligand binding, as well as for consecutive integrin outside-in signaling leading to postadhesion events, including cytoskeletal reorganization and platelet spreading. Platelet spreading, a process needed for increasing the platelet surface, was shown to be reduced in human and murine neonatal platelets. Isolated mouse platelets from fetuses showed decreased relative platelet area and perimeter changes upon seeding on fibrinogen and stimulation with thrombin.3 The study by Caparrós-Pérez et al also showed decreased spreading response in platelets from healthy full-term neonates upon seeding on poly-l-lysine.21 This was linked to a downregulation of the Munc18b–syntaxin-11 complex (as also shown for degranulation).21 With respect to postadhesion steps, recently, platelet migration was put into focus.41 However, the functional relevance of this observation, as well as potential connections to immune responses, needs further examination in neonates, as well as in adults.
Whole-blood hemostasis
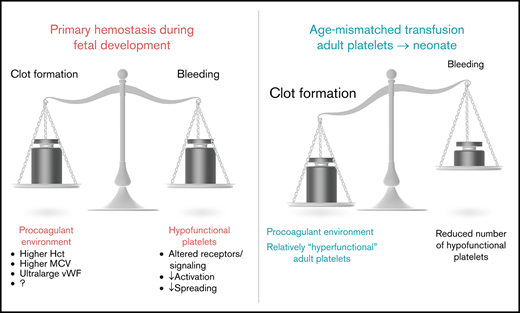
Independent of the aforementioned findings consistently demonstrating a hyporeactive phenotype of fetal or neonatal platelets, it is dangerous to assume a generally reduced hemostatic capacity in the neonate. Hyporeactivity in these studies was demonstrated primarily by flow cytometry for P-selectin surface expression or PAC-1 binding42 or by using assays in isolated platelet samples. Results from whole-blood hemostatic assays show that, depending on the stimuli used, normal or even increased adhesion/aggregation responses can be observed in healthy term neonates. Indeed, a recent study showed that full-term neonatal platelets exhibit similar adhesion and aggregate-formation capabilities in an in vitro flow chamber assay.18 Studies using the PFA-100 System showed reduced closure times in whole-blood samples from healthy neonates,43 even though these data were affected by gestational age.44 In line with this, bleeding times of healthy term neonates were also reported to be shorter compared with adults,45 whereas Del Vecchio et al again emphasized an age dependency, with a prolongation of bleeding times in preterm neonates.46 In addition to the impact of gestational age on whole-blood assays, as discussed above, the presence of plasmatic components is most likely crucial for the interpretation of contradicting results. Caparrós-Pérez and colleagues observed reduced platelet adhesion and aggregation in plasma-free medium,21 whereas Shenkman et al reported an increased deposition of platelets from full-term neonates on subendothelial extracellular matrix in whole-blood samples.19 This points toward the importance of plasmatic components for a functional whole-blood hemostatic capacity in the neonate. Indeed, an interesting study revealed an impressive procoagulant activity of neonatal whole blood. Ferrer-Marin et al placed adult platelets into platelet-depleted neonatal whole blood and assessed closure times using the PFA-100 System.4 The PFA-100 System replaces the assessment of bleeding times and is commonly used in pediatrics to assess the primary hemostatic capacity, because the system involves shear stress and agonist-induced activation of platelets in whole blood to reach the formation of a stable platelet plug (closure time). When adult platelets were placed into neonatal blood, closure times were dramatically shorter compared with platelets placed into adult blood.4 These results show that neonatal blood possesses procoagulant properties in which adult platelets reach a hyperreactive hemostatic potential, whereas neonatal (per se defined as hyporeactive) platelets might reach a level of functionally sufficient hemostasis. Factors responsible for this prothrombotic phenotype might be the presence of unusually large VWF multimers, an increased hematocrit, or increased mean corpuscular volume.47 In this respect, Muntean and colleagues suggested that shorter closure times in the PFA-100 System assay relied on the presence of VWF multimers in the neonate rather than on an increased hematocrit.48 These important in vitro findings were amended by in vivo observations in the mouse in which isolated adult platelets were transfused into E14.5 mouse fetuses. This resulted in fast platelet adhesion and thrombus formation at various sites, whereas the rate of thrombus formation was lower when age-matched E14.5 platelets were transfused.3 These findings exemplify the prohemostatic potential of fetal and neonatal blood with imminent implications on transfusion strategies, which will be discussed in “Clinical implications.” Interestingly, clot firmness was reduced in term neonates to below adult levels, as well as to below comparative pediatric levels, pointing out that functional clot formation does not imply adequate thrombus stability,49 which is in line with reduced thrombus stability observed in the mouse fetus.3
Platelet counts and thrombopoiesis
The quality, as well as the quantity, of platelets is critical for adequate hemostatic function.50 Tober et al investigated the first emergence of platelets into the mouse circulation and reported a subsequent increase in the number from E10.5 onward.51,52 Interestingly, platelet counts remain significantly below adult levels until postnatal days 10 to 14 in the mouse.3,53 According to Liu et al, platelet counts and the overall platelet mass increase around birth as a result of changes in platelet lifespan.53 A study of 47 000 patients emphasized an age-dependent regulation of platelet counts in humans as well.54 With regard to size, platelets from premature infants born before 31 weeks of gestation had a higher mean platelet volume (MPV) compared with late preterm and term neonates, whereas newborns generally exhibited a postnatal increase in MPV.54 Likewise, MPV and the platelet large cell ratio were inversely correlated with gestational age and were highest in E13.5 murine fetuses compared with later developmental stages.3 Similar to increased mean corpuscular volumes in erythrocytes of premature infants and neonates, high fetal MPV is considered a sign of cellular immaturity and might outweigh the dramatically reduced platelet numbers to reach a higher platelet mass (MPV × number × volume) relative to the existing vessel surface.
Remarkably, megakaryopoiesis also appears to be ontogenetically regulated (reviewed by Elagib et al55 ). Recent studies found an increase in platelet-forming functions, with upregulation of transcription factors (eg, STAT5A and ETS2) during gestation. In addition, megakaryocyte ploidy shows only a slow increase during fetal life. Finally, an overall change toward a hemostasis-supporting and platelet-producing phenotype was noted with upregulation of ITGA6, CXCR4, VWF, SLC6A4, and MAOB.55,56
Platelet function beyond hemostasis
In addition to their crucial role in hemostasis, platelets have been described to be involved in a multitude of inflammatory, antibacterial, (lymph-)angiogenic, and lymphatic processes.57-60 Because platelets are capable of guiding leukocytes to extravasation sites61 and are important mediators of immune defense, eg, in the lung,62 we have recently investigated whether platelets interact with leukocytes in mouse fetal microvessels.3 Our in vivo results in the mouse fetus showed that platelets did not interact with fetal leukocytes at E14.5. Because platelet–leukocyte interactions rely heavily on platelet P-selectin and leukocyte-expressed PSGL-1, we also analyzed surface expression of these adhesion molecules. We found a strong downregulation of P-selectin on fetal platelets in the mouse and a moderate reduction in PSGL-1 on fetal leukocytes, which might explain the absence of platelet leukocyte interactions in the mouse fetus.3,63,64 More recently, attention has been focused on platelets for their antimicrobial properties41,65,66 ; however, platelet migration and internalization of bacteria by platelets have not been studied in the fetus. Similarly, effects of neonatal platelets on T cells, such as regulation of T-cell polarization, have not been thoroughly examined in the fetus.67 Finally, lymphatic vessel growth and lymph node integrity depend, in part, on megakaryocytic and platelet CLEC-2.68-70 Preliminary data show reduced CLEC-2 expression in neonatal platelets, although it remains unclear what level of expression is needed to reach sufficient lymphatic development in the human fetus.16
Clinical implications
Decreased platelet counts and a hyporeactive platelet phenotype in the developing fetus might put the fetus at risk for severe bleeding complications; however, major bleeding is rarely observed in the growing fetus while in its physiologic environment. This delicately balanced system is challenged in the case of premature birth: postnatal bleeding risk increases dramatically and correlates inversely with gestational age.71 The reason for this is only partially clear.
Neonatal thrombocytopenia and association of platelet counts with hemorrhage
Thrombocytopenia is a frequent finding in neonates, affecting roughly 20% to 30% of newborns admitted to the neonatal intensive care unit (NICU).72-74 The prevalence is inversely correlated with gestational age and can increase to up to 85% in extremely low birth weight infants.75,76 In most clinical settings, platelet counts <150 × 109/L are used for the diagnosis of neonatal thrombocytopenia, with distinction of mild (100-150 × 109/L), moderate (50-100 × 109/L), and severe (<50 × 109/L) forms.77 However, as demonstrated by Wiedmeier et al, who examined >47 000 neonates from 22 to 42 weeks of gestation, platelet counts depend on gestational age, with a lower reference range (fifth percentile) of 104 × 109/L in neonates born before 32 weeks of gestation.54 Therefore, platelet counts between 100 × 109/L and 150 × 109/L might still be within the normal range in very preterm infants, leading to a relative “overdiagnosis” of thrombocytopenia in this patient population.54
The greatest concern with neonatal thrombocytopenia is the development of severe bleeding, especially intraventricular hemorrhage (IVH), which is frequently seen in premature infants.78 Several, mostly retrospective, studies have linked thrombocytopenia to an increased risk for IVH79-83 ; however, this does not prove causality. As shown by Stanworth et al in the prospective PlaNeT-1 study, 91% of observed neonates developed no or only minor hemorrhage, despite severe thrombocytopenia (defined as platelet count <60 × 109/L).84 Furthermore, IVH is detected before the onset of thrombocytopenia in many preterm neonates, and IVH incidence and progression do not correlate with the degree of thrombocytopenia.81,82,84,85 Thus, it was suggested that thrombocytopenia might be a marker for the severity of underlying illness rather than an actual contributor to increased bleeding risk itself.86
At the same time, platelet numbers within the normal reference range are not necessarily equivalent to a functioning hemostatic system. As evidenced in an earlier study by Andrew et al, many very low birth weight infants weighing <1500 g had prolonged bleeding times, despite nearly normal platelet counts.79 As described in the first part of this review, distinct functional differences exist between neonatal and adult platelets, which are even more pronounced in preterm infants.5,17,20,87,88 Therefore, platelet counts alone are likely not a sufficiently appropriate marker to evaluate bleeding risks in neonates and, especially, in preterm infants. In the Neo-HAT study, Deschmann et al are investigating whether closure time ADP measured with a PFA-100 System, as a global in vitro test of primary hemostasis, is a better predictor of bleeding in thrombocytopenic neonates than platelet counts alone.89
Platelet transfusions in neonates: potential benefits and harms
Platelet transfusions are still the treatment of choice for neonatal thrombocytopenia.90 Because of the fear of higher-grade IVH and other hemorrhagic complications, the large majority of platelet transfusions administered in the NICU (up to 98% in some studies) are prophylactic84,91-93 ; however, the benefit of platelet transfusions to prevent bleeding in neonates is still questionable.94 Twenty-five years ago, Andrew et al performed a controlled trial to investigate the effect of early prophylactic platelet transfusion on IVH incidence and/or extension in 152 preterm neonates with mild or moderate thrombocytopenia during the first 72 hours of life.95 They did not find any difference in the rate or extent of IVH between the treatment group receiving platelet transfusions to maintain counts >150 × 109/L until day 7 or the control group, which was only transfused if platelet counts fell below 50 × 109/L, indicating that early prophylactic platelet transfusion is not beneficial in this setting. Just recently, the results of the PlaNet-2 study addressing the question of whether prophylactic platelet transfusions are useful to prevent bleeding in severely thrombocytopenic neonates and beyond the first week of life have been published.96,97 In the trial, 660 preterm neonates (median gestational age 26.6 weeks) were randomized to receive platelets at a transfusion threshold of 50 × 109/L or 25 × 109/L, and death or new major bleeding within 28 days after randomization was evaluated as primary outcome. Remarkably, the investigators found that death or major hemorrhage occurred significantly more often in the high-threshold group compared with the low-threshold group (26% vs 19%), whereas the rate of serious adverse events was comparable in both groups.
In view of these concerning results, the questionable benefit of platelet transfusions should be carefully weighed against its potential harms90 (Figure 3). Several studies reported an increase in neonatal mortality rates with an increasing number of administered platelet transfusions, with mortality reaching as high as 50% in patients who received >20 transfusions.6,75,84,91,98 Although this association is surely confounded by the severity of the underlying illness, a sensitivity analysis using data from 1600 thrombocytopenic NICU patients suggested that platelet transfusions per se are responsible, to some degree, for increasing mortality.6 It is well documented that the relative rate of adverse events, including febrile and allergic reactions, transmission of infections, and TRALI, is highest after transfusion of platelets compared with other labile blood components.99-101 This may be explained, in part, by the underlying medical condition of the recipients and the storage of platelet concentrates at room temperature. However, as already described, it is increasingly recognized that platelets are key players in hemostasis, as well as important regulators of physiological and pathological processes, such as inflammation and angiogenesis.57,59 Platelets contain vast amounts of bioactive mediators, such as inflammatory cytokines, vasoactive agents, coagulation factors, adhesion molecules, and growth factors, which are released or shed from the surface upon activation and have been linked to the development of transfusion-related febrile reactions and TRALI.102
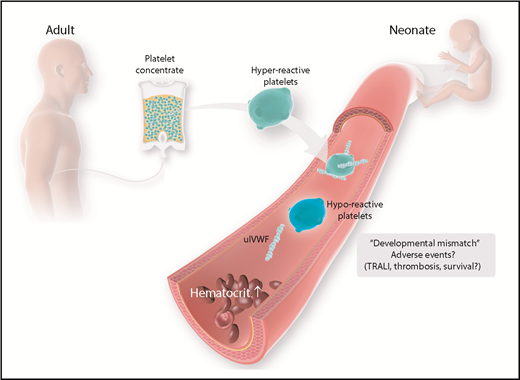
Age-mismatch transfusion of platelet concentrates and possible adverse events. Currently, platelet concentrates are collected from adult donors. Adult platelets feature a distinct phenotype that is different from the physiologic properties of neonatal platelets. Possible harmful effects have to be considered and investigated in more detail. TRALI, transfusion-related acute lung injury; ulVWF, unusually large VWF.
Age-mismatch transfusion of platelet concentrates and possible adverse events. Currently, platelet concentrates are collected from adult donors. Adult platelets feature a distinct phenotype that is different from the physiologic properties of neonatal platelets. Possible harmful effects have to be considered and investigated in more detail. TRALI, transfusion-related acute lung injury; ulVWF, unusually large VWF.
As shown by Stainsby et al, neonates are especially prone to experiencing an adverse outcome following transfusion.103 Possible factors predisposing neonates and, especially, preterm infants to transfusion-related adverse events are the small total blood volume and the functional immaturity of organs and the immune system. Furthermore, children and, particularly, neonates are confronted with a unique situation in transfusion practice, given the fact that there is a developmental difference between recipient and donor because labile blood components are generally derived from adults. As brought forward by Sola-Visner, this “developmental mismatch” might lead to specific problems associated with neonatal platelet transfusions.104 As mentioned above, healthy full-term neonates have shorter bleeding times and shorter closure times compared with adults, as determined using the PFA-100 System, despite the hyporeactivity and decreased adhesive capacity of neonatal and fetal platelets.45,47 Thus, it was hypothesized that the neonatal hemostatic system, instead of being defective, is a finely balanced system in which the developmental differences in platelet function are counterbalanced by a relative “hypercoagulability” of the neonatal blood.77 However, this balance may be disturbed when adult (relatively hyperreactive) platelets are transfused into neonatal blood, potentially leading to thrombotic complications following neonatal platelet transfusion.
Outlook
Recent work has revealed profound differences in the regulation of platelet function and primary hemostasis between the fetal and adult organism. This has wide-reaching implications for clinical decision making in neonatal medicine. Future studies have to address possible therapeutic benefits on the one hand and the potential risks of platelet transfusions on the other hand. Although evidence is still lacking, this view can even be expanded to immunologic and other cellular functions of platelets, taking into account transfusion-related adverse events, such as thrombosis and TRALI. Large randomized controlled trials are needed to fully understand the developmental aspects of platelet function and the possible damage arising from age-mismatched platelet transfusion in neonatology. Modification of adult platelets by pretreatment with specific neonatal-mimicking inhibitory cocktails might be one approach for priming adult platelets to be transfused to match their ontogenetic requirements. New techniques, such as the age-matched in vitro production of platelet concentrates could prove beneficial for clinical applications.105 Nonetheless, it still remains unclear whether priming toward a more active or inactive state is desired for fetal platelet function. Yet, these discoveries hold the potential to also translate findings from neonatal medicine into adult care, where distinct modulations of platelet-reaction properties toward a hyporeactive phenotype might be helpful. Clinically, in view of the possible undesirable side effects of platelet transfusions in the neonate and the worrisome association with increased mortality, efforts should be undertaken to keep the amount of platelet transfusions as low as possible. In light of the recent results of the PlaNeT-2 study,97 lower transfusion thresholds might be combined with parameters other than platelet counts to guide transfusion decisions. In addition, novel experimental techniques might allow a more detailed workup of platelet functions in in vivo fetal animal models, as well as in prematurely born humans.
Conclusions
Platelet function is tightly balanced to achieve functional and effective hemostasis. Recent evidence suggests an ontogenetic regulation of platelet function in the neonate. This involves a maturation-dependent increase in platelet numbers, as well as changes in expression and signaling patterns of platelet surface receptors, that ultimately results in a physiologically diminished capacity for thrombus formation in the young fetus. These findings have critical relevance for clinical decisions on the use of platelet transfusions to treat thrombocytopenia in the NICU. These decisions have to be made with caution and reevaluated in light of the possible adverse effects of “age-mismatched” transfusions to the newborn. New techniques, such as the in vitro production of donor platelets, could help to overcome these limits. Yet, future research is needed to further elucidate the molecular mechanisms governing ontogeny of platelet functions in vivo.
Acknowledgments
This work was supported by research funding from Deutsche Forschungsgemeinschaft Sonderforschungsbereich (SFB) 914 (project B1) (M.S.), associated Integrated Research Training Group of SFB 914 (A.M. and M.S.), scholarship program Förderung Forschung und Lehre FöFoLe (A.M. and M.S.), and the Centre for Interdisciplinary Research (IZKF Münster, SEED 12/18) (A.M.).
Authorship
Contribution: A.M., C.N., and M.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Sperandio, Walter Brendel Centre of Experimental Medicine, Ludwig-Maximilians-Universität München, Großhaderner Str 9, 82152 Planegg-Martinsried, Germany; e-mail: markus.sperandio@lmu.de.