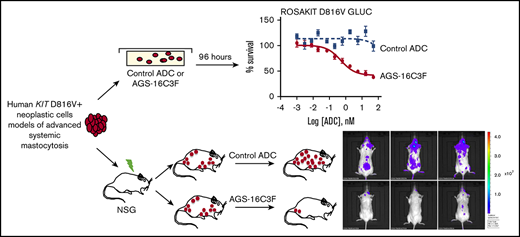
Key Points
CD203c was investigated as a novel treatment target for advanced systemic mastocytosis, using ADC AGS-16C3F.
AGS-16C3F induced apoptosis in human KIT D816V+ neoplastic cells and had antitumor activity in xenografted mice.
Abstract
Antibody-drug conjugates (ADCs) are a new class of therapeutics that use antibodies to deliver potent cytotoxic drugs selectively to cancer cells. CD203c, an ecto-nucleotide pyrophosphatase-phosphodiesterase 3, is overexpressed on neoplastic mast cells (MCs) in systemic mastocytosis (SM), thus representing a promising target for antibody-mediated therapy. In this study, we have found that human neoplastic MC lines (ROSAKIT D816V and ROSAKIT D816V-Gluc), which express high levels of CD203c, are highly and specifically sensitive to the antiproliferative effects of an ADC against CD203c (AGS-16C3F). In these cell lines, AGS-16C3F induced cell apoptosis at very low concentrations. To characterize the effects of AGS-16C3F on leukemia progression in vivo, ROSAKIT D816V-Gluc NOD-SCID γ mouse models of advanced SM (AdvSM) were treated with AGS-16C3F or an ADC control for 2 weeks. Whereas AGS-16C3F had no apparent toxicity in xenotransplanted mice, in vivo neoplastic MC burden significantly decreased in both hematopoietic and nonhematopoietic organs. Furthermore, animals treated with AGS-16C3F had prolonged survival compared with the animals treated with control ADC, and AGS-16C3F efficiently prevented disease relapse. In conclusion, these preclinical studies identified CD203c as a novel therapeutic target on neoplastic MCs, and AGS-16C3F as a promising ADC for the treatment of patients with AdvSM.
Introduction
Mastocytosis is a heterogeneous group of diseases affecting both children and adults, and is characterized by an accumulation of abnormal (neoplastic) mast cells (MCs) in 1 or several organs.1 Most adult patients present with a systemic involvement (systemic mastocytosis [SM]) characterized by an accumulation of abnormal MCs in bone marrow (BM) and in other extracutaneous organs.2 According to the World Health Organization, SM is classified into 5 major categories3 : patients with indolent SM (ISM) have a good prognosis and a nearly normal life expectancy and usually require only symptomatic therapies,4,5 whereas patients with smoldering SM present with a high MC burden and an intermediate prognosis.6 The 3 other SM categories (namely, SM with an associated hematologic neoplasm, aggressive SM, and MC leukemia) are collectively termed advanced SM (AdvSM). Patients with AdvSM share a poor prognosis and usually require cytoreductive treatment.1 These patients are rarely responsive to conventional chemotherapy, or even to targeted therapies such as KIT-targeted tyrosine kinase inhibitors.4 Thus, there is an urgent medical need to find both new targets on neoplastic MCs and new targeted therapies to treat these patients.
KIT (CD117), the tyrosine kinase receptor for stem cell factor, the major growth factor for the MC lineage, is significantly involved in the pathophysiology of mastocytosis; the majority of patients carry KIT mutations.7 In particular, recurrent activating KIT D816V mutations are found in more than 80% of all patients with SM7 and induce sustained proliferative and antiapoptotic signaling in neoplastic MCs.8 Although KIT-tyrosine kinase inhibitors are available for patients with AdvSM, they do not exhibit true curative potential.9
Recent studies suggest that antibody (Ab)-drug conjugates (ADCs) are efficacious as anticancer therapies, as they deliver highly effective cytotoxic agents specifically to the tumor, thereby inducing clinically meaningful responses in patients.10 The specificity of an ADC arises from the Ab component, which binds an antigen that ideally is highly expressed on tumor cells and minimally or not expressed elsewhere.11-14
The cell surface marker CD203c, ectonucleotide pyrophosphatase/phosphodiesterase 3, has been found to be specific for basophils and MCs among cells of the hematopoietic lineage.15,16 CD203c is highly expressed in renal cell carcinoma (RCC), but has restricted expression in normal tissues, with the exception of the kidney.17-20 Cell surface expression of CD203c by basophils and mast cells is upregulated on allergic activation of these cells, and seems to regulate allergic inflammation on these cells.21 Overexpression of CD203c has been reported in neoplastic MCs in mastocytosis22 ; however, the potential of CD203c as a target in this disease has not yet been investigated.
AGS-16C3F is an ADC consisting of an anti-CD203c Ab conjugated to the antimicrotubule agent, monomethyl auristatin F (MMAF), which has demonstrated potent antiproliferative activity on RCC in mouse xenograft models.17 AGS-16C3F was shown to bind to CD203c in human basophils without causing activation, as measured by histamine release. Furthermore, AGS-16C3F has also been tested in a phase 1 clinical trial in subjects with advanced metastatic RCC.23 The results showed that AGS-16C3F can be administered safely at 1.8 mg/kg every 3 weeks, and has antitumor activity in a heavily pretreated, refractory mRCC population. In the present study, we evaluated the effects of AGS-16C3F on a human KIT D816V+ neoplastic MC cell line expressing the CD203c antigen, and tested the in vivo effects of AGS-16C3F in a recently established xenograft model of AdvSM.24
Materials and methods
Reagents
ADCs used in this study, including AGS-16C3F and the control ADC cHmLYS-1c3.G2k-mcMMAF directed against a nonhuman protein (hen egg white lysozyme), were provided by Agensys, Inc., an affiliate of Astellas Pharma, Inc. (Santa Monica, CA).
Human mast cell lines
The recently characterized stem cell factor–independent, KIT D816V+ neoplastic MC line ROSAKIT D816V was cultured in Iscove modified Dulbecco medium (Thermo Fisher, Montigny-le-Bretonneux, France), supplemented with 10% heat-inactivated fetal bovine serum (PAA Laboratories, Velizy Villacoublay, France) and other reagents, as previously described.25 ROSAKIT D816V-Gluc cells expressing the secreted Gaussia princeps luciferase (Gluc) were obtained as previously described by stable transduction of the gene encoding Gluc into ROSAKIT D816V cells, and were cultured in the same medium.24 The human MC leukemia cell line human mast cell (HMC)–1 was provided by J. H. Butterfield (Mayo Clinic, Rochester, MN). Two subclones of the HMC-1 cell line were used (HMC-1.1 expressing the mutant KIT V560G, and HMC-1.2 cells harboring both KIT V560G and KIT D816V mutants), and were cultured exactly as previously described.26
Evaluation of CD203c expression at the cell membrane of human MC lines
The membrane expression of CD203c on the various human MC lines was measured by flow cytometry on a FACSCalibur (BD Biosciences, Rungis, France), using APC anti-human CD203c Ab (Ozyme, Montigny-le-Bretonneux, France).
In vitro measurement of cell proliferation
Cells were incubated in triplicate in medium containing AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF (0 [Control], 0.001, 0.003, 0.008, 0.02, 0.07, 0.21, 0.62, 1.82, 5.57, 16.67, 50, 150, 450, and 1350 nM) in a 5% CO2 incubator at 37°C for 96 hours. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide assay was conducted as described previously.26 Results are expressed as the percentage of control and represent the mean ± SD of triplicates. The half maximal inhibitory concentration (IC50) values for each ADC were calculated using Prism GraphPad 6.0 software after plotting log concentration vs response.
Assessment of apoptosis in vitro
Flow cytometric analysis of apoptosis was measured by combined Annexin V/propidium-iodide (PI) staining as previously described.27 Apoptosis was expressed as a percentage of Annexin V+/PI− and Annexin V+/PI+ cells.
Xenogeneic transplantation of ROSAKIT D816V-Gluc cells in NOD-SCID IL-2R γ−/− mice
NOD-SCID γ mice were bred and maintained under specific pathogen-free conditions at the animal facility of the Gustave Roussy Institute. Animal experiments were performed in accordance with guidelines established by the Institutional Animal Committee. Five million cells of the neoplastic human MC line (ROSAKIT D816V-Gluc) were injected in the tail vein of immune-deficient NOD-SCID γ mice 24 hours (n = 10 per group) after 2.5 Gy X-ray irradiation of the mice.
Detection of xenografted cells
ROSAKIT D816V-Gluc cell burden was determined by flow cytometry analysis and cell numeration in mouse peripheral blood (PB), BM, and spleen samples, using phycoerythrin-conjugated monoclonal antibodies (mAbs) directed against human KIT (CD117; Beckman Coulter, Villepinte, France), phycoerythrin/Cy7 conjugated anti-human CD45 mAbs, and an APC-Cy7-conjugated rat anti-mouse CD45 mAb, both from BD Biosciences. Engrafted ROSAKIT D816V-Gluc cells were defined as hCD45+/KIT+ cells.
Measurement of Gluc activity ex vivo in peripheral blood
Blood samples were collected in EDTA-precoated vials (Thermo Fisher). Gluc activity was determined in a 5-μL sample of plasma by adding 100 μL water-soluble Gluc substrate coelenterazine (50 μg/mL), followed by the immediate acquisition of photon counts, using a luminometer (PerkinElmer EnSpire).28
In vivo treatment with ADC targeting CD203c
AGS-16C3F and control ADC were formulated in a sterile 5% dextrose solution before dosing, and stored at 4°C. Six weeks after reconstitution, xenografted mice were randomly assigned into 4 groups according to their level of secreted luciferase and percentage of circulating MCs for treatment, as follows: a group of 10 mice treated with a non-CD203c targeting ADC (cHmLYS-1c3.G2k-mcMMAF, control ADC) at 4 mg/kg IV ×4 doses, with 1 dose every 4 days (4 mg q4day ×4); a group of 10 mice treated with ADC targeting CD203c (AGS-16C3F) at 4 mg/kg IV ×4 doses, with 1 dose every 4 days (4 mg q4day × 4); a group of 10 mice treated with a non-CD203c targeting ADC (control ADC) at 1 mg/kg IV ×4 doses, with 1 dose every 4 days (1 mg q4day × 4); and a group of 10 mice treated with ADC targeting CD203c (AGS-16C3F) at 1 mg/kg IV ×4 doses, with 1 dose every 4 days.
In vivo bioluminescence imaging
At the end of the treatment, in vivo bioluminescence imaging was performed. Briefly, mice were anesthetized using isoflurane, and Gluc imaging was performed immediately after IV injection of 100 μL water-soluble coelenterazine (4 mg/kg body weight), using a charge-coupled device (CCD) camera (IVIS 50) and analyzed with Living Image Software, as previously described.24
Histopathology and immunohistochemistry analysis
Four mice were euthanized 4 days after the last injection of AGS-16C3F. BM, spleen, and liver were fixed in 10% neutral buffered formalin for 48 hours and then transferred to 70% ethanol. Fixed tissues were stained with a mouse anti-human CD45 mAb (Dako, Trappes, France), as previously described.29 Staining was visualized by Histomouse Kit (Thermo Fisher). The tissue sections were then counterstained with hematoxylin and examined using a Zeiss Axiophot microscope (Carl Zeiss, Marly-le-Roy, France).
Tryptase measurement in serum
At the moment of euthanasia, blood samples were collected in EDTA-precoated vials. Tryptase levels were measured in the plasma of mice from each treatment group by fluorescent enzyme-linked immunoassay following the manufacturer's recommendations (ImmunoCAP Tryptase; Thermo Fisher).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 software. Data are reported as the mean ± SD. Differences in the distribution of continuous variables between categories were analyzed by the Student t test. The Kaplan-Meier method was used for survival analysis. The log-rank test was used to check for equality of the survival distributions. In all evaluations, differences were considered significant if P < .05.
Results
CD203c is differentially expressed on various human neoplastic MC lines
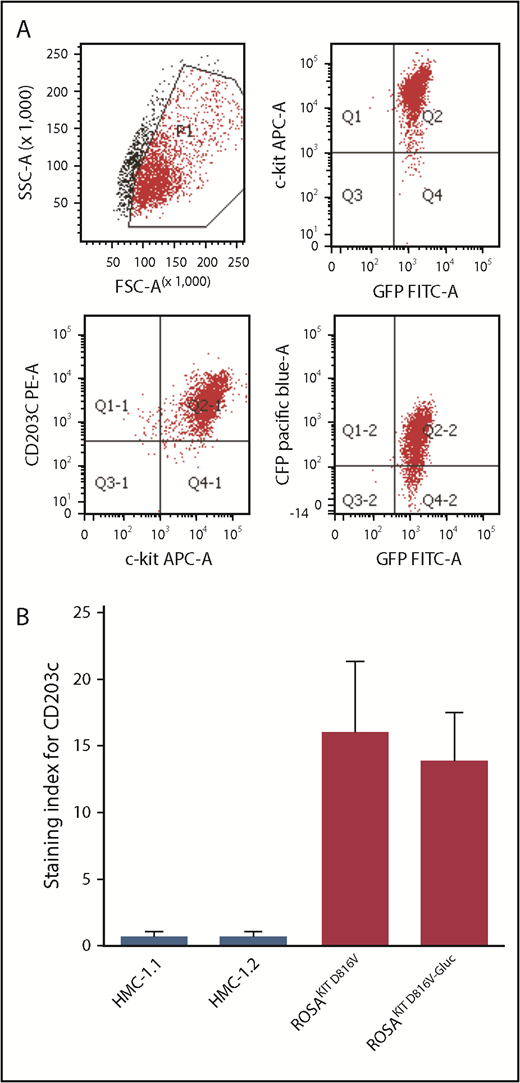
As described in our previous work, we transduced the human neoplastic MC line, ROSAKIT D816V, that stably expressed KIT D816V mutant and GFP, with a lentiviral vector expressing Gluc cyan florescent protein (CFP), LV-82987 Gluc-CFP.24 As shown in a representative flow cytogram (Figure 1A), CD203c was found to be expressed at high levels on ROSAKIT D816V-Gluc (GFP+CFP+KIT+) cells. In addition, analysis of the staining index demonstrates a consistent expression of CD203c on both KIT D816V+ ROSA cell lines (ROSAKIT D816V and ROSAKIT D816V-Gluc), whereas the staining index was found to be very low, if any, on human MC lines HMC-1.1 and HMC-1.2 (Figure 1B).
CD203c expression in human neoplastic mast cell lines. (A) Representative flow cytometric evaluation of CD203c expression on ROSAKIT D816V-Gluc cells. (B) Assessment of surface expression of CD203c by flow cytometry on HMC-1.1, HMC-1.2, ROSAKIT D816V, and ROSAKIT D816V-Gluc cells. Results are expressed as staining index (SI, median fluorescence intensity values of tested Ab relative to its isotype control) and represent the mean ± standard deviation of triplicates. APC-A, allophycocyanin α; GFP, green fluorescent protein; SSC-A, side-scatter area.
CD203c expression in human neoplastic mast cell lines. (A) Representative flow cytometric evaluation of CD203c expression on ROSAKIT D816V-Gluc cells. (B) Assessment of surface expression of CD203c by flow cytometry on HMC-1.1, HMC-1.2, ROSAKIT D816V, and ROSAKIT D816V-Gluc cells. Results are expressed as staining index (SI, median fluorescence intensity values of tested Ab relative to its isotype control) and represent the mean ± standard deviation of triplicates. APC-A, allophycocyanin α; GFP, green fluorescent protein; SSC-A, side-scatter area.
Effects of different ADCs on the proliferation of human neoplastic MC lines
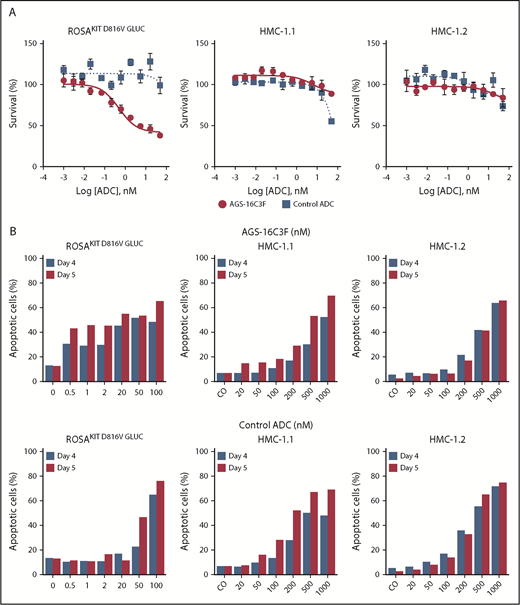
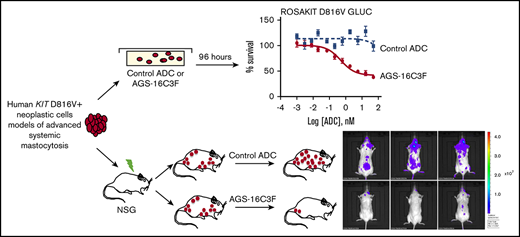
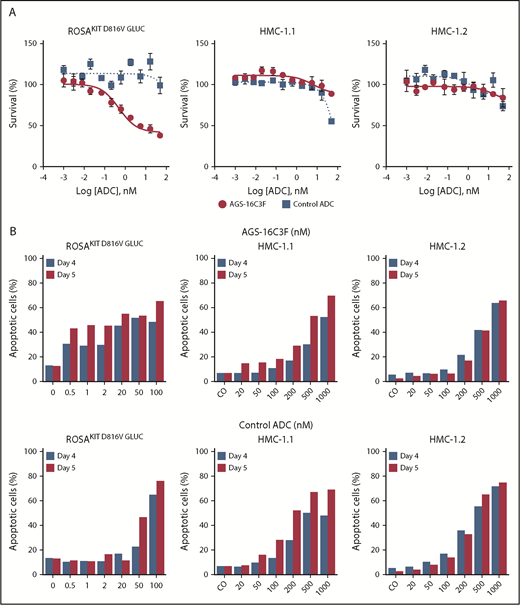
Although AGS-16C3F inhibited the proliferation of the human neoplastic MC line ROSAKIT D816V-Gluc in a dose-dependent manner, as was expected, it had only slight effects on HMC-1.1 and HMC-1.2 cells (Figure 2A). The IC50 values obtained after a 5-day treatment with AGS-16C3F for ROSAKIT D816V and ROSAKIT D816V-Gluc cells were 1.1 and 2.6 nM (Table 1), respectively, similar to what was reported for tumor cells.17
Antiproliferative and pro-apoptotic effects of AGS-16C3F and cHmLYS-1c3.G2k-mcMMAF on human neoplastic mast cell lines. (A) ROSAKIT D816V-Gluc, HMC-1.1, and HMC-1.2 cells were treated with or without different concentrations of AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF. On day 4 of treatment, viability was evaluated. Results are expressed as the percentage of control and represent the mean ± standard deviation of triplicates. (B) ROSAKIT D816V-Gluc, HMC-1.1, and HMC-1.2 cells were incubated in their medium (control) or with different concentrations of AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF for 4 and 5 days. Results are expressed as the percentage of apoptotic cells, as measured by Annexin V/PI labeling.
Antiproliferative and pro-apoptotic effects of AGS-16C3F and cHmLYS-1c3.G2k-mcMMAF on human neoplastic mast cell lines. (A) ROSAKIT D816V-Gluc, HMC-1.1, and HMC-1.2 cells were treated with or without different concentrations of AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF. On day 4 of treatment, viability was evaluated. Results are expressed as the percentage of control and represent the mean ± standard deviation of triplicates. (B) ROSAKIT D816V-Gluc, HMC-1.1, and HMC-1.2 cells were incubated in their medium (control) or with different concentrations of AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF for 4 and 5 days. Results are expressed as the percentage of apoptotic cells, as measured by Annexin V/PI labeling.
AGS-16C3F also induced apoptosis in KIT D816V+ ROSA cell lines more potently compared with HMC-1 cells (Figure 2B). The apoptosis-inducing effect of AGS-16C3F was found to be dose-dependent in all cell lines and, in addition, was found to be time-dependent in ROSAKIT D816V (data not shown), ROSAKIT D816V-Gluc, HMC-1.1, and HMC-1.2 cells. In addition, the control ADC (cHmLYS-1c3.G2k-mcMMAF) was less potent at inducing apoptosis compared with AGS-16C3F (Figure 2B).
The number of ROSAKIT D816V-Gluc cells is reduced in xenotransplanted mice treated with AGS-16C3F
The experimental design for the evaluation of AGS-16C3F is described in supplemental Figure 1. Mice were separated into 4 groups (n = 10) according to the luciferase activity in plasma and the percentage of ROSAKIT D816V-Gluc cells in PB (supplemental Figure 2A-D). Two doses (4 mg/kg or 1 mg/kg) of AGS-16C3F and control ADC were used. After 2 weeks, no effect on the weight of the mice was observed with either ADC (supplemental Figure 3).
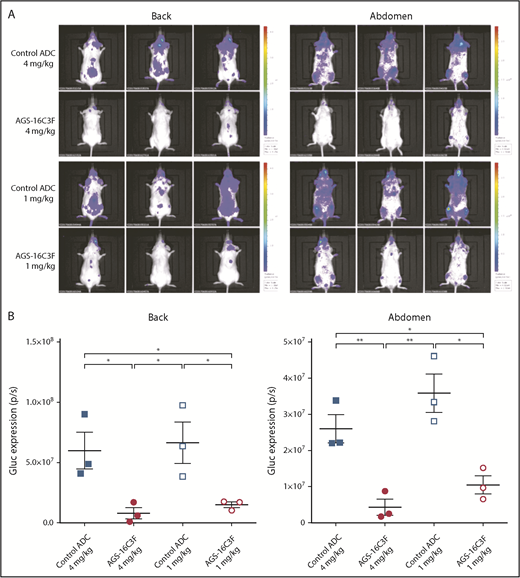
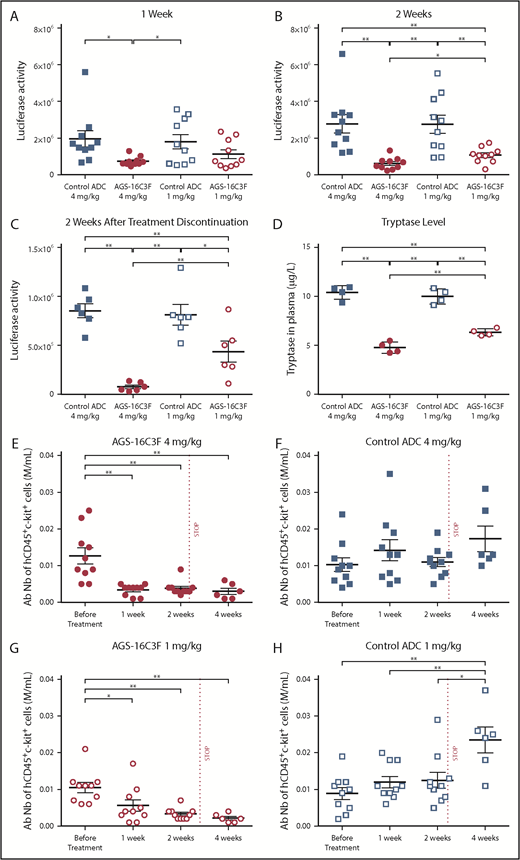
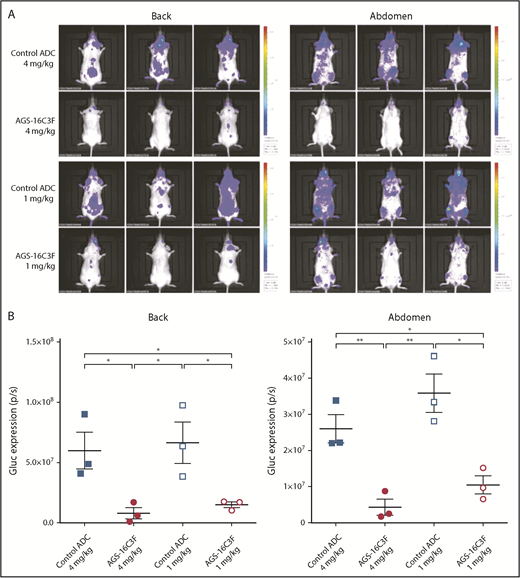
Antitumor effects of AGS-16C3F and control ADC were initially assessed by in vivo bioluminescence imaging (IVIS). Compared with ADC control, AGS-16C3F resulted in a significant decrease in bioluminescence (Figure 3A-B). Plasma Gluc activity, which reflects total neoplastic MCs in the entire animal, increased progressively during the 2 weeks of treatment in the control ADC-treated groups (Figure 4A-B; supplemental Figure 4). However, only a slight increase in plasma Gluc activity was detected in mice treated with the highest dose of AGS-16C3F after 1 and 2 weeks of treatment. The Gluc activity in the group treated with the low dose of AGS-16C3F was lower than that of control, although the difference was not statistically significant (Figure 4A-B). Furthermore, the effect of AGS-16C3F on plasma luciferase activity was dose-dependent, suggesting a dose-dependent inhibition of disease progression. Of note, weeks after treatment discontinuation, luciferase activity continued to increase in mice previously treated with control ADC, whereas it was maintained at the same level in mice that received high doses of AGS-16C3F (Figure 4C). In contrast, an increase of luciferase activity was observed in mice that received the low dose of AGS-16C3F, suggesting disease recurrence after treatment discontinuation (Figure 4C).
AGS-16C3F decreased luciferase activity in vivo after 2 weeks of treatment. (A) IVIS showing localization and density of Gluc in treated mice. Units in rainbow color scales are photons per second per centimeter squared per steradian (p/s/cm2/sr). (B) Total relative units per second were calculated for Gluc intensity shown in panel A by reactive oxygen intermediates analysis. Left, back; right, abdomen. Each group comprises 3 mice. Data for each animal are represented; the horizontal bar is the average. *P < .05; **P < .01 assessed by Student t test.
AGS-16C3F decreased luciferase activity in vivo after 2 weeks of treatment. (A) IVIS showing localization and density of Gluc in treated mice. Units in rainbow color scales are photons per second per centimeter squared per steradian (p/s/cm2/sr). (B) Total relative units per second were calculated for Gluc intensity shown in panel A by reactive oxygen intermediates analysis. Left, back; right, abdomen. Each group comprises 3 mice. Data for each animal are represented; the horizontal bar is the average. *P < .05; **P < .01 assessed by Student t test.
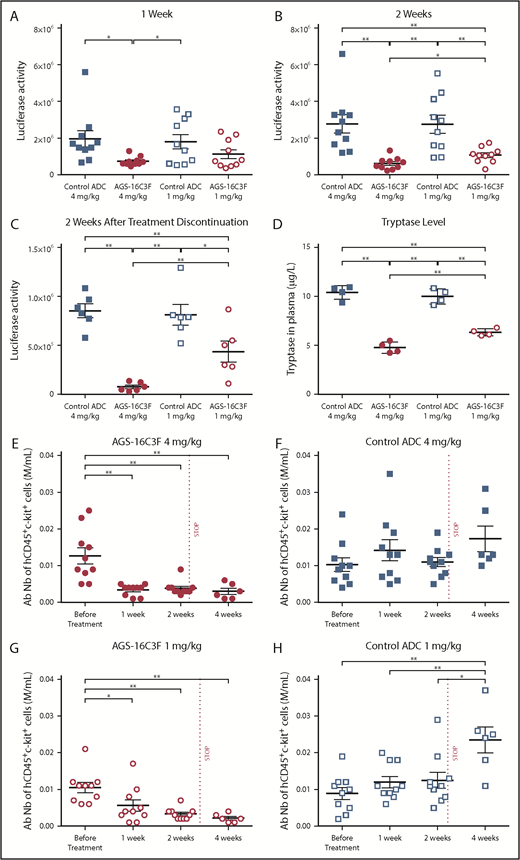
Luciferase and tryptase activity are reduced in plasma, and absolute numbers of ROSAKIT D816V-Gluccells are reduced in PB of AGS-16C3F-treated xenotransplanted mice. (A) Luciferase activity after 1 week of treatment, (B) after 2 weeks, and (C) 2 weeks after stopping treatment. (D) Tryptase level measured at 4 days after the last injection of AGS-163CF. Each group comprises 4 mice. (E-H) Absolute neoplastic MC numbers in PB calculated by the equation: total WBC × % of hCD45+/KIT+ cells (fluorescence-activated cell sorter analyses of percentage of hCD45+/KIT+ in PB). Each group comprises 10 mice. Data for each animal are represented; the horizontal bar is the average. *P < .05; **P < .01 assessed by Student t test.
Luciferase and tryptase activity are reduced in plasma, and absolute numbers of ROSAKIT D816V-Gluccells are reduced in PB of AGS-16C3F-treated xenotransplanted mice. (A) Luciferase activity after 1 week of treatment, (B) after 2 weeks, and (C) 2 weeks after stopping treatment. (D) Tryptase level measured at 4 days after the last injection of AGS-163CF. Each group comprises 4 mice. (E-H) Absolute neoplastic MC numbers in PB calculated by the equation: total WBC × % of hCD45+/KIT+ cells (fluorescence-activated cell sorter analyses of percentage of hCD45+/KIT+ in PB). Each group comprises 10 mice. Data for each animal are represented; the horizontal bar is the average. *P < .05; **P < .01 assessed by Student t test.
As elevated serum tryptase levels in patients with SM reflect neoplastic MC burden, we evaluated the serum tryptase level in the xenotransplanted mice 4 days after the last injection. Lower levels of tryptase were detected in plasma of AGS-16C3F-treated mice compared with control-treated mice (Figure 4D). This result mirrored the result observed for luciferase activity. In addition, in the AGS-16C3F-treated group, the percentage (supplemental Figure 5) and the absolute number of hCD45+/KIT+ cells was significantly reduced relative to the level before treatment (Figure 4E,G). In contrast, an increase in the percentage of hCD45+KIT+ cells was observed after 2 weeks of treatment with control ADC. Two weeks after treatment discontinuation, both the percentage (supplemental Figure 5) and the total number (Figure 4E,G) of MCs remained very low in the PB of AGS-16C3F-treated mice. In contrast, both the percentage (supplemental Figure 5) and the total number (Figure 4F,H) of neoplastic MCs in PB increased in control ADC-treated mice compared with the level observed before treatment.
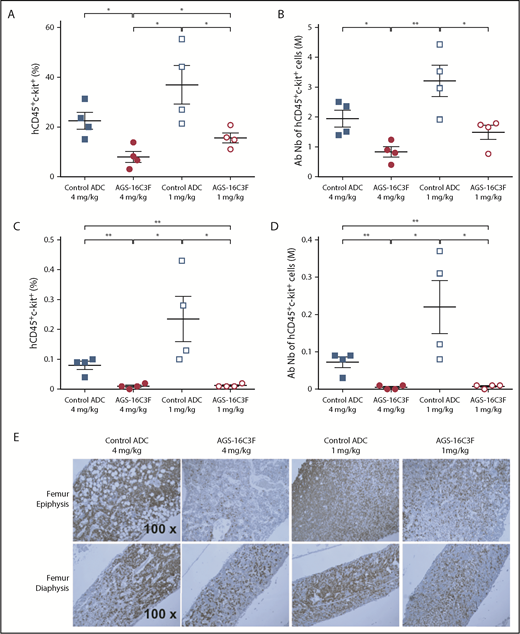
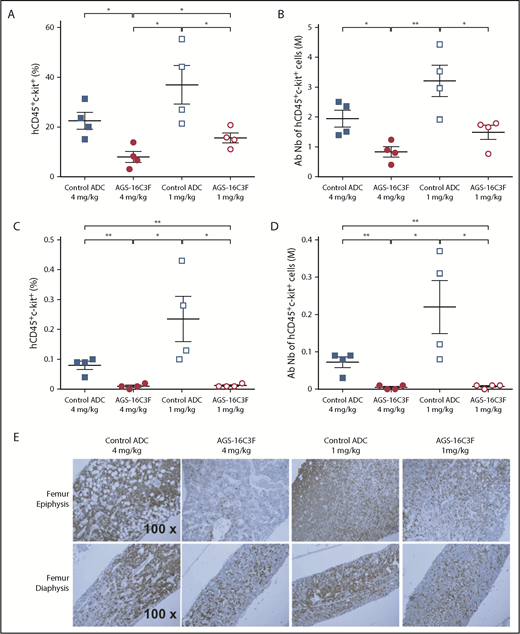
We also analyzed the neoplastic MC burden in hematopoietic organs. Similar to the results obtained on PB cells, both the percentage (Figure 5A,C) and the total number (Figure 5B,D) of neoplastic MCs in the BM of AGS-16C3F-treated mice were significantly reduced by the 2 doses of AGS-16C3F compared with control ADC-treated mice. Immunohistochemical analyses using anti-human CD45 Ab to detect ROSAKIT D816V-Gluc cells revealed that MCs occupied almost the entire femur cavity, especially the epiphysis region in the control animals (Figure 5E). In contrast, only scattered human cells were observed within normal murine hematopoietic cells in AGS-16C3F-treated mice BM. The difference was even more pronounced in the epiphysis region. Rare scattered human cells were present in the spleen and liver of control-treated mice, but were absent in the spleen or liver of AGS-16C3F-treated mice (supplemental Figure 6).
Treatment with AGS-16C3F decreased the number of ROSAKIT D816V-Gluccells in hematopoietic organs. Mice were euthanized 4 days after the last injection of AGS-16C3F. (A,C) Fluorescence-activated cell sorter analyses of the percentage of hCD45+/KIT+ in BM (A) and spleen (C). (B,D) Absolute numbers of neoplastic MCs in BM (B) and spleen (D), calculated by: total cell number in 1 femur and in total spleen × % of hCD45+/KIT+ cells in BM and spleen. (E) The femurs from 4 mice were collected and stained immunohistochemically with anti-human CD45 Ab. The sections were counterstained with hematoxylin. *P < .05; **P < .01.
Treatment with AGS-16C3F decreased the number of ROSAKIT D816V-Gluccells in hematopoietic organs. Mice were euthanized 4 days after the last injection of AGS-16C3F. (A,C) Fluorescence-activated cell sorter analyses of the percentage of hCD45+/KIT+ in BM (A) and spleen (C). (B,D) Absolute numbers of neoplastic MCs in BM (B) and spleen (D), calculated by: total cell number in 1 femur and in total spleen × % of hCD45+/KIT+ cells in BM and spleen. (E) The femurs from 4 mice were collected and stained immunohistochemically with anti-human CD45 Ab. The sections were counterstained with hematoxylin. *P < .05; **P < .01.
AGS-16C3F treatment induced in vivo apoptosis of ROSAKIT D816V-Gluc cells and increased mice survival
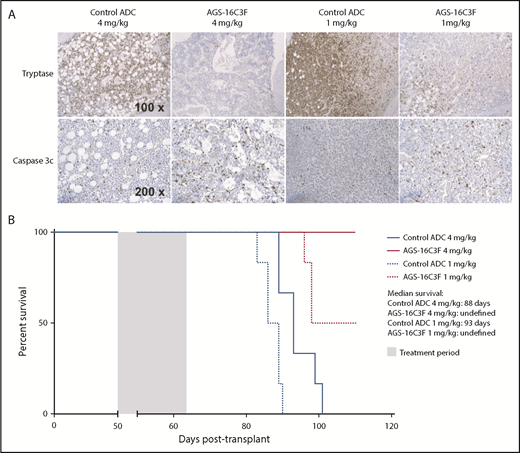
Finally, we tested the effect of AGS-16C3F treatment on apoptosis on neoplastic MCs in vivo. At the time of euthanasia, both anti-CD45 (Figure 4E-H) and tryptase staining (Figure 6A, upper panels) were sharply decreased in PB and in the femurs of AGS-16C3F-treated mice compared with control. This decrease was associated with an increase in the frequency of caspase 3-labeled cells, indicating that AGS-16C3F efficacy is related to apoptosis induction of neoplastic MCs in vivo (Figure 6A, lower panels). Accordingly, an increase in overall survival was observed for AGS-16C3F-treated mice compared with control ADC-treated mice (Figure 6B).
Treatment with AGS-16C3F increased apoptosis of ROSAKIT D816V-Gluccells in hematopoietic organs and increased survival of mice. Mice were euthanized 4 days after the last injection of AGS-16C3F. (A) Tryptase staining (upper line) and cleaved caspase 3 (lower line) in femur from control ADC or from AGS-16C3F-treated mice. (B) AGS-16C3F prolonged life span of mice. Survival analyses (n = 6 animals/group). Treatment was performed between days 50 and 64, as indicated in the blue box. Statistical difference: P = .02, between the high and low dose of control-treated mice; P < .001 between AGS-16C3F and high- or low-dose control-treated mice independently (log-rank test).
Treatment with AGS-16C3F increased apoptosis of ROSAKIT D816V-Gluccells in hematopoietic organs and increased survival of mice. Mice were euthanized 4 days after the last injection of AGS-16C3F. (A) Tryptase staining (upper line) and cleaved caspase 3 (lower line) in femur from control ADC or from AGS-16C3F-treated mice. (B) AGS-16C3F prolonged life span of mice. Survival analyses (n = 6 animals/group). Treatment was performed between days 50 and 64, as indicated in the blue box. Statistical difference: P = .02, between the high and low dose of control-treated mice; P < .001 between AGS-16C3F and high- or low-dose control-treated mice independently (log-rank test).
Discussion
CD203c is consistently expressed at high levels on neoplastic MCs in patients with SM, including patients with advanced disease phenotypes,22 suggesting that an Ab specifically targeting this antigen, and particularly an ADC, may have antineoplastic activity in such contexts. In our study, we tested the in vitro effects of the anti-CD203c ADC AGS-16C3F on proliferation and apoptosis, using the KIT D816V+CD203c+ human MC line (ROSAKIT D816V) that we recently characterized as a model of neoplastic MCs found in patients with AdvSM.25 We observed that AGS-16C3F inhibited the proliferation of CD203c+ ROSA cells in vitro at an IC50, comparable to what has been reported for other tumor cells such as RCC,17 and that the growth-inhibitory effect of AGS-16C3F was associated with induction of apoptosis. The activity of AGS-16C3F was more evident in the RosaKIT D816V cell lines than in the HMC1.1 and HMC1.2 cell lines, which was likely because of the differential expression of CD203c levels, with CD203c being more strongly expressed in the RosaKIT D816V cell lines. Furthermore, to study the effects of AGS-16C3F in vivo, we used the unique in vivo model of aggressive SM/MC leukemia that we have recently established by IV injection of ROSAKIT D816V-Gluc cells in NOD-SCID γ mice.24
AGS-16C3F inhibited the proliferation of neoplastic MCs in hematopoietic organs and prolonged the life span of treated mice. This result is consistent with a previous report describing the antitumor activity of AGS-16C3F in RCC xenograft models.17 Analysis of the percentage and absolute number of MCs in the PB showed the same inhibitory profile with the high and low doses of AGS-16C3F during the treatment or 2 weeks after treatment. However, we detected lower levels of luciferase activity at 2 weeks of treatment with the high doses compared with the low doses of AGS-16C3F. This difference became even more pronounced 2 weeks after treatment discontinuation, suggesting a dose-dependent inhibitory effect of AGS-16C3F. Indeed, treatment discontinuation resulted in a rapid increase in luciferase activity in the PB of animals treated with the low dose of AGS-16C3F, suggesting disease relapse. This is probably because of the resumption of the proliferation of residual neoplastic MCs in the BM, reflected immediately by an increase of luciferase activity in the PB. Interestingly, late leakage of neoplastic MCs from BM to PB led to an increase in the percentage of MCs in the PB at the point of last treatment and 2 weeks after last injection, suggesting that luciferase activity assay is more reliable for detecting the early relapse of the disease than the percentage of MCs in PB in our study. Similar results were obtained from the tryptase measurement in the serum. However, tryptase determination needs higher PB volume than the luciferase assay (50 μL for tryptase assay and 5 μL for luciferase activity assay), making it inconvenient, if not impossible, to use in monitoring the disease over time in mice. In addition, Gluc intensity measured by IVIS was decreased in AGS-16C3F-treated mice, suggesting that tumor burden was decreased in all organs. This result was further confirmed by immunohistochemistry analyses with anti-human CD45 Ab in mice femur, spleen, and liver. Because this is a xenograft model, the immune response is likely to be minimal, but some residual macrophage function is likely preserved in these mice.
The antitumor activity of some ADCs can arise not only from the delivery of a highly potent toxin to tumor cells but also from the modulation of the target, as well as Ab-dependent cellular cytotoxicity.30 However, for AGS-16C3F, a previous nonclinical study has shown that the unconjugated Ab AGS16-7.8 had no significant antitumor activity.17 Therefore, in our study, the effect of AGS-16C3F is likely to be related to delivery of the highly effective cytotoxic agent Cys-mcMMAF, specifically to the ROSAKIT D816V-Gluc cells. Importantly, AGS-16C3F was well-tolerated in a phase 1 study in patients with advanced renal carcinoma, providing clinical validation of the safety of the CD203c target, justifying clinical testing of AGS-16C3F in patients with systemic mastocytosis.23
In conclusion, the data presented here identify, for the first time, CD203c as a novel potential target on human neoplastic MCs, using relevant animal models of human AdvSM in vitro and in vivo. AGS-16C3F, which specifically targets CD203c and has potent activity against human CD203c+ neoplastic MC cell lines, may therefore be a new emerging agent for the treatment of patients with advanced categories of systemic mastocytosis.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank J. Ulrich for his assistance with the animal experiments and P. Gonin and K. Ser-Le Roux from the Service Commun d'Expérimentation Animale for providing excellent animal colony care. The authors are grateful to P. Rameau and Y. Lecluse for cell sorting and flow cytometry analysis. The authors are also grateful to P. Opolon and O. Bawa for performing the immunohistostaining experiments.
This work was funded in part by Inserm and by French grants from the Foundation de France (FDF n°2013 00038290) and Agence nationale de la recherche (ANR-08-BIOT-0231-01/VACTHERA). Agensys has provided grants to F.L., G.W., and Y.Z. in support of this work. Financial support for the development of this manuscript, including medical writing and editorial assistance under the authors’ guidance, was provided by Regina Switzer from SuccinctChoice Medical Communications (Chicago, IL) and was funded by Astellas Pharma, Inc. (Northbrook, IL).
Authorship
Contribution: Y.Z. designed and performed experiments, analyzed data, and helped prepare the initial draft of the manuscript; G.W., L.H., M.W., F.B., V.B., and S.B performed experiments; J.-M.L performed experiments and analyzed data; M.A. designed the work, supervised experiments, and wrote the manuscript; F.L. designed the work, supervised experiments, and wrote the manuscript; F.D. and H.K.-M provided materials, analyzed data, and revised the manuscript; and all authors are accountable for all aspects of the work and approved the manuscript for submission.
Conflict-of-interest disclosure: At the time of the research, H.K.-M. and F.D. were both employees of Agensys, Inc. The remaining authors declare no competing financial interests.
Correspondence: Fawzia Louache, Inserm U1170 Hématopoïèse normale et pathologique, Institut National de la Santé et de la Recherche Médicale, UMRS-1170, CNRS GDR 3697 Micronit, Gustave Roussy, Université Paris-Sud, Université Paris-Saclay, 94805 Villejuif, France; e-mail: fawzia.louache@gustaveroussy.fr.