Abstract
The association between malignancy and thrombosis has been recognized for over a century and a half. Patients with cancer have an elevated risk of both initial and recurrent venous thromboembolism (VTE) compared with patients without cancer owing to cancer- and patient-specific factors. Recurrent VTE is common despite anticoagulation, presenting additional management challenges. Patients with cancer also have an increased risk of bleeding when on anticoagulants compared with patients without cancer. This bleeding risk is heightened by the thrombocytopenia common in patients with hematologic malignancies and those treated with intensive myelosuppressive chemotherapy regimens. Despite the advancements in cancer-directed therapy made over the past 15 years, numerous large studies have confirmed that bleeding and VTE recurrence rates remain high in cancer patients. Balancing the increased and competing risks of clotting and bleeding in these patients can be difficult, because management of cancer-associated thrombosis requires anticoagulation despite known increased risks for bleeding. In the context of challenging illustrative cases, this review will describe management approaches to clinical scenarios in which data are sparse: cancer patients with recurrent VTE despite anticoagulation and cancer patients with a new VTE in the setting of severe thrombocytopenia.
Managing initial and recurrent venous thromboembolism in cancer patients
Clinical case I
A 52-year-old man presents with a 2-month history of fatigue, back pain, and unintentional weight loss. On abdominal computed tomography (CT), he is found to have a pancreatic mass. He is referred to oncology providers and ultimately diagnosed with a borderline resectable pancreatic ductal adenocarcinoma. Chest and pelvis CTs are normal. The complete blood count is normal, creatinine is 0.8 mg/dL, and he weighs 67 kg. During the initial oncologic evaluation, left lower extremity swelling is noted. Bilateral lower extremity compression ultrasound reveals a partially occlusive thrombus in the left femoral vein, with no evidence of thrombus in the right leg. He is started on rivaroxaban 15 mg twice a day, with plans to start neoadjuvant chemotherapy.
Anticoagulant choice in cancer patients
For >15 years, low-molecular weight heparin (LMWH) has been accepted as optimal anticoagulant therapy for cancer-associated thrombosis (CAT) after clinical trials demonstrated decreased rates of venous thromboembolism (VTE) recurrence with LMWH compared with vitamin K antagonists.1,2 Recent data suggest that direct oral anticoagulants (DOACs) are also acceptable treatment of CAT. The results of a quality improvement initiative evaluating >1000 carefully selected cancer patients with VTE treated with rivaroxaban demonstrated a low VTE recurrence rate of 4.2% (95% confidence interval [95% CI], 2.7%-5.7%), similar to historical rates in patients treated with LMWH.3 The 6-month bleeding rates were 2.2% (95% CI, 1.1%-3.2%) for major bleeding and 5.5% (95% CI, 3.7%-7.1%) for clinically relevant nonmajor bleeding (CRNMB). Patients with increased risk of bleeding, including those with ongoing gastrointestinal (GI) tract bleeding as well as known untreated luminal GI tract, luminal genitourinary tract, and central nervous system lesions, were excluded. Of the major bleeds, 73.3% occurred in the GI tract. DOACs were found to be as effective as LMWH in 2 recent large randomized, controlled trials. The SELECT-D (Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism) trial compared rivaroxaban with dalteparin in 400 patients,4 and the Hokusai VTE Cancer trial evaluated the use of edoxaban (after 5 days of LMWH) vs dalteparin in 1050 patients.5 Although both DOACs were associated with lower numeric rates of recurrent VTE, rates of clinically relevant major bleeding and CRNMB were increased compared with dalteparin in these trials, with statistically significant increases in major bleeding in the Hokusai VTE Cancer trial and CRNMB in both trials. Halfway through the SELECT-D trial, the Data Safety Monitoring Board halted enrollment of patients with upper GI tract malignancies owing to an imbalance in bleeding in those patients, whereas a subanalysis of the Hokusai VTE Cancer trial found that the increased risk of major bleeding with edoxaban occurred primarily in patients with GI tract malignancies.6 The ongoing Caravaggio trial (NCT03045406) is assessing the efficacy and safety of apixaban vs dalteparin, with enrollment completed in June 2019.7 Publication of the final results of the ADAM-VTE (Apixaban, Dalteparin, in Active Cancer Associated Venous Thromboembolism) trial, which randomized 300 patients to apixaban or dalteparin, is awaited. The results of these studies will add to our knowledge of the risks and benefits of DOAC treatment of VTE in patients with cancer.
Consensus guideline statements have been updated to reflect the emerging clinical trial data. National Comprehensive Cancer Network (NCCN) 2019 V1 guidelines state that LMWH or edoxaban (after a 5-day LMWH lead in) is preferred for the first 6 months in patients with proximal deep venous thrombosis (DVT) or pulmonary embolism (PE) and for prevention of recurrent VTE in patients with advanced metastatic cancer. Rivaroxaban is also given as an option (category 1 recommendation); apixaban is listed as an acceptable alternative for patients in whom LMWH is refused or should be avoided.8 The International Society for Thrombosis and Haemostasis (ISTH) suggests that the use of edoxaban or rivaroxaban is acceptable in cancer patients with acute VTE, low bleeding risk, and no drug-drug interactions.9 The American Society of Clinical Oncology (ASCO) recommendations are similar, with a strong recommendation for using initial anticoagulation with LMWH, unfractionated heparin (UFH), fondaparinux, or rivaroxaban for 5 to 10 days followed by LMWH, edoxaban, or rivaroxaban for the next 6 months because of high-quality evidence.10 The 2019 International Initiative on Thrombosis and Cancer (ITAC) clinical practice guidelines recommend LMWH as initial anticoagulation in patients with a creatinine clearance ≥30 mL/min, with either edoxaban (after a lead in with LMWH) and rivaroxaban also being appropriate choices in patients without a high risk of GI or genitourinary bleeding.11 The ISTH, NCCN, ASCO, and ITAC guideline recommendations for anticoagulant choice for acute VTE in cancer patients are summarized in Table 1. Our pragmatic approach to anticoagulant choice in cancer patients with VTE is detailed in Table 2.
Clinical case I describes a young patient with a pancreatic tumor, no intraluminal GI tract lesions, and no obvious increased risk for bleeding. In this patient, anticoagulation with rivaroxaban is an acceptable alternative to LMWH.
Clinical case I (continued)
Just 10 days after starting chemotherapy with fluorouracil, oxaliplatin, irinotecan, and leucovorin (FOLFIRINOX), he presents with new right lower extremity swelling. Imaging reveals both DVT and superficial venous thrombosis in the right leg. He denies missing any doses of rivaroxaban but notes nausea and vomiting with mild diarrhea for several days after his first infusion. After some discussion, rivaroxaban is discontinued, and enoxaparin 1 mg/kg (70 mg) twice a day is begun.
Anticoagulation challenges.
Identifying recurrent VTE in cancer patients can be difficult. Recurrence may occur because of a number of patient-, tumor-, or clot-related factors. Patient factors include noncompliance, poor injection technique (for LMWH or fondaparinux), or failure to achieve or maintain adequate plasma drug concentrations. Tumor-related factors include the consequences of disease progression, which may result in new vascular compression or modified tumor biology and increased release of procoagulant mediators. Vascular compression or other tumor-related vessel obstruction may necessitate placement of venous stents. Asymptomatic early thrombus propagation can occur in any patient despite adequate anticoagulation.12 This usually occurs in patients with high clot burden in whom anticoagulation has not had adequate time to be effective. In patients with cancer and the question of recurrent VTE, multiple factors need to be assessed. In this patient with new symptoms and the finding of thrombus in the opposite leg, the use of DOACs in the setting of nausea and vomiting is concerning for failure to maintain adequate plasma drug concentrations. In the CLOT (Venous Thromboembolism in Patients with Cancer) trial, 37% of recurrent VTE events occurred in patients on warfarin with an international normalized ratio of <2.0.1 Although warfarin effect is often labile, the anorexigenic and emetogenic effects of systemic anticancer treatments can impact the effectiveness of any oral anticoagulant.13 Periprocedural anticoagulation interruption also contributes to recurrence risk. Recent data suggest that cancer patients have higher rates of periprocedural VTE recurrence and bleeding than patients without cancer.14,15 Although edoxaban and rivaroxaban have been shown to have decreased risks of recurrent VTE compared with dalteparin, the 6-month recurrence rates for each of these DOACs in clinical trials were still 6.5% and 4%, respectively.4,5 Additional factors that can affect DOAC efficacy include obesity, drug-drug interactions, and poor GI absorption.16 Although drug-drug interactions may be more common in patients with cancer,17 this patient does not have apparent drug-DOAC interactions. Although potential drug-DOAC interactions are based on the effects of concomitant drugs on induction or inhibition of metabolic pathways for the DOAC, the true clinical significance of these interactions has not been evaluated. In this patient with normal weight, normal renal function, and no expected drug-DOAC interactions, the nausea, vomiting, and diarrhea are concerning for inadequate GI absorption of rivaroxaban. Moving to full-intensity anticoagulation with a parenteral agent bypasses the possible poor GI tract absorption and is a good next step.
Primary thromboprophylaxis for cancer patients.
VTE prophylaxis with LMWH for all ambulatory cancer patients was not widely adopted because of the overall low prevalence of VTE in unselected cancer patient populations. Selection of patients at increased VTE risk based on patient- and cancer-specific factors might improve the utility of prophylaxis. Published CAT risk stratification tools (such as the Khorana, Ottawa, Vienna-CATS, and PROTECHT [Prophylaxis of Thromboembolic Events in Cancer Patients Receiving Chemotherapy] risk assessment scores) include tumor site of origin, with pancreatic cancer considered among the highest-risk tumor types.18 The patient in clinical case I could have been considered for thromboprophylaxis with a DOAC had he not presented with a VTE at the time of cancer diagnosis. Results from the AVERT (Apixaban for the Prevention of Venous Thromboembolism in Cancer Patients) and CASSINI (Rivaroxaban for Preventing Venous Thromboembolism in High-Risk Ambulatory Patients with Cancer) trials demonstrate decreased risk of developing VTE in high-risk ambulatory cancer patients receiving chemotherapy using prophylactic doses of rivaroxaban and apixaban.19,20 The ISTH suggests that DOACs be used for primary prophylaxis in patients with cancer and a Khorana risk score of ≥2 unless there are concerns for drug interactions or GI bleeding, in which case LMWH should be used.21 The ASCO suggests that similar high-risk cancer patients with Khorana score of ≥2 may be offered thromboprophyalxis with apixaban, rivaroxaban, or LMWH, with intermediate quality of evidence and moderate strength of the recommendation.10
Clinical case I (continued)
After 2 cycles of chemotherapy, restaging CT scans reveal a decrease in size of the primary tumor, but 2 segmental pulmonary emboli are seen in the left lung base. On questioning, he admits that he has noted increased dyspnea on exertion and an increased resting heart rate measured by his smartwatch for the past 4 days, but he attributed it to deconditioning over the past few months since the diagnosis of cancer. He has been compliant with twice-daily enoxaparin injections. The enoxaparin dose is increased to 80 mg twice daily.
Management of breakthrough VTE.
Cancer patients can have recurrent VTE despite full-intensity anticoagulation, especially patients with high-risk tumors, such as pancreatic and gastric adenocarcinomas. A prospective cohort study of patients with cancer and VTE (the DALTECAN [Evaluation of Dalteparin for Long-term Treatment of Blood Clots in Subjects With Cancer] study) demonstrated that rates of VTE recurrence beyond the initial 6 months of treatment were far greater than bleeding risks during that same time period.22 Patients with advanced-stage disease are more likely to develop recurrent VTE.23 It is critical to ensure that the detected VTE is a new event by comparing past imaging with a radiologist to confirm. D-dimer measurement is less helpful in patients with cancer because of baseline D-dimer elevations in many cancer patients. In patients confirmed to have a new event, limited data are available to guide management. Two small retrospective studies have assessed dose escalation of LMWH to 120% to 125% of the full dose.24,25 In the first study of 70 patients, those on less than full-dose anticoagulation were increased to full dose and those on full-dose LMWH were increased to 120% to 125% of full dose for 4 weeks, with an acceptable major bleeding rate of 4.3%.24 The second study included 55 patients and used a similar strategy of dose escalation for a longer duration of time (4-12 weeks), with a major bleeding rate of 5.5%.25 In practice, this dose escalation approach is common as demonstrated by a more recently published prospective registry study collecting data on management of “breakthrough” VTE. Most patients were on full-dose LMWH at the time of breakthrough VTE, and LMWH dose was increased in 31% of patients.26 Evidence to guide treatment in patients who develop recurrence on dose-escalated (120%-125% dose intensity) parenteral anticoagulation is limited. Published case reports describe success with a dual anticoagulation approach with addition of a second anticoagulant with a distinct mechanism of action, such as addition of dabigatran to dose-escalated fondaparinux.27-29
Because dalteparin is the only LMWH that has Food and Drug Administration and European Medicines Agency approval for monotherapy of CAT for a 6-month treatment period, dalteparin has been the comparator LMWH used in all of the large published clinical trials examining LMWH vs oral anticoagulants. The approved dosing strategy is 200 U/kg once daily for the first month followed by 150 U/kg once daily for the remainder of treatment.1,5,30 We consider dalteparin 150 U/kg once daily and enoxaparin 1.5 mg/kg once daily to be less than full-intensity anticoagulation. Patients on these dosing regimens who experience recurrent VTE should be escalated to full-dose weight-based LMWH or fondaparinux. In this patient with recurrent VTE on full-dose parenteral anticoagulation, increasing to 120% to 125% of the dose is the next step. Enoxaparin was increased to 80 mg twice daily, ∼1.2 mg/kg twice daily, or 120% of standard full intensity. Our approach to dose escalation in patients with breakthrough VTE is given in Table 3.
Future approaches to risk stratification and VTE prevention.
Developments in understanding tumor biology indicate that molecular aberrations often dictate tumor behavior and clinical prognosis. Specific mutations or mutational signatures may predispose to the development of CAT. The heterogeneity in the mutations found in different tumors may explain the variability in thrombotic potential noted between different tumor types and between different patients with the same tumor type. Tumors harboring the EML4-ALK rearrangement may be associated with higher thrombotic risk than other types of nonsmall cell lung cancer (NSCLC). One retrospective analysis of 98 patients with anaplastic lymphoma kinase (ALK)-rearranged NSCLC found a 36% VTE incidence, considerably higher than the 8% to 15% incidence seen in unselected NSCLC patients.31 Multiple case reports of fulminant disseminated intravascular coagulation or dramatic hypercoagulability on presentation of newly diagnosed ALK-rearranged NSCLC28,32,33 support this hypothesis. Current risk stratification models do not seem to adequately distinguish between patients at moderate and high VTE risk for some tumor types. Refined models are needed and may benefit from incorporating tumor genomic signatures and other biomarkers to improve risk stratification and consideration for primary thromboprophylaxis.34
Thrombocytopenia and bleeding in the cancer patient
Clinical case II
A 58-year-old man with a 6-year history of immunoglobulin G κ multiple myeloma is seen in outpatient clinic on day 15 after an autologous hematopoietic stem cell transplant. He complains of new shortness of breath and is found to have segmental pulmonary emboli in both right and left lower lobar arteries. Bilateral lower extremity compression ultrasound is negative for DVT. He has mild to moderate right-sided chest pain and dyspnea on exertion. His heart rate is 108/min, blood pressure is 128/78 mm Hg, and oxygen saturation is 91% on room air. Renal and hepatic functions are normal. Platelet count is 7000/µL. The transplant team calls you requesting advice on how to manage the VTE.
Assessment of competing risks in the cancer patient requiring anticoagulation.
Patients with cancer receiving anticoagulation have a 2- to 3-fold increase in major bleeding risk compared with anticoagulated patients without cancer.15,35 Bleeding from unresected primary tumors, particularly GI tract, genitourinary tract, and gynecologic malignancies, is common. Factors that increase the risk of VTE can also increase the bleeding risk with anticoagulation, including tumor site of origin,36 advanced/metastatic disease,37 cytotoxic agents, radiation therapy, and surgery. Patients with acute lymphoblastic leukemia develop thrombocytopenia as a result of tumor site of origin (bone marrow) and cytotoxic agents used to treat the disease but simultaneously have an increased thrombotic risk because of the disease, endothelial damage from cytotoxic agents, and acquired prothrombotic risk associated with asparaginase treatment.38
Two primary factors need to be assessed and balanced when approaching any cancer patient with active bleeding or increased risk for bleeding requiring anticoagulation: the risk of withholding anticoagulation and the risk of bleeding with anticoagulation. The location and significance of the VTE are major factors; pulmonary emboli are the most concerning because of potential death from pulmonary or cardiac compromise. Less concerning for risk of death or compromise are distal lower extremity thrombosis and central venous access line–associated upper extremity thrombosis, although patients with these clots may have associated pain and experience symptomatic relief with anticoagulation. Time from diagnosis of thrombosis is also a factor, with acute thrombosis, diagnosed within 12 weeks, the most concerning for risk of propagation.
In considering these 2 competing risks, the expected duration of bleeding or risk for bleeding, such as thrombocytopenia, is highly relevant. In this patient, the duration of thrombocytopenia is expected to be short, making limited duration platelet transfusion support to allow full-intensity anticoagulation feasible. Patients with expected ongoing bleeding or prolonged thrombocytopenia require a different approach. Limited duration of full-dose anticoagulation with platelet transfusion support for the first 4 weeks is often used with subsequent reduction in anticoagulation intensity and discontinuation of platelet transfusions.
Risk of withholding anticoagulation.
Historic rates for PE-associated mortality in the general population when anticoagulation was not given were ∼25% to 30%; however, more recent assessments of patients with a missed PE diagnosis in the emergency room suggest a lower mortality rate of ∼5%.39 In cancer patients, VTE is associated with increased morbidity and mortality,23,40 and it is a major cause of death.41 Rates of recurrent VTE risk are 3- to 4-fold higher in cancer patients.15,42 The RIETE (Registry of Patients with Venous Thromboembolism) registry, an ongoing international prospective voluntary registry of VTE patients, found that 2.6% of patients with CAT developed fatal PE within the first 3 months of treatment despite anticoagulation, considerably higher than in patients without cancer, whereas fatal bleeding occurred in only 1.0%.35 A large prospective observational study of ambulatory cancer patients receiving chemotherapy demonstrated an annualized death rate because of VTE of 448 per 100 000 patients, a 47-fold elevation over the annualized death rate for VTE in the general population.41 Both incidentally detected VTE and subsegmental PE have been found to have the same risk of recurrence and morbidity as symptomatic VTE or more proximal PE in patients with cancer43,44 ; anticoagulation should not be withheld in patients with these findings.
Risk of bleeding with anticoagulation.
Significant thrombocytopenia is common in patients with hematologic malignancies and solid tumors. Many agents that cause thrombocytopenia, such as platinum-based chemotherapy and gemcitabine, are also associated with increased thrombotic risk.45 Multiple studies have demonstrated high rates of initial and recurrent VTE despite thrombocytopenia, yet platelet transfusions are associated with high rates of adverse events when given to allow anticogulation.46,47 In an RIETE registry study of patients treated for VTE with a platelet count of <80 000/µL, the major bleeding rate was 5.8%.48 Patients with platelet counts of <80 000/µL were found to have >2- to 4-fold higher odds ratios for major bleeding and fatal bleeding (2.70 and 3.70, respectively) than those with normal platelet counts. Invasive procedures also contribute to increased bleeding in cancer patients that may not be alleviated with periprocedural platelet transfusion in patients.49
Clinical case II (continued)
The patient is expected to have recovery of platelet count with engraftment. Given the symptomatic PE and severe thrombocytopenia, he is admitted and treated with intravenous UFH and platelet transfusion. He tolerates the anticoagulation without bleeding. He has a good response to platelet transfusion, with a posttransfusion platelet count of 65 000/µL, but his platelet count is down to 11 000/µL 2 days later, requiring additional platelet transfusion. After 3 days, he is discharged to home on enoxaparin 1 mg/kg twice a day with plans for outpatient platelet transfusion 3 times weekly until count recovery.
Triage of VTE patients with high bleeding risk.
Clinical practice guidelines for determination of mortality risk and management of patients with PE were developed for the general population and require additional validation in cancer patients.50 In clinical case II, the concern for bleeding because of profound thrombocytopenia supports initiation of anticoagulation in a monitored setting. In other patients with terminal cancer for whom quality of life is a major consideration and time outside of the hospital is precious, shared decision making between patient and provider regarding anticoagulation and transfusion support is advised.51
Platelet count thresholds for anticoagulation.
Dedicated studies evaluating efficacy and safety of anticoagulant dose modifications in severely thrombocytopenic cancer patients are sparse.52-54 In the CLOT trial, dalteparin was reduced by ∼25% for platelet counts between 50 000 and 100 000/µL, and anticoagulation was held for platelet counts of <50 000/µL.1 Platelet thresholds for discontinuing the DOACs for treatment of CAT, however, have varied. Edoxaban was held for a platelet count threshold of <30 000/µL in the Hokusai VTE Cancer trial, with no dose reduction for platelets <100 000/µL, whereas a platelet count cutoff of <50 000/µL was used for rivaroxaban in the SELECT-D trial. Because <5% of the patients in the Hokusai VTE Cancer trial had thrombocytopenia (platelet counts of 50 000-100 000/µL) at enrollment,5 determination of the safety of edoxaban in thrombocytopenic patients is not possible. Consensus guideline statements from the ISTH, the NCCN, and the ASCO recommend full-intensity anticoagulation in patients with a platelet count of >50 000/µL.8,55-57 The ISTH guidance suggests full-dose anticoagulation in patients with high risk of thrombus propagation defined as acute proximal or recurrent thrombosis and transfusion support to maintain platelet count of >40 000-50 000/µL. A recent health claims database analysis of >400 000 patients, however, suggests that, even in patients with a platelet count of 50 000 to 100 000/µL, there is a higher risk of bleeding than if the platelet count is >100 000/µL.58 Careful follow-up of patients on full-dose anticoagulation with platelet counts in this range is required.
For patients with lower risk of thrombus progression, such as distal location or older thrombus and platelets between 25 000 and 50 000/µL, intermediate-dose anticoagulation can be used; if platelets are <25 000/µL, the ISTH suggests holding anticoagulation.57 This approach is supported by the findings of a quality assessment initiative in which 99 patients with 140 episodes of platelet counts ≤50 000/µL had guideline-driven dose reductions with no episodes of recurrent VTE or major bleeding when the LMWH dose was decreased or held.54 Guidelines from the American Society of Hematology on the management of CAT, including in thrombocytopenic patients with cancer, are awaited.
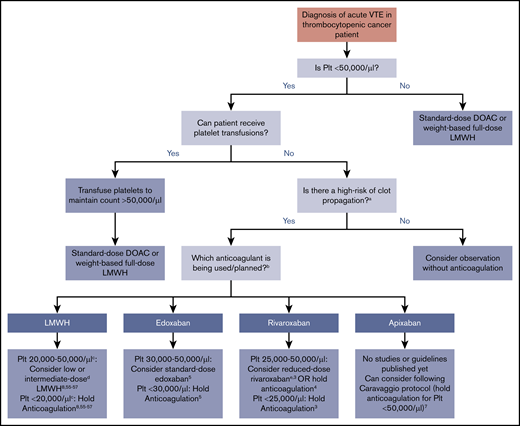
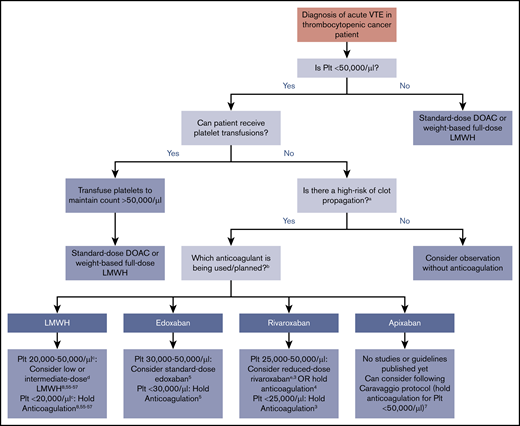
In clinical practice, severely thrombocytopenic patients with acute CAT are often initiated on low- or intermediate-dose anticoagulation with close patient observation. Inferior vena cava filter use is discouraged55,56 and reserved only for the rare circumstance of an acute large proximal lower extremity DVT and an inability to safely treat with any dose of anticoagulant. In general, DOACs can be considered if there are no concerns for impaired absorption, GI bleeding, or drug-drug interactions, although the half-life is longer than LMWH. Figure 1 illustrates an evidence -and consensus guidelines–based approach to anticoagulation in thrombocytopenic cancer patients with acute VTE (<12 weeks since event). Platelet threshold for stopping full-intensity anticoagulation varies by anticoagulant choice based on limited data. For patients who cannot be transfused with platelets, monitoring with careful anticoagulant choice and dose selection is needed. Patients with chronic VTE (>12 weeks from event) and significant thrombocytopenia are less likely to have clot propagation and recurrent thrombosis than those with acute VTE, although data for management are lacking. Multiple options can be considered in patients with platelet counts of 20 000 to 50 000/µL: from withholding anticoagulation to consideration of reduced-dose DOAC or LMWH. An individualized approach and shared decision making with the patient, with particular attention to bleeding risks, are advised.
Summary of evidence and guidelines for treatment of acute VTE in the thrombocytopenic cancer patient. Recommendations for each anticoagulant and platelet thresholds are based on published consensus guidelines or major published trials and studies. For patients that cannot be transfused, timing of onset and severity of VTE must be considered. aHigh risk for thrombus propagation is defined as acute proximal or recurrent thrombosis. bDabigatran is not displayed as an option because of the lack of data, studies, or discussion in guideline statements. cThe ISTH and NCCN guidelines recommend holding anticoagulation at platelet counts of <25 000/µL, whereas the ASCO guidelines use a threshold of 20 000/µL. dLow-dose LMWH is generally defined as prophylactic dosing (eg, 30-40 mg enoxaparin daily or 5000 U dalteparin daily), and intermediate-dose LMWH is variably defined as enoxaparin 0.5 mg/kg twice daily or 1 mg/kg once daily (Table 2). eDefined in this study as rivaroxaban 10 mg twice daily during the first 3 weeks of treatment or 10 mg once daily after the first 3 weeks of treatment.3 Plt, platelet count.
Summary of evidence and guidelines for treatment of acute VTE in the thrombocytopenic cancer patient. Recommendations for each anticoagulant and platelet thresholds are based on published consensus guidelines or major published trials and studies. For patients that cannot be transfused, timing of onset and severity of VTE must be considered. aHigh risk for thrombus propagation is defined as acute proximal or recurrent thrombosis. bDabigatran is not displayed as an option because of the lack of data, studies, or discussion in guideline statements. cThe ISTH and NCCN guidelines recommend holding anticoagulation at platelet counts of <25 000/µL, whereas the ASCO guidelines use a threshold of 20 000/µL. dLow-dose LMWH is generally defined as prophylactic dosing (eg, 30-40 mg enoxaparin daily or 5000 U dalteparin daily), and intermediate-dose LMWH is variably defined as enoxaparin 0.5 mg/kg twice daily or 1 mg/kg once daily (Table 2). eDefined in this study as rivaroxaban 10 mg twice daily during the first 3 weeks of treatment or 10 mg once daily after the first 3 weeks of treatment.3 Plt, platelet count.
In this case, the patient has newly diagnosed bilateral segmental PE and is considered high risk for clot propagation. After initial inpatient anticoagulation and platelet transfusions, he was discharged on full-dose LMWH with planned platelet transfusion support until platelet recovery expected in the next 2 weeks.
Clinical case II (continued)
The patient initially does well with platelet transfusions and enoxaparin injections, with improvement in shortness of breath over the next several days. Five days after discharge, he feels lightheaded and has a large bowel movement with bright red blood. He is seen in the emergency department, where he is found to have a hemoglobin of 6.1 g/dL, which down from 10.2 g/dL 1 week before. He last administered enoxaparin 4 hours before arrival to the emergency department. Platelet count is 44 000/µL, and anti-Xa activity is 0.97 IU/mL. Red cell transfusion, platelet transfusion, and protamine sulfate are administered; posttransfusion platelet count is 105 000/µL. Colonoscopy reveals diverticula. One diverticulum exhibiting oozing is treated with epinephrine injections and endoscopic clipping of the bleeding vessel. Platelet transfusions are used to maintain a daily platelet count of >50 000/µL. Anticoagulation is held for 48 hours and then, restarted with intravenous UFH. He is observed for another 48 hours on intravenous UFH, transitioned to enoxaparin 1 mg/kg once a day, and discharged for close outpatient follow-up. Platelet count is noted to be sustained at 90 000/µL 1 week later, and with no additional GI bleeding, enoxaparin dose is increased to 1 mg/kg twice a day.
Etiology and management of bleeding in patients with malignancy
Patients with solid tumors without thrombocytopenia may also be at risk for bleeding, which can be exacerbated with anticoagulation. Bleeding may occur at the site of the primary tumor or metastatic lesions, especially necrotic or friable tumor; at sites of tumor invasion and erosion into the GI or genitourinary tract or airways; or at normal tissue sites owing to the effects of radiation and chemotherapy. The severity and extent of bleeding in these patients need to be carefully assessed in the context of anticoagulation. Local measures to treat bleeding are indicated, with interruption or decrease in intensity of anticoagulation required and dictated by the degree of bleeding.
Basic principles of bleeding management should be followed. Source control should be obtained whenever possible. Platelet transfusions for significant thrombocytopenia and holding anticoagulation with reversal for life-threatening bleeding may be needed. Other types of coagulopathy should be corrected. Nonspecific hemostatic agents can be considered, but use of recombinant activated factor VII or activated prothrombin complex concentrates is not advised in patients with recent acute thrombosis given thrombotic risk. The use of antifibrinolytic agents, such as tranexamic acid or ε-aminocaproic acid, can be considered.
In cancer patients with acute VTE who have bleeding on anticoagulation, decisions regarding if or when to restart anticoagulation must be individualized. When possible, anticoagulation should be resumed in patients who are <12 weeks from the thrombotic event after durable hemostasis is achieved. Although primarily evaluating patients without cancer, a large retrospective cohort study evaluating 442 warfarin-anticoagulated patients with GI bleeding found that early resumption of warfarin (within 90 days) resulted in fewer thromboembolic events without significantly increased bleeding rates.59 Use of reduced-intensity anticoagulation as for thrombocytopenic patients and use of an agent that can be readily monitored are options that may reduce the risk of bleeding recurrence. In patients beyond the 12-week window, a reduced-intensity approach or cessation of anticoagulation in patients with major bleeding are both reasonable options in the context of the patient’s wishes and overall treatment plan.
Conclusions
Management of thrombosis and bleeding in the cancer patient requires careful consideration of competing risks in each individual patient and sound clinical judgement. Although the past several years have witnessed an expansion of our understanding of the risks and benefits associated with the management of CAT, many questions in this unique and clinically challenging patient population remain unanswered and inadequately studied. Individualized approaches to VTE risk stratification, prophylaxis, and treatment will allow additional improvement in the care of these patients.
Acknowledgments
H.A.-S. is the recipient of the National Hemophilia Foundation-Shire Clinical Fellowship Award and the Harvard KL2/Catalyst Medical Research Investigator Training Award, which provide salary support.
Authorship
Contribution: H.A.-S. contributed to the first draft of the manuscript, critical writing and revision of the intellectual content, and final approval; and J.M.C. developed the concept and design, contributed to the first draft of the manuscript, critical writing and revising the intellectual content, and final approval.
Conflict-of-interest disclosure: H.A.-S. is a consultant for and received research funding from Agios, is a consultant for and received research funding from Dova, and is a consultant for Moderna. J.M.C. is on the scientific advisory board and a consultant for and received personal fees from Bristol-Myers Squibb, is on the data safety monitoring board for Unum Therapeutics, and is on scientific advisory boards for Portola. Off-label drug use: None disclosed.
Correspondence: Jean M. Connors, Division of Hematology, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02215; e-mail: jconnors@bwh.harvard.edu.
References
Author notes
This article was selected by the Blood Advances and Hematology 2019 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2019. It is reprinted in Hematology Am Soc Hematol Educ Program. 2019;2019:71-79.