Abstract
Over the last decade, there have been numerous developments and changes in treatment practices for the management of patients with immune thrombocytopenia (ITP). This article is an update of the International Consensus Report published in 2010. A critical review was performed to identify all relevant articles published between 2009 and 2018. An expert panel screened, reviewed, and graded the studies and formulated the updated consensus recommendations based on the new data. The final document provides consensus recommendations on the diagnosis and management of ITP in adults, during pregnancy, and in children, as well as quality-of-life considerations.
Introduction
In 2010, an international group of experts published an International Consensus Report on the investigation and management of primary immune thrombocytopenia (ITP).1 The following year, the American Society of Hematology published practice guidelines for ITP.2 These guidelines provided evidence-based recommendations and identified gaps where evidence was lacking.2 The 2010 consensus report offered recommendations based on the expert opinion of the investigators to provide a practical viewpoint.1
The literature search for the consensus report was performed in 2008.1 Since then, the field has been very active, and more information on ITP has become available, including new treatment options and trial results for established therapies. However, there are still areas where data are unavailable and, thus, the opinion of expert practitioners remains valuable.
The panel for this update includes experts from around the world, to obtain a global perspective on ITP. In addition to the regions represented in the previous consensus report, here perspectives from Australia, China, and Japan are included. Another addition to the panel is a patient support expert, who provided insights from the patients’ viewpoint and facilitated the inclusion of a section dedicated to the quality of life of ITP patients.
Therefore, this article provides an update to the previous consensus report, including up-to-date evidence, expert opinion from around the world, and the incorporation of a new focus on the patients’ perspective.
Methods
The panel included 22 members with recognized clinical and research expertise in ITP representing North America (United States, 4; Canada, 1), Europe (13), Australia (1), China (2), and Japan (1). There were 3 pediatric hematologists and 18 adult hematologists (2 with expertise in obstetric hematology). Two members were experts in clinical trials methodology. There was 1 patient representative.
The methodology of the original consensus report was closely followed. A literature search of the electronic database PubMed was performed in July 2018. The following search terms were used: “immune thrombocytopenic purpura,” “idiopathic thrombocytopenic purpura,” “autoimmune thrombocytopenic purpura,” “autoimmune thrombocytopenia,” “idiopathic thrombocytopenia,” “immune thrombocytopenia,” and “ITP.” Corresponding MeSH terms were used, in addition to searching titles and abstracts.
The search was restricted to articles published from 1 January 2009 to 23 July 2018 to capture articles published since the literature search for the original consensus report. The following filters were applied: humans, English, clinical study, clinical trial, clinical trial phase 3, clinical trial phase 4, comparative study, controlled clinical trial, multicenter study, observational study, pragmatic clinical trial, and randomized controlled clinical trial. Conference abstracts were not included. The search results were screened, and the final selection was reviewed by the authors. Any articles not identified on screening but deemed relevant by the authors were also included. Evidence levels of the articles were assigned and reviewed by the authors following the same scoring system as used previously.1 These evidence levels then determined the grades of recommendation, again using the same system as used previously.1
All authors participated in the literature search and review; grading of evidence was required of all of the contributors. Grading of recommendations was as in the original consensus report (Tables 1 and 2), as follows: Grade A requires ≥1 randomized controlled trial (RCT) as part of a body of literature of overall good quality and consistency addressing specific recommendation (evidence levels Ia, Ib), Grade B requires the availability of well-conducted clinical studies but no randomized clinical trials on the topic of recommendation (evidence levels IIa, IIb, III), and Grade C requires evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. It indicates an absence of directly applicable clinical studies of good quality (evidence level IV).
Evidence levels
| Evidence level . | Definition . |
|---|---|
| Ia | Evidence obtained from meta-analysis of RCTs |
| Ib | Evidence obtained from ≥1 RCT |
| IIa | Evidence obtained from ≥1 well-designed controlled study without randomization |
| IIb | Evidence obtained from ≥1 other type of well-designed quasi-experimental study* |
| III | Evidence obtained from well-designed nonexperimental descriptive studies, such as comparative studies, correlated studies, and case studies |
| IV | Evidence obtained from expert committee reports or opinions and/or clinical experience of respected authorities |
| Evidence level . | Definition . |
|---|---|
| Ia | Evidence obtained from meta-analysis of RCTs |
| Ib | Evidence obtained from ≥1 RCT |
| IIa | Evidence obtained from ≥1 well-designed controlled study without randomization |
| IIb | Evidence obtained from ≥1 other type of well-designed quasi-experimental study* |
| III | Evidence obtained from well-designed nonexperimental descriptive studies, such as comparative studies, correlated studies, and case studies |
| IV | Evidence obtained from expert committee reports or opinions and/or clinical experience of respected authorities |
Refers to a situation in which implementation of an intervention is not under the control of the investigators, but an opportunity exists to evaluate its effect.
Grading of evidence
| Grade of recommendation . | Definition . | Level of evidence . |
|---|---|---|
| A | Requires ≥1 RCT as part of a body of literature of overall good quality and consistency addressing specific recommendation | Evidence levels Ia, Ib |
| B | Requires the availability of well-conducted clinical studies but no randomized clinical trials on the topic of recommendation | Evidence levels IIa, IIb, III |
| C | Requires evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. Indicates an absence of directly applicable clinical studies of good quality. | Evidence level IV |
| Grade of recommendation . | Definition . | Level of evidence . |
|---|---|---|
| A | Requires ≥1 RCT as part of a body of literature of overall good quality and consistency addressing specific recommendation | Evidence levels Ia, Ib |
| B | Requires the availability of well-conducted clinical studies but no randomized clinical trials on the topic of recommendation | Evidence levels IIa, IIb, III |
| C | Requires evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. Indicates an absence of directly applicable clinical studies of good quality. | Evidence level IV |
Adapted from the National Guidelines Clearinghouse (www.guideline.gov).
Two panel meetings were held to discuss the identified data, draft consensus statements, and finalize the updated consensus-based recommendations. Updates were made based on the identified evidence, following the principles laid out in the CheckUp guidelines.3 All authors provided input on each draft of the manuscript and approved the final version for submission.
Although 100% consensus was not attained on every recommendation, 85% of recommendations achieved 85% agreement within the expert group (supplemental Table 1).
Given the rate of development of new treatments for ITP, the consensus report leadership will review the need for updates each year. In addition to publication in professional journals, implementation of the consensus recommendations will be encouraged through distribution via patient support organizations (eg, Platelet Disorder Support Association [PDSA], UK ITP Support Association), presentations at international meetings, and through generation of an ITP treatment Web site.
Diagnostic approach in patients with suspected ITP
Recommendations for diagnosis of primary ITP in children and adults
The diagnosis of ITP is based principally on the exclusion of other causes of isolated thrombocytopenia using patient history, physical examination, blood count, and evaluation of the peripheral blood film (to exclude other hematological conditions, including hereditary thrombocytopenia and pseudothrombocytopenia). If therapy is administered, platelet count should be closely monitored for response as a diagnostic aid.
A complete history, physical examination, full blood count, and an expert analysis of the peripheral blood film should be evaluated at initial diagnosis (Grade C recommendation). Based on the evidence currently available, when there is isolated thrombocytopenia and no abnormal features present on physical examination or examination of the blood smear, a bone marrow examination is not required in the initial diagnosis (Grade B recommendation), whether or not treatment is recommended.
The detection of Helicobacter pylori infection, with the urea breath test or the stool antigen test, should be included in the initial work-up in appropriate geographical areas (evidence level IIa; Grade B recommendation).
The majority of authors routinely test for hepatitis B virus (HBV), HIV, and hepatitis C virus (HCV) in all adult patients (evidence level IIb).
Quantitative immunoglobulin (Ig) level testing is indicated to exclude an immune deficiency syndrome (evidence level IV; Grade C recommendation) or before treatment with IVIg. In children, Ig level testing may be considered at baseline and should be measured in those children with persistent or chronic ITP as part of a reassessment evaluation.
Bone marrow examination could be appropriate in those relapsing after remission, in patients not responding to initial treatment options, where splenectomy is considered, or if other abnormalities are detected in the blood count or morphology (evidence level III; Grade C recommendation). This examination should ideally include an aspirate, biopsy, flow cytometry, and cytogenetics (evidence level IV; Grade C recommendation).
ITP may be classified as primary or secondary to other medical conditions present at diagnosis. Furthermore, it may be further classified as newly diagnosed (0-3 months), persistent (>3-12 months), or chronic (>12 months).
Diagnostic tools for adults and children with suspected ITP are grouped into 3 sets of recommendations (Table 3). The diagnosis of ITP can be challenging, with the differential diagnosis of thrombocytopenia being extensive (Table 4); secondary ITP needs to be excluded.4 A presumptive diagnosis of ITP is made when the history, physical examination, complete blood count (CBC), and examination of the peripheral blood smear do not suggest other thrombocytopenia etiologies. No “gold standard” test exists to reliably establish the diagnosis. Response to ITP-specific therapy (eg, IVIg, IV anti-D Ig [anti-D], or steroids) supports the diagnosis but does not exclude secondary ITP, because many secondary ITPs respond to IVIg.
Recommendations for the diagnosis of ITP in children and adults
| Basic evaluation in all patients . | Tests of potential utility in the management of an ITP patient . | Tests of unproven or uncertain benefit* . |
|---|---|---|
| Patient history | Glycoprotein-specific antibody (can be used in difficult cases, has poor sensitivity, and is not a primary diagnostic test) | TPO level |
| Family history | Anti-phospholipid antibodies (including anti-cardiolipin and lupus anticoagulant) if there are clinical features of antiphospholipid syndrome | Reticulated platelets/immature platelet fraction |
| Physical examination | Anti-thyroid antibodies and thyroid function | |
| CBC and reticulocyte count | Pregnancy test in women of childbearing potential | Bleeding time |
| Peripheral blood film | Antinuclear antibodies | Serum complement |
| Quantitative Ig level measurement† | Viral PCR for EBV, CMV, and parvovirus | |
| Blood group (Rh) | Bone marrow examination (in selected patients; refer to text) | |
| HIV‡ | Direct antiglobulin test | |
| HCV‡ | H pylori‡ | |
| HBV |
| Basic evaluation in all patients . | Tests of potential utility in the management of an ITP patient . | Tests of unproven or uncertain benefit* . |
|---|---|---|
| Patient history | Glycoprotein-specific antibody (can be used in difficult cases, has poor sensitivity, and is not a primary diagnostic test) | TPO level |
| Family history | Anti-phospholipid antibodies (including anti-cardiolipin and lupus anticoagulant) if there are clinical features of antiphospholipid syndrome | Reticulated platelets/immature platelet fraction |
| Physical examination | Anti-thyroid antibodies and thyroid function | |
| CBC and reticulocyte count | Pregnancy test in women of childbearing potential | Bleeding time |
| Peripheral blood film | Antinuclear antibodies | Serum complement |
| Quantitative Ig level measurement† | Viral PCR for EBV, CMV, and parvovirus | |
| Blood group (Rh) | Bone marrow examination (in selected patients; refer to text) | |
| HIV‡ | Direct antiglobulin test | |
| HCV‡ | H pylori‡ | |
| HBV |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; PCR, polymerase chain reaction; PTT, partial thromboplastin time; Rh, rhesus; TPO, thrombopoietin.
These tests have no proven role in the differential diagnosis of ITP from other thrombocytopenias and do not guide patient management.
Quantitative Ig level measurement should be considered in children with ITP and is recommended in children with persistent or chronic ITP as part of the reassessment evaluation.
Recommended by the majority of the panel for adult patients in the appropriate geographic setting.
Differential diagnoses of thrombocytopenia
| Previously diagnosed or possible high risk for conditions that may be associated with immune thrombocytopenia (eg, infections [HIV, HCV, HBV]), autoimmune/immunodeficiency disorders (CVID, systemic lupus erythematosus, or APS), and malignancy (eg, lymphoproliferative disorders) |
| Liver disease (including cirrhosis or portal hypertension) |
| Splenomegaly |
| Drugs (prescription or nonprescription), including heparin, alemtuzumab, PD-1 inhibitors, abciximab, valproate, alcohol abuse, consumption of quinine (tonic water), exposure to environmental toxins, or chemotherapy |
| Bone marrow diseases, including myelodysplastic syndromes, leukemias, other malignancies, metastatic disease, myelofibrosis, aplastic anemia, megaloblastic anemia, myelophthisis, and Gaucher disease |
| Recent transfusions (rare possibility of posttransfusion purpura) and recent vaccinations |
| Inherited thrombocytopenia: TAR syndrome, radioulnar synostosis, congenital amegakaryocytic thrombocytopenia, Wiskott-Aldrich syndrome, MYH9-related disease, Bernard-Soulier syndrome, type IIB VWD, or platelet-type VWD |
| Other thrombocytopenic disorders (DIC, TTP, HUS, Evans syndrome) |
| Previously diagnosed or possible high risk for conditions that may be associated with immune thrombocytopenia (eg, infections [HIV, HCV, HBV]), autoimmune/immunodeficiency disorders (CVID, systemic lupus erythematosus, or APS), and malignancy (eg, lymphoproliferative disorders) |
| Liver disease (including cirrhosis or portal hypertension) |
| Splenomegaly |
| Drugs (prescription or nonprescription), including heparin, alemtuzumab, PD-1 inhibitors, abciximab, valproate, alcohol abuse, consumption of quinine (tonic water), exposure to environmental toxins, or chemotherapy |
| Bone marrow diseases, including myelodysplastic syndromes, leukemias, other malignancies, metastatic disease, myelofibrosis, aplastic anemia, megaloblastic anemia, myelophthisis, and Gaucher disease |
| Recent transfusions (rare possibility of posttransfusion purpura) and recent vaccinations |
| Inherited thrombocytopenia: TAR syndrome, radioulnar synostosis, congenital amegakaryocytic thrombocytopenia, Wiskott-Aldrich syndrome, MYH9-related disease, Bernard-Soulier syndrome, type IIB VWD, or platelet-type VWD |
| Other thrombocytopenic disorders (DIC, TTP, HUS, Evans syndrome) |
This table lists frequent examples of differential diagnoses of ITP and possible alternative causes of thrombocytopenia identified by patient history.
APS, antiphospholipid syndrome; CVID, common variable immunodeficiency; DIC, disseminated intravascular coagulation; HUS, hemolytic-uremic syndrome; MYH9, myosin heavy chain 9; PD-1, programmed cell death protein 1; TAR, thrombocytopenia-absent radius; TTP, thrombotic thrombocytopenic purpura; VWD, von Willebrand disease.
Patient history
The differential diagnosis of thrombocytopenia (eg, ethanol abuse, infection, drugs, liver disease, and primary hematologic disorders) (Table 4) and secondary causes of ITP should be investigated. A previous infection has been reported in 55% of pediatric cases,4 and an increased ITP risk was associated with measles-mumps-rubella vaccination in children.5,6 However, in adults with newly diagnosed ITP, a case-control study found “no evidence of an increased odds of ITP after vaccination” in the previous 6 or 12 months (odds ratio, 1.0; 95% confidence interval, 0.7-1.4) (evidence level IIb).7
Important historical patient information should include bleeding after previous surgery, dentistry or trauma, prior blood counts, drug and toxin exposure, recent foreign travel and vaccinations, recent infections, needle stick accidents, and prior transfusions with blood products. Incidence and degree of bleeding (eg, menstrual bleeding) is important. If a diagnosis of ITP is established, contraindications to or cautions about corticosteroid therapy (eg, diabetes) should be noted. Inherited thrombocytopenia should be considered in all patients with long-standing thrombocytopenia unaffected by treatment, in those with a family history of thrombocytopenia or bleeding disorders, or if there are other features atypical of ITP (eg, orthopedic abnormalities, retardation, renal disease, hearing disorders, or malignancy). A prospective observational cohort study found no association of family history of autoimmune disease with development of ITP (evidence level III).8
The possibility of physical abuse must be considered by emergency department staff when dealing with a patient presenting with bruising and purpura for the first time (evidence level IV).
Fatigue may be part of the ITP syndrome.9-11
Basic evaluation
Physical examination.
Physical examination should be normal aside from bleeding manifestations. Moderate or massive splenomegaly suggests an alternative cause. Constitutional symptoms (eg, fever or weight loss, hepatomegaly, splenomegaly, or lymphadenopathy) might indicate an underlying disorder (HIV, chronic liver disease, systemic lupus erythematosus [SLE], lysosomal storage disease, or a lymphoproliferative disease).
Peripheral blood count.
CBC is usually normal except for isolated thrombocytopenia. Microcytic anemia from blood loss, if present, should be proportional to the amount and duration of bleeding; it may indicate iron deficiency (evidence level IV). The reticulocyte count may be used to identify the cause of anemia. Severe vitamin B12 and folate deficiency can result in thrombocytopenia.12,13
Evaluation of peripheral blood smear.
A peripheral blood smear should be evaluated by a qualified hematologist or pathologist. Pseudothrombocytopenia due to EDTA-dependent platelet agglutination should also be excluded (evidence level III).14 A peripheral blood smear may demonstrate abnormalities inconsistent with ITP (eg, schistocytes in thrombotic thrombocytopenic purpura or hemolytic uremic syndrome, or leukocyte inclusion bodies in myosin heavy chain 9–related disease). Excessive numbers of giant or small platelets may indicate an inherited thrombocytopenia.
Bone marrow examination.
Bone marrow examination may be informative in patients with systemic symptoms, abnormal signs, or with suspicion of a different diagnosis. Bone marrow examination can be performed during consideration of splenectomy or before starting a new treatment.15-18 If performed, the bone marrow examination should include an aspirate, a biopsy, flow cytometry, and cytogenetic analysis (evidence level IIb-IV) to help to distinguish ITP from lymphoproliferative disorders, myelodysplastic syndrome, or primary bone marrow disorders.19 In elderly patients or those not responding to corticosteroids or IVIg, next-generation sequencing (NGS) panels should be considered to assess for genes associated with clonal malignancy. Approximately one third of ITP patients have increased bone marrow reticulin; however, this is not correlated with disease severity, clinical features, or comorbidities and, thus, should not dispute the diagnosis unless there are significant amounts of type I collagen seen on the trichrome stain (evidence level III).20-22
H pylori testing.
The detection of H pylori infection with the urea breath test or the stool antigen test should be considered in adults with typical ITP, in those with digestive symptoms, and those from areas of high prevalence; evidence does not support routine testing in ITP patients outside of these areas (evidence level IIa).23 Serologic detection may be used, but it is less sensitive and less specific than the other tests and may produce false-positive results after IVIg therapy.
HIV and HCV testing.
Viral testing should be performed at baseline, before giving blood products (including Ig) (evidence level IV). Thrombocytopenia associated with HIV and HCV infections may be clinically indistinguishable from primary ITP and can occur several years before other symptoms develop.24 Routine serologic evaluation for HIV and/or HCV infection in adult patients with suspected ITP is recommended, regardless of local background prevalence and personal risk factors. Controlling these infections may result in complete hematologic remission (evidence level IIa).24
Quantitative Ig level testing.
Baseline Ig levels (IgG, IgA, and IgM) should be measured in adults (evidence level IV) and in children. They should also be measured in children with persistent or chronic ITP during reassessment. Low levels may reveal conditions including common variable immunodeficiency (CVID).
Direct anti-globulin test.
A positive direct anti-globulin test (DAT)/direct Coombs test is found in ∼20% of patients with ITP25 ; if positive, it should be followed by a haptoglobin, lactate dehydrogenase, bilirubin, and reticulocyte count to assess for hemolysis. A DAT is generally appropriate if anemia associated with a high reticulocyte count is found and if treatment with anti-D is being considered.
Blood group Rh(D) typing.
This is essential if treatment using anti-D is being considered.
Tests of potential utility
Antiplatelet antibody assays: glycoprotein-specific antibody testing.
Assays for antibodies to specific platelet glycoproteins are not routinely recommended. They have high specificity and low sensitivity but may be helpful in complex and difficult cases.26,27 Tests based on immunocapture, such as monoclonal antibody-specific immobilization of platelet antigens and modified antigen capture enzyme-linked immunosorbent assays, could be useful in complex cases and should be performed in reference centers. In contrast, platelet-associated IgG is unhelpful, because it is elevated in immune and nonimmune thrombocytopenia (evidence level IV). However, recent glycoprotein-specific assays have shown that the sensitivity and specificity of a positive test for diagnosis of active ITP (N = 228 patients) were 90% and 78%, respectively.26,27 Studies, which require confirmation, suggest it may possibly be prognostic for treatment response in the future.28,29
Anti-phospholipid antibodies.
Anti-phospholipid antibodies (APLAs), including anti-cardiolipin antibodies and lupus anticoagulant, can be found in up to 46% of otherwise typical adults with ITP,30 although most studies report rates of 25% to 30%.31-33 APLAs do not appear to affect the response to ITP treatment. Routine testing is not recommended in the absence of symptoms of antiphospholipid syndrome (eg, venous/arterial thrombosis or history of fetal loss), but it may be useful if there is concern about thrombosis or other aspects of antiphospholipid syndrome.
Anti-nuclear and extractable nuclear antigen antibodies.
A positive antinuclear antibody (ANA) test is present in 9% of children and 33% of adults with ITP and may be a predictor of chronicity (evidence levels IIb, III);34,35 hydroxychloroquine may be an effective treatment if ANAs are present, especially in young women.36 ANA testing can be considered before splenectomy because of the increased risk for thrombosis after splenectomy.37-39
Anti-thyroid antibody and thyroid function testing.
ITP was associated with clinical hyperthyroidism in 8% to 14% of patients followed in a longitudinal study.40 Other patients developed antibodies to thyroglobulin with hyper- or hypothyroidism. Mild thrombocytopenia can occur in patients with hyperthyroidism (reduced platelet survival) or hypothyroidism (possible decreased platelet production), often resolving with restoration of the euthyroid state. Antibodies to thyroglobulin, free T4, and thyroid-stimulating hormone may be measured to identify at-risk patients.
Testing for other acute and persistent infections.
There has been anecdotal association between vaccination and ITP41 ; a prospective case-control study found no increased incidence of ITP following vaccination (evidence level IIb).7 Acute viral infections and some vaccinations (with live attenuated virus) have been associated with (usually transient) thrombocytopenia. Some acute or persistent infections (eg, parvovirus, Epstein-Barr virus, and cytomegalovirus) can cause and perpetuate thrombocytopenia.
Thrombopoietin level.
Thrombopoietin (TPO) levels are generally normal in ITP patients and markedly elevated in thrombocytopenia patients as a result of bone marrow failure.42,43
Reticulated platelets/immature platelet fraction.
Reticulated platelets/immature platelet fraction are thought to reflect platelet production, and these values are elevated in most patients with ITP. However, these assays are not standardized yet and have limited availability.44,45
Coagulation tests.
Classification of ITP
ITP may be classified as primary or secondary; this article focuses only on primary ITP. In many cases, patients with primary or secondary ITP are treated similarly. However, if ITP is secondary to an underlying disease (eg, HCV, HIV, or a lymphoproliferative disease), treatment may focus on this instead of the ITP.
ITP in adults
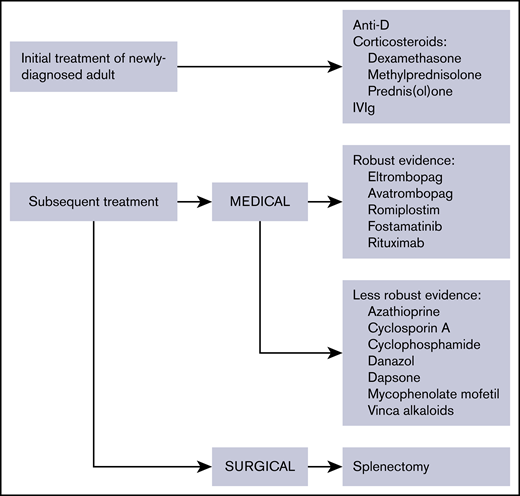
The treatment landscape has changed considerably; rituximab, eltrombopag, avatrombopag, and romiplostim are well studied, and high-dose dexamethasone may be an alternative to prednisone. Splenectomy is now recommended only after failure of medical therapies and depending on patient age and comorbidities. Here, an updated comprehensive approach to treatment is provided with evidence published since 2010 (Figure 1; Table 5). Treatment options are listed per the authors’ assessment of clinical benefit. Treatment is divided into the following categories: initial treatment, subsequent treatment, and patients failing multiple treatments.
Overview of therapies for the treatment of adult ITP. The evidence available for medical therapies is indicated.
Overview of therapies for the treatment of adult ITP. The evidence available for medical therapies is indicated.
Therapies for the treatment of ITP
| Clinical situation . | Therapy option . |
|---|---|
| Initial treatment of newly diagnosed ITP | Corticosteroids |
| Dexamethasone | |
| Methylprednisolone | |
| Prednis(ol)one | |
| IVIg | |
| Anti-D (licensed and available for ITP in only a few countries) | |
| Subsequent treatment | Medical therapies |
| Medical therapies with robust evidence | |
| Rituximab | |
| TPO-RAs: eltrombopag, avatrombopag, romiplostim | |
| Fostamatinib | |
| Medical therapies with less robust evidence | |
| Azathioprine | |
| Cyclophosphamide | |
| Cyclosporine A | |
| Danazol | |
| Dapsone | |
| Mycophenolate mofetil | |
| TPO-RA switch | |
| Vinca alkaloids | |
| Surgical therapies | |
| Splenectomy | |
| Treatment of patients failing multiple therapies | Accessory splenectomy |
| Alemtuzumab | |
| Combination of initial and subsequent therapies | |
| Combination chemotherapy | |
| Clinical trials | |
| HSCT | |
| Splenectomy, if not already performed | |
| Supportive care |
| Clinical situation . | Therapy option . |
|---|---|
| Initial treatment of newly diagnosed ITP | Corticosteroids |
| Dexamethasone | |
| Methylprednisolone | |
| Prednis(ol)one | |
| IVIg | |
| Anti-D (licensed and available for ITP in only a few countries) | |
| Subsequent treatment | Medical therapies |
| Medical therapies with robust evidence | |
| Rituximab | |
| TPO-RAs: eltrombopag, avatrombopag, romiplostim | |
| Fostamatinib | |
| Medical therapies with less robust evidence | |
| Azathioprine | |
| Cyclophosphamide | |
| Cyclosporine A | |
| Danazol | |
| Dapsone | |
| Mycophenolate mofetil | |
| TPO-RA switch | |
| Vinca alkaloids | |
| Surgical therapies | |
| Splenectomy | |
| Treatment of patients failing multiple therapies | Accessory splenectomy |
| Alemtuzumab | |
| Combination of initial and subsequent therapies | |
| Combination chemotherapy | |
| Clinical trials | |
| HSCT | |
| Splenectomy, if not already performed | |
| Supportive care |
Treatment options for ITP are listed in alphabetical order and do not imply a preferred treatment option.
HSCT, hematopoietic stem cell transplantation; TPO-RA, TPO receptor agonist.
Treatment choice is impacted by cost, financial resources of the patient, or the publicly funded health care system, national licensing and availability, and country-specific treatment guidelines. The higher cost of newer treatments is counterbalanced by the fact that some new treatments are not immunosuppressive, have undergone rigorous RCTs, have high efficacy, and may lead to periods of treatment-free remission.
Who should be treated?
Treatment should always be tailored to the patient, because many factors contribute to treatment decisions. Impacting management decisions are the extent of bleeding, age, comorbidities predisposing to bleeding, complications of specific therapies, activity and lifestyle, fatigue (see “Quality of life of adults with ITP”), tolerance of side effects, need for interventions with a bleeding risk, accessibility of care, patient expectations, patient worry or anxiety, and patient need for non-ITP medications that may create a bleeding risk. Female sex, exposure to nonsteroidal anti-inflammatory drugs (NSAIDs), platelet count <20 × 109/L, and exposure to anticoagulant drugs were associated with bleeding at diagnosis;50 bleeding and infection contribute equally to mortality.51 Although bleeding risk is rarely related to any distinct threshold platelet count, bleeding risk appears to increase when platelets are <20 × 109/L.50 Based on this and the consensus of the authors, treatment is rarely indicated in patients with platelet counts >20 × 109/L in the absence of bleeding due to platelet dysfunction or another known or unknown hemostatic defect, trauma, surgery,1,50 clearly identified comorbidities for bleeding, mandated antiplatelet or anticoagulation therapy, or fatigue or other nonhemorrhagic complications of ITP, as well as in persons whose profession or lifestyle predisposes them to trauma. Patient preference must be considered when discussing treatment options.
Several case series reported successful use of eltrombopag or romiplostim as a “bridging” therapy to increase platelet counts prior to surgery or another procedure (evidence level III).52-54 Consensus-based recommendations for patients with ITP undergoing surgery are presented in Table 6.
Consensus-based recommendation for target platelet counts for surgery or medical therapy in adults
| Type of surgery . | Target platelet count, × 109/L . |
|---|---|
| Dental prophylaxis (descaling, deep cleaning) | ≥20 to 30 |
| Simple extractions | ≥30 |
| Complex extractions | ≥50 |
| Regional dental block | ≥30 |
| Minor surgery* | ≥50 |
| Major surgery | ≥80 |
| Major neurosurgery | ≥100 |
| Splenectomy | See “Subsequent therapy: surgical” |
| Obstetrics | See “Thrombocytopenia presenting during pregnancy” |
| Single antiplatelet agent or anticoagulant (ie, 1 antiplatelet agent, warfarin, or TSOAC) | ≥30 to 50 |
| Dual antiplatelet or anticoagulant (ie, 2 antiplatelet agents or 1 antiplatelet agent plus warfarin or TSOAC) | ≥50 to 70 |
| Type of surgery . | Target platelet count, × 109/L . |
|---|---|
| Dental prophylaxis (descaling, deep cleaning) | ≥20 to 30 |
| Simple extractions | ≥30 |
| Complex extractions | ≥50 |
| Regional dental block | ≥30 |
| Minor surgery* | ≥50 |
| Major surgery | ≥80 |
| Major neurosurgery | ≥100 |
| Splenectomy | See “Subsequent therapy: surgical” |
| Obstetrics | See “Thrombocytopenia presenting during pregnancy” |
| Single antiplatelet agent or anticoagulant (ie, 1 antiplatelet agent, warfarin, or TSOAC) | ≥30 to 50 |
| Dual antiplatelet or anticoagulant (ie, 2 antiplatelet agents or 1 antiplatelet agent plus warfarin or TSOAC) | ≥50 to 70 |
Evidence level IV. Adult patients with ITP considered to be at “typical” bleeding risk from surgery. Target platelet count depends on the clinical situation and urgency and need for procedure.
TSOAC, target-specific oral anticoagulants.
Cataract surgery with laser technique has no bleeding risk.
Goals of therapy
Recommendations for treatment goals
Treatment goals should be individualized to the patient and the phase of the disease.
Treatment should prevent severe bleeding episodes.
Treatment should maintain a target platelet level >20-30 × 109/L at least for symptomatic patients (because risk for major bleeding increases below this level).
Treatment should be with minimal toxicity.
Treatment should optimize health-related quality of life (HRQoL).
Together with patient-individual and disease phase–specific treatment, patients must be educated on clinical treatment goals and reassured so that they can continue normal activities. The clinical goals should be to resolve bleeding events or to prevent severe bleeding through providing adequate hemostasis. The platelet count should be improved to attain a minimum of 20 to 30 × 109/L, but there is no need to normalize the platelet count; however, this level may not be appropriate for patients who are active or older than 60 years of age (several studies have shown increased rates of bleeding, thrombosis, and death in patients older than 60 years),55-58 patients with other comorbidities, or those on anticoagulants. Depending on a treatment response, the most appropriate additional treatments should be selected, treatment side effects should be minimized, and patients should be selected for further treatment as needed. Finally, patients’ complaints of fatigue should be respected if not offset by increased toxicity of treatment (for more information on the impact of fatigue, see “Quality of life of adults with ITP”).
Therapy for ITP can be given as an outpatient in most situations, unless there is active bleeding or other medical variables (anticoagulant therapy), the patient requires close monitoring, or it is the initial presentation for thrombocytopenia and platelets are ≤20 × 109/L.
Initial treatment of newly diagnosed patients
Recommendations for initial treatment of newly diagnosed patients
Corticosteroids are the standard initial treatment for adults with ITP who need treatment and do not have a relative contradiction: predniso(lo)ne at 1 mg/kg (maximum dose 80 mg, even in patients weighing >80 kg) for 2 weeks, to a maximum of 3 weeks, or dexamethasone 40 mg/d for 4 days, repeated up to 3 times.
If a response is seen (eg, platelets >50 × 109/L), the predniso(lo)ne should be tapered, aiming to stop predniso(lo)ne by 6 weeks (maximum 8 weeks), even if the platelet count drops during the taper.
If there is no response to the initial dose within 2 weeks, the predniso(lo)ne should be tapered rapidly over 1 week and stopped.
Longer courses of steroids should be avoided, although occasional patients may benefit from continuous low-dose corticosteroids (eg, ≤5 mg/d). This type of ongoing low-dose corticosteroid treatment should be based on the individual patient’s needs, prior therapies, and so forth.
Use of IVIg (1 g/kg on 1 or 2 consecutive days or 0.4 g/kg per day for 5 days), or IV anti-D (50-75 µg/kg once) where available, may be appropriate in patients with bleeding, at high risk for bleeding, who require a surgical procedure, or who are unresponsive to predniso(lo)ne. If using anti-D, consideration needs to be exercised over potential triggering of DIC or hemolysis. Steroid premedication should be considered for anti-D to minimize acute infusion reactions (eg, headaches, fever-chills, and/or intravascular hemolysis).
Certain patients may have relevant contraindications to high-dose corticosteroid therapy (eg, insulin-dependent diabetes, uncontrolled diabetes, psychiatric disorders, active infection) and may be managed with only IVIg or IV anti-D as initial therapy.
TPO receptor agonists (TPO-RAs) and rituximab are not considered initial therapies.
Initial treatments for adults newly diagnosed with ITP are presented in supplemental Table 2.
Corticosteroid therapy.
Corticosteroids remain the standard initial treatment of newly diagnosed patients and should be used for a limited time. Corticosteroids have multiple beneficial hemostatic effects on platelets by decreasing platelet clearance59 and increasing platelet production.60 Additionally, they may reduce bleeding, independent of the platelet count increase, via a direct effect on blood vessels.61,62 Although the consensus panel believed that some patients were able to maintain a platelet response to a daily dose of predniso(lo)ne ≤ 5 mg, the side effects of corticosteroids outweigh their benefits in the long-term.1
Prednisone and dexamethasone.
There are multiple small trials on prednisone and dexamethasone with various outcomes.63-69 In a meta-analysis of high-dose dexamethasone vs prednisone, there was no difference in platelet count response at 6 months; however, high-dose dexamethasone led to faster responses without additional toxicity.70 Acyclovir may be given with dexamethasone to minimize the potential for herpes virus reactivation (evidence level IV).
Dexamethasone plus rituximab.
Since 2010, 5 studies (1 in children), including 2 large RCTs, have assessed the efficacy of rituximab in combination with dexamethasone.71-75 Additionally, a large meta-analysis on rituximab plus standard of care published in 2015 showed that adding rituximab may increase the response rate earlier; however, after 6 to 12 months, there is limited evidence for a sustained response.76 The panel believed that the potential benefit of adding rituximab to corticosteroids did not warrant the added toxicity and costs but that with further study this regimen may be proven to be the optimal way of using these 2 therapies.
Methylpredniso(lo)ne.
High-dose methylpredniso(lo)ne was used in various regimens to treat patients failing first-line therapies,77,78 with 80% response rates; however, maintenance therapy with oral corticosteroids may be required as a result of the short-term response (evidence level IV). There are no new data since 2010 to recommend methylpredniso(lo)ne over dexamethasone or prednisone.
IVIg.
Seven studies assessing IVIg were identified.79-85 IVIg as a standard ITP therapy is well documented.1 Newer research focused on optimizing delivery of IVIg infusions to reduce the burden of treatment and HRQoL80 or trialing different formulations or rates.79,82,83 IVIg 10% is as effective as IVIg 5%, with response rates ranging from 72.2% to 80.7% (evidence level IIa-IIb).79,82,83 Adverse events (AEs) were comparable to those seen for IVIg 5% (evidence level IIa-IIb).79,81-83
Several studies have examined the relationship between IVIg responses and the type of antiplatelet antibody. Two have found a reduced IVIg response in patients with only anti–GPIb-IX antibodies.86,87 In 1 study, IVIg therapy (0.4 g/kg per day for 5 days) in treatment-naive adults with severe ITP resulted in responses (platelet count ≥30 × 109/L) in 36.4% of patients with anti–GPIb-IX antibodies and in 80% of those negative for anti–GPIb-IX autoantibodies.85 Although anti–GPIb-IX antibodies may predict poor response to IVIg (evidence level IIb), this finding has not been confirmed by other studies.88 Moreover, these tests are not readily available, and most patients have multiple antibodies; rarely do they have only anti–GPIb-IX antibodies.
Subcutaneous Ig does not appear to be as effective as IVIg in ITP.
IV anti-D.
In addition to the previous studies,1 1 small study (20 patients) evaluated anti-D treatment (50 µg/kg) in adults with ITP. In newly diagnosed patients, the overall response rate was 65%, with a median duration of remission of 25 days; however, administration of anti-D was associated with chills, pyrexia, a decrease in hemoglobin count, and an increase in bilirubin, with clinical and laboratory evidence of hemolysis in all patients (evidence level III).89 This drug should be used with caution in patients with active autoimmune hemolysis and anemia. IV anti-D carries a black box warning for a risk for intravascular hemolysis in ITP leading to death, anemia, multisystem organ failure, and acute respiratory distress syndrome, as well as serious complications, including severe anemia, acute renal insufficiency, renal failure, and DIC.90 Administering IV anti-D with steroid premedication greatly minimizes the side effects indicated.91 IV anti-D is not available in Europe.
Emergency treatment
Recommendations for life-threatening bleeding
A combination of initial treatments, including IV corticosteroids and, usually, IVIg, should be used in emergency situations in which there is an urgent need to increase the platelet count within 24 hours (Grade C recommendation). Platelet transfusions may be helpful and must not be postponed in cases of life-threatening bleeding, especially intracranial hemorrhage (ICH).
In the case of life-threatening bleeding and the absence of a significant response to IVIg and platelet transfusion in a patient on corticosteroids, the use of a TPO-RA should be considered.
Additional options may include IV anti-D, vincristine or vinblastine, antifibrinolytics in combination with other initial therapies (Grade C recommendation), and, rarely, emergency splenectomy.
No study was found reporting on treatments aimed at urgently increasing platelet counts in patients requiring urgent surgery, those at high risk for bleeding, or those with active central nervous system, gastrointestinal, or genitourinary bleeding.
Although changing from corticosteroids to IVIg or anti-D alone may be effective in emergency settings, adding either to corticosteroids may be appropriate for the emergency treatment of patients with uncontrolled bleeding. High-dose methylprednisolone may also be useful. Other therapies that work rapidly include platelet transfusion, possibly combined with IVIg, and emergency splenectomy. Some evidence shows a rapid (peak effect in 7-9 days) response to vinca alkaloids.92
Although TPO-RAs take ≥5 days to initiate a response, and rituximab usually takes 3 to 4 weeks, early administration of either might be considered, especially in patients who are not surgical candidates or who have major contraindications to other therapies. Also, because almost all other treatments will be short-lived, administration of these agents may limit the need to revisit the emergency department 2 weeks later.
Definition of life-threatening bleeding.
Patients presenting with severe bleeding manifestations, particularly if the platelet count is ≤20 × 109/L,50 demand immediate treatment. Definition of bleeding severity is largely a subjective judgment by the clinician, but the use of a standardized bleeding assessment tool may represent a general guide. For example, Khellaf et al used a very simple score based on subjective judgment of external bleeding manifestations at onset to decide between treatment based on corticosteroids only or corticosteroids plus IVIg.93 An International Working Group (IWG) proposed a more elaborate standardized bleeding assessment tool in which 3 distinct domains (skin, visible mucosae, organ) were scored based on the worst manifestation.94
General measures.
These include cessation of drugs reducing platelet function, control of blood pressure, inhibition of menses, and efforts to minimize trauma (evidence level IV). However, there may be instances in which oral anticoagulation (eg, in patients with some prosthetic heart valves, atrial fibrillation, or with coronary stents) is required, and this necessitates raising the threshold platelet count for treatment. In patients with reduced renal function, hemostasis may be improved with estrogens or desmopressin and by maintaining hemoglobin at ≥10 g/dL.
Platelet transfusions with or without IVIg.
Platelet transfusion in ITP has been poorly studied but is widely recommended in bleeding patients failing other therapies.95,96 Platelet transfusion increases the posttransfusion platelet count by >20 × 109/L in 42% of bleeding ITP patients and may reduce bleeding.97 In a retrospective study of 40 patients (evidence level IIb), concurrent administration of platelet transfusions and IVIg was associated with bleeding resolution, rapid restoration of adequate platelet counts, and minimal side effects (evidence level III/IV).98 This combination is widely used, albeit not well substantiated. Similarly, platelet bolus vs continuous infusion is not well studied, but giving a bolus, followed by continuous infusion, seems appropriate in situations in which increasing the platelet count is urgent, especially if the bolus increases the platelet count.
Vinca alkaloids.
Weekly IV doses of vincristine (1-2 mg for 2-4 weeks) or vinblastine (10 mg for 1-3 weeks) are associated with rapid responses of 71% at 7 days and 68% at 1 month in patients with ITP.99-110 Unfortunately, these high rates of response are accompanied by a low rate of durable response (28%) and significant toxicity. However, when combined with other agents, vinca alkaloids may be useful in patients requiring emergency treatment (evidence level IIb).1,111 Repetitive use is commonly associated with peripheral neuropathy, which may favor vinblastine.
Emergency splenectomy.
See “Subsequent therapy: surgical.”
Antifibrinolytics.
Antifibrinolytic agents (eg, oral or IV tranexamic acid and ε-aminocaproic acid) may be useful in preventing recurrent bleeding in patients with severe thrombocytopenia; however, the efficacy has not been evaluated by a randomized trial in ITP patients. A recent Cochrane analysis of patients with many types of thrombocytopenia was inconclusive.112 Tranexamic acid (15 to 20 mg/kg every 8 hours orally) and ε-aminocaproic acid (1-5 g every 4-6 hours [maximum dose, 24 g/d]) may be useful in certain dental or surgical procedures or if there is ongoing or a substantial risk for bleeding. Use of these agents also increases the risk of thrombosis.
Emergency treatments that are not indicated.
Plasmapheresis and recombinant factor VIIa are not recommended.1
Subsequent treatment options for adult patients with persistent and chronic ITP
Most adult patients relapse upon cessation of steroid treatment. Although few patients may be maintained on daily low doses of steroids (eg, ≤2.5-5 mg prednisone) for prolonged periods of time without incurring major side effects, subsequent therapy is indicated for most other patients. Probably the most consistent and prevalent error in ITP management is overusage and reliance on steroids. Currently, no study has addressed the correct sequence of subsequent therapies; as such, the panel presents the following consensus-based recommendations.
Recommendations for subsequent therapy strategy
There are many medical therapy options with few AEs.
Not all therapies are available in all countries; thus, the recommendations should be modified based on available resources and patient preference.
Some medical options may require ongoing continued treatment.
Up to one third of patients may remit in 1 year,113 and up to 80% may remit in 5 years.114,115 If possible, splenectomy should be deferred for ≥1 year to allow for remission.113,115
Since 2010, the main changes are in the use of rituximab, the TPO-RAs (eltrombopag, avatrombopag, and romiplostim), and fostamatinib, for which numerous studies have been published; the main evidence is described here. Furthermore, splenectomy is dealt with as a separate section (see “Subsequent therapy: surgical”). Physicians should make individual judgments about the nature of subsequent treatments based on the patient’s profile (bleeding history, comorbidities, and compliance), patient preference, and availability of drugs.
The main goal of subsequent treatment is to attain a sustained increase in the platelet count that is considered hemostatic for the individual patient while minimizing AEs and allowing for the possibility of attaining a remission.
The concept of remission in ITP has not been rigorously defined. In several articles it has been defined as a platelet count ≥30 × 109/L in the absence of treatment. It occurs in up to 85% of ITP patients without splenectomy after 5 years,115 in 32% of ITP patients within 1 year if treated with romiplostim within the first 6 months of diagnosis,113 and in 28% of patients with chronic ITP treated with romiplostim for >6 months.116 For this consensus, remission will be regarded as a platelet count ≥30 × 109/L in the absence of any ITP-specific treatment.
Available treatment modalities have different mechanisms of action and can be broadly categorized into those that are given only once (or for only 1 course) and are intended to induce a long-term response (rituximab, splenectomy) and those that need continued or chronic administration (low-dose corticosteroids, immunosuppressive agents, TPO-RAs). Patients on agents in the latter category may improve at any time, but who will improve and when is unpredictable (supplemental Table 3).
Subsequent therapy: medical
Medical therapies with robust evidence
TPO-RAs (eltrombopag, avatrombopag, romiplostim) have provided excellent responses (>60%) in splenectomized and nonsplenectomized patients (Grade A recommendation, evidence level Ib). Response to continued TPO-RAs persists for up to 6 to 8 years117 and often allows other ITP therapy to be reduced or discontinued. Cessation of treatment will lead to the return of thrombocytopenia in most cases, but some patients (10%-30%) may achieve a durable response after TPO-RAs are tapered and withdrawn.
Evidence from a systematic review of multiple uncontrolled trials and RCTs shows a response to rituximab in 60% of patients. Long-term durable responses occur in 20% to 25% of adult patients (Grade B recommendation, evidence level IIa). Prior to treatment, hepatitis B status should be determined, and vaccination against encapsulated gram-positive bacteria should be given (Grade C recommendation, evidence level IV).
Fostamatinib offers an alternative mechanism for reducing platelet destruction; it may provide response rates of 43% but stable responses of only 18%.
TPO-RAs: romiplostim, eltrombopag, avatrombopag.
TPO-RAs increase platelet production (supplemental Table 3).65,66 Romiplostim and eltrombopag are approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of adults (and children, see below) with chronic ITP who have had an insufficient response to corticosteroids, Igs, or splenectomy.65-68 Avatrombopag has only recently (27 June 2019) been approved by the FDA for adult patients with chronic ITP who have had an insufficient response to a previous treatment.118 Romiplostim is administered at an initial dose of 1 µg/kg per week subcutaneously, with dose adjustments up to 10 µg/kg per week according to platelet response.65,67 Eltrombopag is administered at an initial dose of 25 or 50 mg/d, depending on patient age, Asian ancestry, and presence of hepatic impairment, up to a maximum of 75 mg/d.66,68 Avatrombopag is administered initially as 1 20-mg pill daily with dose increases up to 40 mg/d, depending on subsequent platelet counts.118
Due to their mechanism of action, TPO-RAs are considered a maintenance therapy. Upon cessation of treatment, many patients return to lower platelet counts (10% transiently falling below baseline platelet counts); however, nearly one third of newly diagnosed patients69 and ∼15% to 30% of chronic patients70 are able to eventually discontinue treatment successfully, for various periods. In studies, most AEs were reported as mild. These drugs are associated with a possible increase in bone marrow reticulin fibrosis, which has been a concern, but the risk of clinically meaningful fibrosis appears to be very low.119-121 Bone marrow fibrosis has been assessed in prospective studies with romiplostim and eltrombopag; it was found to be increased by treatment in 6.9% of patients on romiplostim22 and in 3.8% of those on eltrombopag.21 Most patients were able to remain on treatment; fibrosis was reversed in those who stopped.
Recent studies have shown no significant increased rate of cataracts and a low (<15%) rate of mild liver function test abnormalities with eltrombopag; however, 3% of patients have had to discontinue the drug for this reason.117
Bone marrow examinations are not mandatory prior to initiating TPO-RAs, but regular monitoring of blood counts is needed. Bone marrow biopsy should be performed if there is no response to treatment within the expected timeframe or if the diagnosis is not certain.
TPO-RAs can be associated with arterial and venous thrombosis. In the long-term studies, 6% of patients developed arterial or venous thrombosis. However, when directly compared with placebo, none of these drugs resulted in an increased rate of thromboembolism,117,122 recognizing that the risk of thromboembolism is increased in ITP patients not so treated.123 However, there is some evidence that thrombotic events are higher in ITP patients on romiplostim, especially those older than 60 years.56
Romiplostim.
Compared with placebo, romiplostim has been shown to significantly increase and maintain platelet counts. Response rates of 74% to 96% have been reported, with some studies showing no differences in splenectomized patients and others reporting lower responses (evidence level Ib-III).122,125,127,128,130,137 Responses were shown to be slightly higher in patients ≥65 years old vs <65 years old132 and are achieved after 1 week of treatment,128 and time to response is shorter with a 3-µg/kg dose compared with a 1-µg/kg dose (1 vs 2 weeks).125 A lengthened dose interval of romiplostim from once weekly to once every 2 weeks has not been shown to be effective (evidence level III).136 Responses remained stable for ≥3 weeks in 82% of patients without a dose change (evidence level III), and patients had a platelet response > 50 × 109/L on a median of 92% of visits; evidence level III).130 Treatment-free remissions occurred in 28% of patients after a median of 1.8 years and was more likely in splenectomized patients than in those who had failed previous treatments (evidence level III).116
Use of rescue medication (9% to 47%) varied among studies125,127,128,130,134,137 ; it appeared to be used more frequently in splenectomized patients and declined significantly over time on romiplostim treatment.122,130 Concomitant Ig use was reduced by 5.31-fold in 1 study (evidence level Ib)129 and was reported to be rarely used in another (evidence level III)130 ; additionally, concurrent ITP treatments were discontinued or reduced in 54% to 100% of patients.127,130,137 Bleeding events have been shown to decrease.124,126 Adverse drug reactions were shown to be comparable with standard of care or placebo135 with a rate of thromboembolic events of 3% to 6.5%, the same as placebo.122,125,127,130,134 Bone marrow reticulin was reported in 4 to 17 patients in studies125,130,134,135 ; an increase of bone marrow reticulin by ≥2 grades was reported in 6.9% of patients in a phase 4 study.22
Romiplostim should be discontinued if the platelet count does not increase sufficiently after 4 weeks of treatment at the maximum dose of 10 µg/kg per week.119,120 If response to romiplostim is lost, testing for an anti-romiplostim antibody should be considered.
Alternative dosing strategies.
Although the recommended starting dose is 1 µg/kg per week, escalation to the maximum dose of 10 µg/kg could take 10 weeks. The average weekly romiplostim dose in most studies is 3 to 5 µg/kg, and ≥1 study has used 3 μg/kg as the initial dose.113,131,139 Suboptimal platelet response may be improved by adding low doses (2.5-5 mg) of prednisone to romiplostim.140
Eltrombopag.
Several studies and 1 meta-analysis on eltrombopag have been published since the previous consensus recommendations.
Eltrombopag is administered orally ≥2 hours before or 4 hours after products containing polyvalent cations (eg, calcium-containing dairy products).141 This is due to its nature as a chelator.121,141 If there is no response after 4 weeks of treatment at the maximum dose, treatment with eltrombopag should be discontinued.121,141
Eltrombopag carries a black box warning for hepatic decompensation in patients with chronic hepatitis C and a risk for hepatotoxicity. Liver tests and CBCs should be performed regularly throughout treatment with eltrombopag.121,141
Persistent and newly diagnosed ITP.
Use of eltrombopag was shown to be effective in a retrospective study of 220 patients with newly diagnosed or persistent ITP. At 15 months, responses occurred in 90% of patients, with complete responses achieved in 75.9% (platelet count ≥30 × 109/L). No differences in responses were seen between the phase of ITP, but there was a trend toward better responses in newly diagnosed patients (93.3% responses and 86.7% complete responses).142 Responses in persistent ITP (83.3% responses and 80.0% complete responses) and chronic ITP (79.4% responses and 73.1% complete responses) were similar (evidence level III).142
Chronic ITP.
Eltrombopag has been well studied in patients with chronic ITP since the previous consensus recommendations.142-152 Eltrombopag has been used at a starting dose of 50 mg daily, as well as 12.5 mg or 25 mg in 1 trial.147,149-151 Response rates vary but have been reported to range from 50% to 88.8%.142-145,149-152 Eltrombopag is associated with a decrease in bleeding rates across studies,142-144,146,150,151 with a 26-fold greater chance of achieving platelet counts ≥50 × 109/L (P < .001) over placebo.144 A median platelet response rate of 75.2% over 15 months has been reported,152 although platelet values have been shown to return to baseline values 2 weeks after treatment cessation.150 Splenectomy status, baseline platelet count, concomitant ITP treatments, and number of previous ITP treatments do not appear to have any effect on response rates.150,152
Longer-term use of eltrombopag (EXTEND study).
Eltrombopag has been shown to be effective in the long term in the open-label EXTEND study conducted in 302 ITP patients. Eltrombopag led to an increase in median platelet counts to ≥50 × 109/L by week 2, which was sustained throughout the median treatment period of 2.37 years (range, 2 days to 8.76 years). Responses were achieved in 85.8% of patients (platelet count ≥50 × 109/L at least once in the absence of rescue therapies), and 52% achieved continuous responses (≥25 weeks). Low platelet counts (<15 × 109/L), more previous therapies, and/or splenectomy led to slightly lower responses. Bleeding symptoms decreased from 57% to 16% at 1 year. Moderate to marked bone marrow reticulin fibrosis was observed in 2 patients (1.7%) (evidence level III).153
Alternative dosing regimen.
Although the prescribed dose is 25 to 75 mg/d, with the daily dose being decreased for platelet counts >200 × 109/L,121,141 an alternative dosing algorithm has been reported in which 75-mg dosing is used in patients without liver dysfunction or Asian ancestry, and the frequency of the 75 mg/d dose is decreased, often to twice a week.140
Discontinuation and sustained responses.
In 1 study, eltrombopag led to complete responses in 201 of 260 patients (evidence level III).154 Of these, 80 discontinued therapy, and 26 of the 49 evaluable patients (53%) maintained their response for ≥6 months). Patients had a median time from ITP diagnosis of 46.5 months, with a median of 4 previous treatments, and 42% were splenectomized.154
Avatrombopag.
Avatrombopag is another TPO-RA that was very recently approved by the FDA for the treatment of chronic ITP in adults.118 Like eltrombopag, avatrombopag binds to the transmembrane region of the TPO receptor and activates signal transduction pathways leading to increased platelet production.155,156 The drug is administered orally, has no interactions with food or cations, does not require monitoring of liver functions, and is three- to fourfold more potent than eltrombopag in stimulating an increase in platelets in healthy volunteers.157,158 Phase 2 studies in humans showed that 93% of adult ITP patients had a platelet response (platelets increasing to >50 × 109/L) 7 days after starting a 20-mg/d dose vs 7% of those on placebo.159 Responses were maintained for 6 months in an extension study. In the subsequent phase 3 study (NCT014339768), adults with chronic ITP were randomized to 20 mg of avatrombopag (32 subjects) or placebo (17 subjects) for 24 weeks; the median cumulative number of weeks with a platelet response >50 × 109/L was 12.4 for avatrombopag and 0.0 for placebo (P < .0001).160 Platelet responses were rapid (66% by day 8) and were maintained for >12 months in an extension study. Efficacy in children has not yet been established. Dose changes are made by increasing the 20-mg starting dose to 40 mg/d or reducing dose frequency.
Rituximab.
Long-term data for rituximab have become available since 2010 (supplemental Table 3). A meta-analysis of 5 trials revealed that complete responses were more likely with rituximab than with standard of care; thus, rituximab can improve platelet count responses at 6 months in ITP patients.76 The meta-analysis showed that rituximab was not associated with reductions in bleeding or an increase in infections. The 5-year outcomes of rituximab at the standard dose were assessed in 376 adults; this study revealed that 215 patients (57%) attained a remission (144 [38%] complete; 71 [19%] partial). Of the initially treated adult patients, only 38% maintained remission at 1 year, and 21% maintained remission at 5 years. No difference in projected outcomes with respect to splenectomy was found, although there was a tendency for splenectomized patients to relapse earlier than nonsplenectomized patients. In adults with responses lasting ≥1 year, relapse rate in partial responders was 53% compared with 31% in complete responders (P < .1) (evidence level III).161 In another study, chronic ITP patients who had not responded to corticosteroids or dexamethasone underwent splenectomy or rituximab therapy at a standard dose; no significant differences in the rate of sustained response to rituximab or splenectomy were observed. There was a propensity for younger patients to have been treated with splenectomy and older patients with rituximab (evidence level IIb).162
In other studies included here, rituximab has primarily been used at a dose of 4 infusions of 375 mg/m2,163-169 although 3 studies have assessed higher dosing schedules and 2 studies have assessed lower dosing schedules (see “Alternative dosing”).168,170-173 Studies indicate that response rates vary from ∼60% to 80%166,169 ; lower response rates appear to be associated with an increased number of failed previous therapies and long duration of disease.163,164 Patients with a shorter duration of disease were statistically significantly more likely to achieve a response (P = .082); patients <40 years old and females were more likely to achieve a complete response (P = .025 and P = .009, respectively).164 Previous splenectomy does not appear to affect the response rate (evidence level III), although there is a tendency to relapse earlier.165 One study showed that responses were better in patients with concomitant corticosteroids compared with rituximab alone (evidence level III).165 Time to relapse has been reported as 36 weeks (evidence level Ib),167 and lasting responses have been reported as 29% at 24 months; a sustained response at 1 year was significantly associated with ITP duration <1 year (P = .02) and a previous transient response to corticosteroids (P = .05) (evidence level III).168 Rituximab may be a good therapeutic option for the treatment of SLE-associated and corticosteroid-dependent ITP.174
Contraindications and long-term safety.
Rituximab carries a black box warning for fatal infusion reactions, severe mucocutaneous reactions, HBV reactivation, and progressive multifocal leukoencephalopathy.175,176 Patients should be screened for HBV infection prior to rituximab administration; those with evidence of prior resolved HBV infection should be monitored during and for several months following rituximab treatment, whereas those with active infection needing rituximab should receive concurrent antiviral treatment.175,176
AEs associated with rituximab in ITP are usually mild or moderate, with a very low incidence of infections of variable severity.76,167,168,177 Previously, there were reports of >50 cases of progressive multifocal leukoencephalopathy (JC virus infection) associated with rituximab treatment in patients with lymphoma, as well as 2 patients with SLE and ITP.1 These cases tended to occur in patients who were heavily immunosuppressed and on combination treatments.
Alternative dosing.
Alternative dosing schedules (1000 mg on days 1 and 15168,170,171 and 100 mg weekly for 4 weeks172,173 ) have been used and showed response rates > 50% (evidence level III).173,178
Rituximab and dexamethasone.
See “Initial treatment of newly diagnosed patients: dexamethasone plus rituximab.”
Rituximab and vaccine effect.
Immunological responses to polysaccharide and conjugated vaccines appear to be impaired in ITP patients who have undergone treatment with rituximab until B-cell return for ≥6 months (evidence level III).179
Rituximab and hypogammaglobulinemia.
Repetitive use of rituximab may cause hypogammaglobulinemia. Ig levels should be monitored in patients receiving repetitive dosing.
Fostamatinib.
Fostamatinib is a small molecule spleen tyrosine kinase inhibitor that was approved by the FDA in April 2018 for treatment of adults with chronic ITP who have had insufficient responses to a previous treatment.180 Two double-blind RCTs in patients who had failed splenectomy, TPO agents, and/or rituximab, and with a median duration of ITP of 8.5 years, have shown that fostamatinib, at an initial dose of 100 mg twice daily (frequently increased to 150 mg twice daily in nonresponders), results in an overall response (platelets ≥50 × 109/L) rate of 43% but with a median stable response (platelets ≥50 × 109/L for 4 of 6 weeks) of only 18% vs 2% of those on placebo (P = .0003). However, the time to response was short, occurring at a median of 15 days (evidence level Ib).181 Patients previously treated with a TPO-RA had a 17% stable response rate.181 More than half of the responders maintained a response on long-term treatment.182 The most common AEs leading to dose reductions were hypertension and diarrhea.181 If no response is seen after 12 weeks, the drug should be discontinued (supplemental Table 3).180
Medical therapies with less robust evidence
Immunosuppressive agents (including mycophenolate mofetil [MMF], cyclosporine A, and azathioprine) may be used in patients failing other therapies. Danazol and dapsone are “corticosteroid-sparing” agents that may be particularly useful in some patients (eg, those in whom splenectomy is contraindicated or if other agents are unavailable) (Grade B recommendation, evidence level IIa/IIb).
Vinca alkaloids are not a chronic therapy option because of neurological toxicity.
Therapies are presented here in alphabetical order; only new data found in the literature search are presented. For many of these agents (eg, cyclosporine A, mycophenolate), there is anecdotal evidence that the addition of corticosteroids or TPO-RA may enhance the response.
Azathioprine.
Limited published data were found since 2010. A retrospective study assessing treatment patterns in ITP patients in Sri Lanka could not demonstrate a significant response to azathioprine (evidence level III).183 Azathioprine has less of a role in patients who could try a TPO-RA or rituximab (supplemental Table 3).
Cyclosporine A.
Cyclosporine A (2.5-3 mg/kg per day) increases the platelet count as a single agent or in combination with predniso(lo)ne. In some patients, the side effect profile restricts its use (Grade B recommendation). Because of its side effect profile, the panel preferred mycophenolate. No new data were found (supplemental Table 3).
Cyclophosphamide.
No new data were found (supplemental Table 3).
Danazol.
One multicenter RCT compared the safety and efficacy of recombinant human TPO (rhTPO) with danazol in patients with persistent ITP; rhTPO plus danazol was more effective than danazol alone and had a slightly shorter time to response (evidence level Ib) (supplemental Table 3).184 In another study of 319 Chinese ITP patients treated with danazol, with or without corticosteroids, the overall response rate was 65%; of the 103 patients treated with danazol alone, 63% attained remission.185
Dapsone.
Two studies have reported on dapsone treatment in ITP patients. Response rates of 55% with a median time to response of 1 month were reported in 20 patients treated with dapsone as a salvage therapy for ITP that had relapsed or failed steroid or rituximab treatments. In all responders, concomitant therapies were discontinued; dapsone was given for a median of 31 months in responders, and responses lasted for 42 months (evidence level III).186 A retrospective analysis reported an overall response rate of 66%, including 24% complete responses, with dapsone in patients with ITP. Response was maintained by 81% of responders after interruption of treatment, for a median of 26 months (evidence level III) (supplemental Table 3).187
MMF.
Two studies have reported on the use of MMF (evidence level III)188,189 ; the studies appear to show that a long duration of daily dosing is required for achieving a response and that MMF can be used effectively many years after an initial ITP diagnosis (supplemental Table 3).
Vinca alkaloids.
One small study investigated the use of vincristine as a combination of rituximab, cyclophosphamide, vincristine, and prednisone in 16 chronic ITP patients who had relapsed or had not responded to rituximab therapy. The treatment was not well tolerated, and there was no benefit observed with regard to response over the standard dose of rituximab (evidence level Ib).190 Because of the neurotoxicity of vinca alkaloids, they are never used as a chronic form of therapy (supplemental Table 3).99,109
Subsequent therapy: surgical
Recommendations for surgical therapy for persistent and chronic ITP in adults
Splenectomy is associated with long-term treatment-free remissions. It is recommended to wait ≥12 to 24 months from diagnosis before performing splenectomy because of the chance of remission or stabilization of a platelet count at a hemostatic level (Grade C recommendation).
When available, indium-labeled autologous platelet scanning may be useful prior to splenectomy to confirm that the spleen is the main site of platelet sequestration (Grade B recommendation).
Laparoscopic splenectomy is as effective as open splenectomy in terms of response and is more comfortable for the patient (Grade B recommendation).
Postoperative thromboprophylaxis should be considered in patients undergoing splenectomy as long as the platelet count is >30 to 50 × 109/L (Grade C recommendation).
Splenectomy should be performed by a surgeon experienced in identifying accessory splenic tissue, which is common and should be removed (Grade C recommendation).
Appropriate vaccination against Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae must be provided ≥2 weeks before splenectomy and maintained according to national guidelines; recent treatment (within 6 months) with rituximab may impair vaccination efficacy.
Patients should be informed of the long-term risks of splenectomy (increased rates of thrombosis, infection, and cancer) and educated to follow advice aimed at mitigating these complications (Grade C recommendation).
Antibiotic prophylaxis should be given as per national guidelines (Grade C recommendation).
Splenectomy.
Splenectomy was previously considered a second-line surgical therapy.1 Here, “Subsequent therapy: surgical” covers the efficacy, patient acceptability, procedure timing, and adverse effects of splenectomy (supplemental Table 3).
When to perform splenectomy.
It is recommended to wait ≥12 to 24 months from diagnosis before performing splenectomy because of the chance of remission or stabilization. One single-center study reported that very low platelet counts (<10 × 109/L) should not necessarily be a contraindication for laparoscopic splenectomy in patients with ITP.191 Ideally, platelet counts should be increased if possible to >50 × 109/L prior to the procedure to reduce postsurgical complications (evidence level IV). Steroids, IVIg, TPO-RAs,66,192 or other effective therapy may be used to increase platelet counts prior to splenectomy.
Response rates.
Initial overall response rates of up to 90% have been reported in studies of splenectomy in patients with ITP,37,192-197 but these data may be exaggerated by spontaneous remission. Response has been maintained for 10 years in 78% of patients,195 and a 20-year relapse-free survival of 67% has also been reported.37 However, up to 19% of patients have no formal response to splenectomy; nevertheless, some attain a hemostatic platelet count (evidence level IIb).37,193,194
Two studies have compared splenectomy with rituximab.162,196 One reported no significant difference in response rate with splenectomy vs rituximab in corticosteroid-refractory patients162 ; the other reported significantly higher rates of response and complete response at 3 and 12 months with splenectomy vs rituximab in patients with persistent/chronic ITP (evidence level IIb).196
Predictors of success.
Retrospective analyses have reported no significant impact of baseline characteristics (including age, sex, and interval from diagnosis to splenectomy) on response to splenectomy (evidence level IIb).37,193,197 However, age ≥60 years is associated with significantly higher rates of relapse and postoperative complications (evidence level IIb).193
Indium-labeled autologous platelet scanning may be able to predict response to splenectomy but is not widely available.198-201
Open vs laparoscopic splenectomy.
Laparoscopic splenectomy is associated with significantly shorter hospitalization, less intraoperative blood loss, and quicker resumption of oral diet compared with open splenectomy (evidence levels IIb-III).194,195,202,203 Postoperative complication rates are reported to be nonsignificantly lower with laparoscopic vs open splenectomy,202,204 and response rates are similar with the 2 methods.194,195,197,202,204
Complications.
Postsplenectomy, patients may have a higher risk for thromboembolism and infections (eg, pneumonia, meningitis, and septicemia).49,205,206 These events may be easily resolved or life-threatening; they may occur within a few days of surgery or after longer follow-up. Age ≥60 years has been reported to be associated with significantly higher complication rates.193 A retrospective observational study reported an association between preoperative use of TPO-RAs and reduced complications (evidence level IIb).192 In another study, computed tomography scans performed 3 to 7 days postsplenectomy showed portal vein or splenic vein clots in 22 of 30 ITP patients; repeat scans done ∼42 days later showed resolution of 80%.207
Due to the risk of bleeding, patients with ITP may be administered prophylactic platelet transfusions. However, a retrospective single-center study of splenectomy in patients with low platelet counts reported no significant difference in volume of blood loss or excessive bleeding incidence between patients with platelet counts <10 × 109/L receiving a platelet transfusion and those with counts ≥10 × 109/L (evidence level IIb).191
Prevention of infection after splenectomy.
Patients who have undergone splenectomy are at a higher risk for severe infection, even 10 years after the procedure.208 Infection risk may be reduced, although not completely,193 through antibiotic prophylaxis and vaccination. Appropriate vaccination against S pneumoniae, N meningitidis, and H influenzae must be provided, preferably before splenectomy. Vaccination should be performed ≥6 months before treatment with rituximab if possible.175 The latest national guidelines (eg, Centers for Disease Control and Prevention, https://www.cdc.gov/vaccines/schedules/index.html) on vaccination presplenectomy and on antibiotic prophylaxis postsplenectomy should be followed.
Partial splenic embolization.
Partial splenic embolization could, if necessary, be performed as an alternative to splenectomy. A retrospective single-center analysis of 91 ITP patients reported an overall response rate of 84% and a 10-year failure-free survival of 52%, concluding that partial splenic embolization generates long-term durable responses (evidence level III).209 The most common AEs included grade 1 nausea, fever, and abdominal pain.209 This single-center experience needs confirmation.
Treatment options for adults failing multiple therapies
Recommendations for adults failing multiple therapies
For patients failing multiple prior therapies, it is important to:
Reconsider the diagnosis
Perform bone marrow examination if not already done
Reassess the need for treatment (consider platelet count/bleeding risk)
Consider referral to an ITP treatment center
Reassess the adequacy of prior therapies (eg, was the full dose of TPO-RA explored? Did the addition of a small dose of corticosteroid improve response?)
Assess the risks and benefits of further treatment
Reassess the possibility of splenectomy if not already performed
Consider other medical therapies if not already attempted (eg, MMF, fostamatinib, rituximab, azathioprine, dapsone, danazol)
Consider enrollment in a clinical trial
In patients who relapse >1 year after responding to splenectomy, a search for accessory spleen should be conducted and, if found, resected (Grade C recommendation).
Switching from 1 TPO-RA to another and sequential therapy have been shown to have a positive effect on response and AEs.
Other therapies that have been used as last resorts include combination chemotherapy, alemtuzumab, and hematopoietic stem cell transplantation (HSCT). The side effects of these treatment options may be severe, and the data supporting their use are limited (Grade B recommendation; evidence level III).
Approximately 20% of patients do not attain a hemostatic platelet count after splenectomy37,192-197 or fail to respond to initial or subsequent medical approaches; an additional 20% to 30% of splenectomy responders eventually relapse (evidence level IIb).37,195 However some of these patients may nevertheless maintain a hemostatic platelet count without necessitating further treatment. The term “refractory” was proposed by an IWG for patients remaining at risk for significant bleeding after failing previous treatments, including splenectomy,48 but the authors eschew the use of this term because many patients will eventually attain a hemostatically effective platelet count without splenectomy. Some patients with ITP have disease that is less responsive to multiple forms of therapy or have rejected splenectomy. These patients may be able to tolerate severe thrombocytopenia (ie, platelet counts as low as 10 × 109/L) relatively well with near-normal HRQoL or choose to live with low platelet counts instead of undergoing treatments that may be toxic (supplemental Table 4). However, some patients have consistent and substantial deficits on HRQoL, bleeding, and increased risk for death. In this situation, the risk of further therapy must be discussed with the patient and compared with the benefit of that therapy. In addition, other potential etiologies for thrombocytopenia should be exhaustively explored. If not recently performed, a bone marrow examination should be done.
Therefore, this section will focus on those who have failed multiple prior therapies (at least TPO-RA and rituximab) and usually splenectomy, as well as those who have failed multiple prior therapies (TPO-RA and rituximab) but who are ineligible for or refuse splenectomy. For those failing multiple therapies and who still require treatment, to date, no treatment algorithm has been evaluated in RCTs. Some patients failing to respond to 1 TPO-RA may respond if switched to another TPO-RA (evidence levels IIb, III).138,148,210,211 If not already done, splenectomy can also be reconsidered, as can searching for an accessory spleen.212 In patients failing 1 medical therapy, changing to another medical treatment may be successful. Rare patients may benefit from HSCT.
Switching TPO-RAs.
New data have shown that switching from 1 TPO-RA to another, as well as sequential therapy, has a positive effect on response and on tolerability (evidence levels IIb, III).138,148,210 Switching was shown to be effective in 50% to 80% of patients, resulting in eradication of platelet fluctuation in 54% of patients and resolution of AEs in all (evidence level IIb).138 Another study showed that switching from romiplostim to eltrombopag led to 100% response rates, but switching from eltrombopag to romiplostim led to 66% response rates, when patients switched as a result of inefficacy of the first treatment. When switching was due to side effects or for preference, 100% of patients responded (evidence level III).210 One study on sequential treatment revealed that sequential romiplostim and eltrombopag led to responses of 80%, and median time to relapse was 5.5 months.211
The panel rejected using both TPO-RAs simultaneously because of excessive costs.
Accessory splenectomy.
In patients who relapse following an initial response to splenectomy, radiographic assessment for accessory spleen should be considered.213 One study of 14 patients with ITP resistant to treatment found a response rate to accessory splenectomy of 50% at 4 weeks in adult and pediatric patients. A positive outcome was correlated with being female, having a favorable response to prednisone at first exposure, and absence of Howell-Jolly bodies prior to intervention. Although the study suggests that laparoscopic accessory splenectomy is a treatment option for patients with ITP that is resistant to splenectomy, the added risk of morbidity and the difficulty of finding an accessory spleen in a previous surgical field must be weighed against the likely effectiveness (50%-60%) of the procedure (evidence level IIb).212
Combination therapy.
Two studies assessed combination therapy. Azathioprine + MMF + cyclosporine was well tolerated and resulted in response rates of 73.7% maintained for a median of 24 months in patients failing multiple prior treatments (evidence level III).214 Three cycles of rituximab + cyclophosphamide, vincristine, and prednisone given every 3 weeks was not well tolerated as an intensified rituximab regimen when given to patients relapsing after a standard rituximab dose or to nonresponders (evidence level Ib), and it showed no benefit over standard-dose rituximab (evidence level Ib).190
Alemtuzumab.
As a single agent, alemtuzumab has rarely shown effect in ITP.215 However, low-dose rituximab (100 mg weekly for 4 weeks) in combination with alemtuzumab (10 mg subcutaneously on days 1-3) has produced responses in all 11 ITP patients that lasted >80 weeks in 60% (evidence level III).216 However, the panel cannot recommend this approach because of the perceived side effects of the dual immunosuppression. Overall, however, lower-dose rituximab may be associated with shorter responses and earlier relapses. For details on alemtuzumab alone see supplemental Table 4.
HSCT.
HSCT is warranted only in patients with severe chronic nonresponsive ITP with bleeding complications unresponsive to other modalities; it should be used only in very limited cases.For details see supplemental Table 4; no new data were found.
Clinical trials.
There are a number of promising new therapies for ITP, including Bruton’s tyrosine kinase inhibitors, neonatal Fc receptor inhibitors, proteasome inhibitors, and complement inhibitors.
Supportive care
Antifibrinolytics.
See “Emergency treatment.”
Inhibition of menstrual bleeding.
To decrease menstrual bleeding frequency and amount, progesterone-containing intrauterine devices and oral contraceptives can be used (evidence level IIb);217 no new data were found.
ITP during pregnancy
Presentation of thrombocytopenia during pregnancy
ITP is estimated to occur in 0.83 of 10 000 pregnant women218 and has a variable course, usually worsening as pregnancy progresses.219 Diagnosis is similar to the nonpregnant patient but the differential diagnosis includes pregnancy-specific conditions, such as gestational thrombocytopenia and pregnancy-induced hypertensive disorders. Both of these develop in late gestation, with ITP being the most common cause of thrombocytopenia early in pregnancy.
Recommendations for investigation of suspected ITP in pregnancy
Patients with a history suggestive of ITP or those with a platelet count <80 × 109/L should be investigated for possible ITP (Grade C recommendation).
As in nonpregnant patients, the diagnosis of ITP is one of exclusion using the patient’s history, physical examination, blood counts, and blood smear examination (Grade C recommendation).
Laboratory evaluation is similar to the nonpregnant patient, but special consideration should be given to rule out hypertensive, microangiopathic, coagulopathic, and hepatic disorders associated with pregnancy. Recommended tests should be based on the clinical features and may include review of the blood smear, reticulocyte count, coagulation screen, liver function, thyroid function, ANAs, and APLAs (Grade C recommendation).
Bone marrow examination is not recommended unless there are atypical features (Grade C recommendation).
Anti-platelet antibody testing does not predict the course of maternal or neonatal thrombocytopenia or distinguish ITP from gestational thrombocytopenia and is not recommended (Grade C recommendation).
Testing of TPO levels is not recommended (Grade C recommendation).
Special considerations in the differential diagnosis of thrombocytopenia in pregnancy.
The mean platelet count in pregnant women is lower than in nonpregnant women.220 A decrease of ∼10% in the second half of pregnancy is caused by a combination of hemodilution, increased platelet activation and clearance, and platelet sequestration in the placenta.220,221 Approximately 10% of women with otherwise uncomplicated pregnancies have gestational thrombocytopenia with a platelet count <150 × 109/L at the time of delivery.220 Platelet counts <100 × 109/L are much less common (1.0%) and counts <80 × 109/L are rare (0.1%) in patients without other etiologies.220
As in nonpregnant adults, ITP is a disorder of exclusion. Examination of the peripheral blood smear is required to rule out the presence of red blood cell fragmentation seen with microangiopathic hemolysis and preeclampsia. Coagulation and liver abnormalities may suggest an alternative diagnosis, such as hemolysis, elevated liver enzymes, and low platelet count syndrome, infection, or DIC. Additional laboratory testing will be guided by a patient history of thrombocytopenia, bleeding, thrombosis, or pregnancy loss or other laboratory or clinical findings. Unlike ITP in nonpregnant patients, TPO levels may be elevated,222 but testing is not routinely recommended. Bone marrow examination is very rarely required for the diagnosis, and testing for platelet autoantibodies is not helpful and may be misleading. See Table 7 for testing recommendations.
Testing recommendations in suspected thrombocytopenia in pregnancy
| Tests . | Notes . |
|---|---|
| Recommended for all patients | |
| CBC and peripheral blood smear review | Macrothrombocytopenia |
| Check for other inherited thrombocytopenias | |
| Depending on family history and smear, consider genetic testing, platelet function testing, testing for type 2b von Willebrand disease and platelet-type von Willebrand disease | |
| Schistocytes may suggest microangiopathy in hemolysis and hypertensive disorders | |
| Reticulocyte count | Elevated in cases of hemolysis and hypertensive disorders |
| Coagulation screening | PTT may be prolonged in patients with a history of thrombosis or pregnancy loss. Consider testing for APLAs, anti-cardiolipin antibodies, and lupus anticoagulant. |
| PT/PTT, fibrinogen | |
| Liver function | Possibly viral infection, if abnormal |
| Check for hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). | |
| Thyroid function | |
| Viral serologies (HIV, HCV) | |
| Renal function | HUS/TTP may manifest for the first time in pregnancy. Consider testing for ADAMTS-13, alternative complement pathway. |
| Consider atypical HUS due to autoantibodies against complement components | |
| To be considered | |
| Anti-nuclear antibody | |
| H pylori testing | H pylori stool or antigen test should be performed in patients with history of thrombocytopenia prior to pregnancy |
| Ig levels | Quantitative Ig test should be performed in patients with a history of thrombosis or pregnancy loss |
| Not recommended | |
| Platelet autoantibody testing | Not predictive of neonatal platelet count |
| Bone marrow examination, peripheral blood flow cytometry | |
| Fetal blood sampling | |
| TPO level | May be of value in the future |
| Tests . | Notes . |
|---|---|
| Recommended for all patients | |
| CBC and peripheral blood smear review | Macrothrombocytopenia |
| Check for other inherited thrombocytopenias | |
| Depending on family history and smear, consider genetic testing, platelet function testing, testing for type 2b von Willebrand disease and platelet-type von Willebrand disease | |
| Schistocytes may suggest microangiopathy in hemolysis and hypertensive disorders | |
| Reticulocyte count | Elevated in cases of hemolysis and hypertensive disorders |
| Coagulation screening | PTT may be prolonged in patients with a history of thrombosis or pregnancy loss. Consider testing for APLAs, anti-cardiolipin antibodies, and lupus anticoagulant. |
| PT/PTT, fibrinogen | |
| Liver function | Possibly viral infection, if abnormal |
| Check for hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). | |
| Thyroid function | |
| Viral serologies (HIV, HCV) | |
| Renal function | HUS/TTP may manifest for the first time in pregnancy. Consider testing for ADAMTS-13, alternative complement pathway. |
| Consider atypical HUS due to autoantibodies against complement components | |
| To be considered | |
| Anti-nuclear antibody | |
| H pylori testing | H pylori stool or antigen test should be performed in patients with history of thrombocytopenia prior to pregnancy |
| Ig levels | Quantitative Ig test should be performed in patients with a history of thrombosis or pregnancy loss |
| Not recommended | |
| Platelet autoantibody testing | Not predictive of neonatal platelet count |
| Bone marrow examination, peripheral blood flow cytometry | |
| Fetal blood sampling | |
| TPO level | May be of value in the future |
HUS, hemolytic-uremic syndrome; PT, prothrombin time; TTP, thrombotic thrombocytopenic purpura.
ITP developing late in gestation may be difficult to distinguish from other causes of thrombocytopenia, but the presenting platelet count is often lower than for gestational thrombocytopenia or hypertensive disorders (P = .000 and P < .05, respectively) (evidence level III).223 The latter occur during late gestation and are not associated with neonatal thrombocytopenia.224
Recommendations for the treatment of maternal ITP
Counseling for women with ITP wishing to become pregnant is recommended (Grade C recommendation).
A platelet count between 20 and 30 × 109/L in a nonbleeding patient is safe for most of pregnancy. A platelet count ≥50 × 109/L (see separate anesthesia recommendation below) is preferred for delivery (Grade C recommendation).
Initial treatment is with oral steroids or IVIg (Grade C recommendation).
IV anti-D in Rh(D)-positive nonsplenectomized women appears to be well tolerated and effective based on results from a small pilot study225 (Grade B recommendation, evidence level IIb); however, this may potentially cause maternal or fetal hemolysis.
IVIg can provide a rapid, but often very transient, increase in platelet count and can be used to urgently increase platelet counts during bleeding or for delivery (Grade B recommendation).
Combining therapies (prednisone with IVIg and/or IV anti-D) may elicit a response in patients refractory to single agents alone (Grade C recommendation). High-dose methylprednisolone, in combination with IVIg and/or azathioprine, is suggested for patients refractory to oral corticosteroids or IVIg alone (Grade C recommendation).
Rituximab can be considered in pregnancy for very severe cases, but perinatal and neonatal immunosuppression and subsequent infection are potential complications and require monitoring (Grade C recommendation).
TPO-RAs may be considered in late pregnancy when other treatments have failed, but published information is limited (Grade C recommendation).
In the rare instances when splenectomy is required, it should be performed in the second trimester (evidence level III; Grade C recommendation).
Vinca alkaloids, danazol, and immunosuppressive drugs not listed in these recommendations should be avoided in pregnancy (Grade C recommendation).
Recommendations for the management of ITP in pregnancy are primarily based on clinical experience and expert consensus, because there are still few RCTs.
A stable platelet count of 20 to 30 × 109/L is safe during most of pregnancy and, unless the patient has symptoms of bleeding or a procedure is planned, patients can often be managed by observation only.219,223,226 Late in the third trimester, a platelet count >50 × 109/L is required in preparation for delivery, which may be achieved most easily by scheduling delivery. The thrombocytopenia may persist postpartum (evidence level III)224 and, in particular, women with previous splenectomy should be closely monitored; in 1 study on pregnant women with preexisting ITP, 54% of cases in which ITP worsened after pregnancy occurred in splenectomized women (P < .001) (evidence level III).219 Anecdotal data suggest that breast feeding may perpetuate maternal postpartum thrombocytopenia.
Known toxicity and limited experience in pregnancy with many of the medications commonly used in ITP limit treatment options. In addition, the mother’s platelet count does not predict fetal platelet count, and therapy of the mother is not known to affect neonatal outcome.223,226,227
Initial treatment
Oral corticosteroids.
Low-dose corticosteroids are first-line therapy, although 1 retrospective study of 98 patients suggests that they may be less effective in pregnancy, with <40% of patients achieving an increase in platelet count with these treatments.226
Prednisone, 20 mg/d, is recommended initially and then adjusted to the minimum dose necessary, because corticosteroids can cause hypertension, hyperglycemia, osteoporosis, weight gain, and psychosis. After delivery, the platelet count should be monitored, and corticosteroids should be tapered slowly to avoid a rapid decrease in platelet count and to ensure that the mother’s mental state is not affected (evidence level IV).
IVIg.
IVIg may increase the platelet count faster than corticosteroids (2 ± 1 days vs 16 ± 19 days [mean ± standard deviation], respectively) (evidence level IIb).226 Infusions are generally well tolerated, although they present a significant volume and protein load. Retrospective studies have shown comparable efficacy of corticosteroids and IVIg (P = .465) (evidence level III),223 as well as similar neonatal outcomes (evidence level IIb).226 Patients may occasionally require IVIg to maintain safe platelet counts throughout pregnancy or especially in preparation for delivery when a rapid platelet increase is required (evidence level IV).
anti-D.
If available, IV anti-D in Rh(D)-positive nonsplenectomized women appears to be well tolerated (by mother and fetus) and is effective in the second and third trimesters based on results from a small pilot study (evidence level III).225 Monitoring is required for neonatal jaundice, anemia, and DAT positivity after delivery. Anti-D is not available in Europe.
Management of pregnant patients with ITP failing initial treatment
Combining initial therapy.
Patients refractory to initial single therapy or whose platelet counts fall despite continued therapy may respond to combining initial therapy agents or adding other agents. For patients refractory to oral corticosteroids, high-dose methylpredniso(lo)ne in combination with IVIg or azathioprine is suggested. Cyclosporine A and azathioprine have been safely used in pregnant patients with orthotopic organ transplants.86,228 They may be effective in patients with ITP refractory to other therapies, but it could take weeks to increase the platelet count. There is increasing experience with rituximab before and during pregnancy; potential complications include perinatal and neonatal immunosuppression and subsequent infection.205 Other immunosuppressive agents, including vinca alkaloids and MMF, have been associated with teratogenicity and should not be used.229,230
TPO-RAs and rhTPO.
TPO-RAs may be considered in exceptional circumstances, ideally only in the third trimester near delivery, although tolerability and toxic effects on the fetus have not been rigorously evaluated. An unpublished safety database (Amgen, Thousand Oaks, CA) exists for >25 women treated with romiplostim during pregnancy without untoward effects. All available published data are from 11 available case reports, and all show a platelet response.119-121,141,231-237 Romiplostim and eltrombopag may cross the placenta and could cause potential harm to the fetus.119,120,227 In the 11 cases reviewed here, there were no safety concerns.119-121,141,231-237 TPO-RAs are not recommended except in very serious cases; if used, romiplostim may be preferred given its fewer off-target effects (lack of hepatic toxicity and iron chelation) and limited safety database.
rhTPO is likely to cross the placenta and was effective in increasing the platelet count in 23 of 31 pregnant women who were unresponsive to steroids without untoward effects on the fetus.227 rhTPO is only available in China.
Splenectomy.
Splenectomy is required in very rare cases. It is best performed in the second trimester, by laparoscopy if possible and with vaccination according to national guidelines.
Venous thromboembolism.
In addition to the known prothrombotic state of pregnancy, some women may have risk factors (eg, anti-cardiolipin antibody syndrome) requiring venous thromboembolism prophylaxis (evidence level IV). A platelet count >50 × 109/L is generally recommended for such patients receiving anticoagulation (evidence level IV).
Preparation for delivery: obstetric analgesia and anesthesia
Recommendations for obstetric analgesia and anesthesia
At a platelet count ≥70 × 109/L, in the absence of other hemostatic abnormalities, regional axial anesthesia can be safely performed (Grade C recommendation).
NSAIDs should be avoided for postpartum or postoperative analgesia in women with platelet counts <70 × 109/L because of increased hemorrhagic risk (Grade C recommendation).
A platelet count ≥50 × 109/L should be obtained for delivery (Grade C recommendation).
All women, despite having ITP, who are at an increased risk for thromboembolism should receive appropriate prophylaxis for venous thromboembolism (Grade C recommendation).
The mother with a rapidly falling platelet count should be observed more closely than those with low, but stable, levels (Grade B recommendation).
The minimum platelet count required for delivery has not been defined, although a count ≥50 × 109/L is generally recommended. Discussion about regional anesthesia should include the obstetric anesthetist and take place before the expected delivery date so a plan is in place for increasing the platelet count if needed. A systematic review of 1524 thrombocytopenic parturients estimated the risk of epidural hematoma. More than 500 women with platelet counts of 70 to 100 × 109/L were included, almost 300 of whom underwent epidural blockade. Although no hematomas occurred, the estimated potential risk of epidural hematoma was 0.2%.238 Limited observations with a count <70 × 109/L are available, and the risk is poorly defined, but no cases requiring surgical decompression were observed. Risk of vascular damage likely decreases proportionately to needle size; consequently, spinal injection may be a safer option than epidural blockade in patients with increased bleeding risk or platelet counts <70 × 109/L. Evaluation before neuraxial anesthesia should include whether there is a history of bleeding, presence of bruising, or abnormal coagulation testing (including prothrombin time, activated PTT, and fibrinogen levels) that may increase the risk of bleeding. The role of thromboelastography or rotational thromboelastometry in assessing global hemostasis is unclear, and their use cannot be recommended. Although a platelet count ≥70 × 109/L is recommended for neuraxial anesthesia, this must be weighed against the risk of general anesthesia for the patient. The trend of the platelet count is important, as well as the absolute value. A mother with a falling count should be observed more closely than one with low, but stable, counts.
Delivery: management of mother and infant
Recommendations for management of delivery and newborn infants
Cordocentesis and fetal scalp blood sampling should be avoided in the management of the fetus/neonate of a mother with ITP in pregnancy (Grade C recommendation).
Neonatal alloimmune thrombocytopenia should be excluded by parental testing if the neonate presents with severe thrombocytopenia (Grade C recommendation).
The mode of delivery should be determined by obstetric indications, not by anticipation of the neonatal platelet count (Grade B recommendation).
Procedures during labor that may be associated with increased hemorrhagic risk to the fetus should be avoided, specifically the use of fetal scalp electrodes, fetal blood sampling, ventouse delivery, and rotational forceps (Grade C recommendation).
Previous splenectomy has been associated with worsening of maternal ITP in pregnancy (evidence level III)219 and a higher risk for neonatal thrombocytopenia (evidence levels IIb, III219 ; Grade B recommendation).
A mother with a previous newborn, thrombocytopenic or not, is likely to have a second baby with a similar platelet count.
Optimal management of delivery is based on obstetric indications with avoidance of procedures that may induce bleeding. The very low risk of ICH in the fetus/neonate of the mother with ITP has not been shown to be lessened by Caesarean section, in part because it is so infrequent.
Management of neonate
Recommendations for management of neonates born to women with ITP
Umbilical cord platelet count should be obtained at the time of delivery or as soon as possible (Grade C recommendation).
Repeat the platelet count as needed depending on platelet levels, trends in the count, and response to treatment (if any). If cord platelet count is <100 × 109/L, repeat the platelet count daily until stable (Grade C recommendation). The incidence of pseudothrombocytopenia is high in neonates because of the difficulties encountered in obtaining unclotted blood with blood draws (Grade C recommendation).
If platelet count is <50 × 109/L at birth, perform a cranial ultrasound. A magnetic resonance imaging for confirmation or clarification can be performed without anesthesia using the sleep and swaddle approach 30 to 60 minutes prior (Grade C recommendation).
In the case of ICH, give IVIg and limited steroids to maintain platelet count >100 × 109/L for 1 week if possible and >50 × 109/L for another week (Grade C recommendation). The use of platelet transfusion may increase neonatal risk.
If there is symptomatic bleeding or if platelet count is <30 × 109/L, with or without platelet transfusion, give IVIg (Grade C recommendation).
If severe thrombocytopenia continues for >1 week in a breast-fed infant, consider pausing breastfeeding for a few days to see whether platelet count increases (Grade C recommendation).
Women who had a splenectomy may have a thrombocytopenic newborn, even if their platelet count is normal (Grade C recommendation).
The only currently reliable predictor of whether a baby will be thrombocytopenic is if a previous sibling was thrombocytopenic (Grade B recommendation).
A platelet count from a postnatal cord sample is recommended and, if low, it should be repeated at 3 to 5 days of age, when the neonatal spleen has matured. The risk for ICH in newborns with platelet counts <30 × 109/L is estimated at <1%,239 but given the significant implications of such an event, IVIg should be given to increase the platelet count. Experience shows that, when the neonatal platelet count oscillates, this may reflect platelet clumping (pseudothrombocytopenia) as a result of difficulty obtaining blood from newborns. Splenectomized women, and especially mothers with previous thrombocytopenic newborns, are at greatest likelihood of having a thrombocytopenic newborn. Anti-platelet antibody in breast milk may contribute to persistence of neonatal thrombocytopenia.239
Counseling for women with ITP or who develop ITP in pregnancy.
Women of child-bearing capacity with ITP should be counseled that ITP is almost never a contraindication for pregnancy. Ideally, management of women with ITP wishing to become pregnant should be performed jointly by an obstetrician experienced in high-risk pregnancies and a hematologist knowledgeable in ITP. In the authors’ experience, serious bleeding complications antepartum or postpartum are extremely rare (evidence level IV).
ITP in children
Diagnostic approach in children with suspected ITP
The diagnosis of ITP in children is one of exclusion. Children with newly diagnosed ITP, especially with atypical features, should be referred to a hematologist experienced in the assessment and treatment of children with ITP. Children and their parents will benefit from contacts, literature, and Web-based information available, especially from ITP support groups.
Recommendations for initial investigation of suspected childhood ITP
A complete history, physical examination, full blood count, and expert analysis of the peripheral blood smear should be performed and carefully evaluated at initial diagnosis (Grade C recommendation).
A DAT is recommended to exclude coexistent autoimmune hemolytic anemia, especially prior to therapy (Grade C recommendation).
Baseline Ig levels, to exclude coexisting immunodeficiency, is recommended prior to therapy (Grade C recommendation).
When the CBC shows isolated thrombocytopenia and no abnormal features beyond thrombocytopenia on examination of the blood smear and signs of bleeding are present on physical examination, a bone marrow aspiration/biopsy is not required in children, even prior to steroid therapy (Grade B recommendation).
Children with newly diagnosed ITP, especially with atypical features, should be referred to, or discussed with, a hematologist experienced in assessment and treatment of children with ITP (Grade C recommendation).
Bone marrow aspiration, biopsy, and cytogenetics should be performed if abnormal or potentially malignant cells are visualized on smear and carefully considered if there are other abnormalities of the hemoglobin and/or white cell count (with the exception of microcytic anemia) or if there is hepatosplenomegaly and/or adenopathy. In addition, failure to acutely respond to ITP therapy merits a bone marrow examination (Grade C recommendation).
Additional investigations are based on clinical assessment and may include a variety of testing, including molecular genetics, autoantibody screening, liver-spleen imaging, and other laboratory testing (Grade C recommendation).
See Table 3 for diagnostic tools for adults and children with suspected ITP.
Differential diagnosis.
Although presentation of ITP in children is generally acute, less commonly, bruising and purpura may develop slowly over weeks or months, suggesting a chronic evolution. It is important to exclude other common disorders that may resemble ITP (Table 4).
If a decision is made to observe the child with presumed newly diagnosed ITP, even in typical cases, a CBC and blood smear should be repeated periodically to exclude the evolution of a serious bone marrow or other hematologic disorder until the diagnosis is clear or recovery has occurred.
Children with familial inherited thrombocytopenias are often misdiagnosed as having ITP.240,241 Inherited disorders should be suspected if thrombocytopenia has been present since early life, a positive family history for a similar disorder is elicited,242 characteristic features are present, or there is failure to respond to first-line treatment. If available, genetic panels for blood and bone marrow can be used to identify specific genetic alterations associated with the thrombocytopenia and may predict future risk of clonal disease evolution. Mean platelet volume may be used to differentiate ITP from inherited thrombocytopenia (evidence level IIb); increased mean platelet volume can be suspected on smear if there are many large platelets.243
Special diagnostic considerations in children.
Older children and those with slow evolution of disease (evidence level Ib-III)5,244-246 may be more likely to develop chronic disease. Other autoimmune diseases associated with thrombocytopenia, including SLE, CVID, autoimmune lymphoproliferative syndrome, and chronic viral infections, should be considered in difficult, persistent, and chronic cases and in those with multiple autoimmune cytopenias (eg, Evans syndrome).
Bone marrow evaluation.
Bone marrow evaluation in children with newly diagnosed ITP is recommended only when abnormalities are present other than isolated thrombocytopenia in the blood count/smear, if systemic features (eg, bone pain) are apparent, or if the patient has an enlarged spleen not secondary to liver disease. Bone marrow evaluation (aspirate and biopsy) should be carefully considered in cases who respond minimally or not at all to first-line therapies247 to exclude bone marrow failure; a high mean cell volume, even without anemia, could indicate marrow failure. Genetic panels for inherited thrombocytopenia or congenital marrow failure should also be considered at the time of marrow evaluation.
H pylori infection.
The rate of H pylori positivity in children with ITP is reported to be 20% to 29%.248,249 There is conflicting evidence on the effect of H pylori eradication in positive children (evidence levels IIa and Ib).248,249 In certain children with ITP, eradication of H pylori may improve the platelet count; however, this is inconsistent and cannot be relied upon.
Recommendations for subsequent investigation of children with persistent or chronic ITP
Repeat history, physical examination, full blood count, and expert analysis of the peripheral blood smear should be performed to reassess diagnosis (Grade C recommendation).
Bone marrow aspiration, biopsy, and cytogenetics should be performed at 3 to 6 months if there has been no spontaneous platelet increase and no response to treatment (Grade C recommendation). NGS or targeted sequencing should be considered if available.
Bone marrow aspiration, biopsy, and cytogenetics should be performed earlier if there is no response to treatment within the expected timeframe (Grade C recommendation). NGS or targeted sequencing should be considered if available.
A bone marrow biopsy is not indicated prior to further therapy (eg, with TPO), unless the diagnosis is not certain (Grade C recommendation).
Additional evaluation could include testing for (Grade C recommendation):
Lupus and other markers of autoimmune diseases that might require specific treatment (eg, test for APLAs, ANAs, anti-cardiolipin antibody, lupus anticoagulant, and serum Igs)
Chronic infections (hepatitis, cytomegalovirus, HIV, and/or H pylori in at-risk populations or when there is no other explanation)
Complex immunodeficiency diseases
Genetic screening for inherited thrombocytopenia and bone marrow failure syndromes
In the setting of increasingly difficult-to-treat persistent or chronic ITP, consideration of bone marrow examination should be included in reevaluation of the diagnosis (Grade C recommendation).
Examination of patients with persistent or chronic ITP.
For patients diagnosed with ITP who have no improvement in platelet count after 3 to 6 months and still require treatment, several evaluations are recommended (see “Recommendations for subsequent investigation of children with persistent or chronic ITP”).
Management of ITP in childhood: general measures
From registry and cohort data, 0% to 4% of children with newly diagnosed ITP have severe (grade 4) bleeding requiring immediate intervention (evidence level III)245,250 ; bleeding that may require treatment is reported in 30% to 56% of newly diagnosed children (evidence level IIb-III).245,250,251 The incidence of ICH in children with ITP is ∼0% to 1% (evidence level III),245,250,252-256 and predicting with confidence which children will develop an ICH is very imperfect. Risk factors for ICH include low platelet count,252 head trauma,252 signs of other bleeding (especially grade 3 and macroscopic hematuria), and possibly other less well-documented factors, including NSAIDs and arteriovenous malformation. Caution should be exercised in the management of children with ITP and coexisting vasculitis or coagulopathies, as may occur in the setting of other illnesses, especially with regard to inciting thrombosis.
Current consensus favors consideration of multiple factors when deciding to treat or not to treat children with ITP, including bleeding symptoms, the platelet count, recent trauma, existence of headache, recent medication use, and psychosocial and lifestyle issues, such as activity profiles and economic impacts. These considerations need to be discussed extensively with and explained to the patient and their family, whose wishes must be incorporated.
The management of children with persistent/chronic ITP is essentially the same as those with newly diagnosed ITP, with the exception that the longer the ITP lasts, the less likely it is to improve spontaneously and the more important the impact of the ITP on the patient’s and family’s quality of life becomes.
Clinical classification of newly diagnosed ITP in children
Recommendations for “clinical classification” of ITP in children
Clinical classification should be based on disease severity, including degree of bleeding, platelet count, comorbidities and their treatment (especially anticoagulation), and impact of ITP or its therapies on the patient’s and family’s HRQoL (evidence level IIb; Grade B recommendation).
Management should be considered based on the clinical classification (Grade C recommendation).
In children, the clinical features and symptoms of ITP are of greater impact in treatment decisions than just the platelet count. This leads to an effort to use a “clinical classification” of children based on degree of bleeding, platelet count, and other disease features. Bleeding scores exist for patients with ITP and should be routinely used for assessing severity of ITP (Table 8).94 These bleeding scores confirm that most children with ITP do not have serious bleeding problems, despite low platelet counts.252,255,256 Of note, the severity of mucocutaneous bleeding does not consistently predict the risk for life-threatening bleeding (eg, ICH).
Updated bleeding scale for pediatric patients with ITP
| Grade . | Bleeding . | Management approach . |
|---|---|---|
| Grade 1 (minor) | Minor bleeding, few petechiae (≤100 total) and/or ≤5 small bruises (≤3 cm in diameter), no mucosal bleeding | Consent for observation |
| Grade 2 (mild) | Mild bleeding, many petechiae (>100 total) and/or >5 large bruises (>3 cm in diameter), no mucosal bleeding | Consent for observation |
| Grade 3 (moderate) | Moderate bleeding, overt mucosal bleeding, troublesome lifestyle | Intervention to reach grade 1 or 2 |
| Grade 4 (severe) | Severe bleeding, mucosal bleeding leading to decrease in Hb > 2 g/dL or suspected internal hemorrhage | Intervention |
| Grade . | Bleeding . | Management approach . |
|---|---|---|
| Grade 1 (minor) | Minor bleeding, few petechiae (≤100 total) and/or ≤5 small bruises (≤3 cm in diameter), no mucosal bleeding | Consent for observation |
| Grade 2 (mild) | Mild bleeding, many petechiae (>100 total) and/or >5 large bruises (>3 cm in diameter), no mucosal bleeding | Consent for observation |
| Grade 3 (moderate) | Moderate bleeding, overt mucosal bleeding, troublesome lifestyle | Intervention to reach grade 1 or 2 |
| Grade 4 (severe) | Severe bleeding, mucosal bleeding leading to decrease in Hb > 2 g/dL or suspected internal hemorrhage | Intervention |
This bleeding scale is based on the one used in the previous consensus report,1 updated based on the authors’ opinion.
Hb, hemoglobin.
Expectant “watch-and-wait” policy
Recommendations for watch-and-wait policy based on clinical classification
At diagnosis, children and adolescents with ITP and mild or even moderate bleeding on a pediatric bleeding assessment tool (grade 1-3) may be managed expectantly with supportive advice and a 24-hour contact point, irrespective of platelet count (Grade B recommendation); those with grade 3 bleeding are more likely to require therapy because of the higher rates of serious bleeding requiring hospital admission and emergency treatment. All patients need regular reevaluation to monitor for worsening, including HRQoL and evolution to a serious bone marrow disorder or a secondary form of ITP (Grade C recommendation). The frequency of clinical and laboratory monitoring should be based on bleeding, HRQoL, trend in platelet counts, and impression of family reliability.
The same monitoring and 24-hour access are needed with persistent and chronic ITP, depending upon the factors listed above, but at less frequent intervals in a stable patient. Observation or watch and wait is less validated in patients with persistent and chronic ITP because it is based on the expectation of spontaneous future improvement (Grade C recommendation).
Bleeding grades are described in Table 8.
Most children with newly diagnosed ITP do not have significant bleeding symptoms or other risk factors and may be managed without treatment at the discretion of the hematologist and the patient’s family (evidence levels II-III).5,245,250,251,254,255 Recent observational registries report that 19% to 35%, and even up to 84%, of children with newly diagnosed ITP can be managed without ITP therapy and without increased rates of severe bleeding.5,245,250,251,254,255 Reasons to institute therapy are associated with increased bleeding severity and with risk factors for ICH. In a recent study, 7 of 42 children with, as well as 1 of 58 children without, grade 3 bleeding at diagnosis were hospitalized within 1 month because of grade 3/4 bleeding.256 There is a trend for increased serious bleeding with increased number of bleeding sites.254
Children with ITP and their parents need to understand the risks of serious or life-threatening hemorrhage with or without treatment. They should also be aware that drug therapy is often effective but may have side effects and, thus, is usually reserved for children at higher risk for serious hemorrhage. The potential negative, as well as positive, impact of treatment on the HRQoL of children and their parents, despite platelet increase, should also be communicated (see “Quality of life of children with ITP”).
Hospitalization.
For children with an established diagnosis of ITP, hospital admission should be reserved for those who have grade 3/4 bleeding. Problematic psychosocial circumstances of child and family (eg, family history of noncompliance, behavioral issues, residence remote from a health care facility) and the effect of the ITP on the child’s and family’s HRQoL should also be considered. Parents should be advised to watch for other signs of bleeding and be given a contact name and telephone number where a physician can be reached at all times.
Management of ITP in children
Recommendations for when to start initial treatment in children newly diagnosed with ITP
Most children can be managed with watchful waiting as described (see “Expectant watch-and-wait policy”; Grade C recommendation).
Any severe (grade 4) bleeding requires immediate hospital admission and treatment to increase platelet levels until bleeding has decreased (Grade C recommendation).
Any moderate (grade 3) bleeding requires hospital review and consideration for admission and therapy (Grade C recommendation).
Treatment should be administered and hospitalization should be strongly considered in the following cases (Grade C recommendation):
Worsening bleeding or significant comorbidities
Risk of ICH (eg, head trauma or unexplained headaches); patients at higher risk for ICH include those with a history of moderate or severe bleed in the preceding 28 days, recent administration (within 8 hours) of NSAIDs, and another clinically significant coagulopathy (eg, von Willebrand disease). In the case of head trauma, treatment should precede a head computed tomography scan.
A change in behavior or mood consistent with significant depression or irritability
Parents are anxious about bleeding and do not believe that they can control (young child) or restrict (older child) their child’s activity.
Parents cannot be relied upon to bring the child back readily if there is an emergency (eg, they live too far away, they cannot afford to return, there are additional social concerns).
Child has not spontaneously improved and must be overly restricted in activities.
Child needs to take an anticoagulant or antiplatelet agent.
Higher risk of bleeding due to another medical or psychological issue
Bleeding grades are described in Table 8.
Most children newly diagnosed with ITP do not require any therapy because the platelet count will often reach safe levels within a few days (see “Expectant watch-and-wait policy”). However, children and adolescents with moderate (grade 3) bleeding are at higher risk for subsequent grade 4 bleeding256 and should be considered for early intervention until bleeding has decreased. Children with grade 4 bleeding should be hospitalized and treated immediately (Table 8).
Recommendations for initial treatment of children with ITP when required
If there is moderate or severe bleeding, IVIg and anti-D can often increase the platelet count to hemostatic levels (>50 × 109/L) within 24 to 48 hours. IVIg is effective when given as a single dose of 0.8 to 1.0 g/kg (Grade A recommendation, evidence level Ib). Anti-D has similar efficacy to IVIg when given as a single dose of 75 µg/kg and is rarely associated with severe hemolysis (Grade A recommendation, evidence level Ib). High-dose steroid premedication is recommended for IV anti-D and is useful for IVIg.
A second dose of IVIg or anti-D may be administered if there is a suboptimal initial response and/or ongoing bleeding (Grade C recommendation).
Predniso(lo)ne should be given at 4 mg/kg per day in 3 or 4 divided doses for 4 days with no taper, with a maximum daily dose of 200 mg or at 1 to 2 mg/kg, with an 80-mg maximum daily dose, even in patients weighing >80 kg, for 1 to 2 weeks (Grade C recommendation).
If a response is seen (eg, platelets > 50 × 109/L), the predniso(lo)ne should be tapered, aiming to stop it by 3 weeks, even if the platelet count drops during the taper (Grade C recommendation).
If there is no response to the initial dose within 2 weeks, the predniso(lo)ne should be tapered rapidly over 1 week and stopped (Grade C recommendation).
In general, corticosteroids are used for grade 1 or 2 bleeding (Table 8) or for patients not responsive to IVIg (Grade C recommendation).
Corticosteroids are relatively contraindicated in the presence of severe infection or recent varicella contact (Grade C recommendation).
IV anti-D can be used if the patient is Rh positive, not splenectomized, does not have a positive direct Coombs test (DAT), and has hemoglobin ≥ 9 g/dL.
Initial treatment options to increase platelet counts in children.
Treatment of newly diagnosed ITP in children consists of steroids, IVIg, and, least commonly, IV anti-D (supplemental Table 5). It is recommended that treatment, especially steroids, should be weaned or stopped once bleeding has ceased, a safe platelet count is reached, or when it is clear that treatment has failed. All initial treatments, if effective, can be repeated as needed, although too frequent anti-D can result in anemia.
Corticosteroids.
Steroids suitable for initial treatment of children with ITP include IV high-dose methylpredniso(lo)ne (30 mg/kg IV up to 1 g), high-dose dexamethasone (28 mg/m2), and prednisone at varying doses (0.5-4 mg/kg per day) with varying rates of taper, including abrupt discontinuation. Because of the serious side effects associated with any prolonged corticosteroid treatment in children with ITP, corticosteroids should be used only to maintain a hemostatic platelet count and for as short a time as possible.
Since the previous consensus report, 3 studies have reported on corticosteroids in children with ITP. As described above, 1 study reported no significant differences among IV anti-D, IVIg, or methylprednisolone 2 mg/kg per day in treatment-naive children with ITP (evidence level Ib).244
A retrospective comparison of methylprednisolone and dexamethasone as initial treatment in children with ITP reported similarly high (96.5%) response rates and minor AEs with both regimens (evidence level IIb).257
The third study reported on the use of high-dose dexamethasone in patients, including children, with acute or chronic ITP. Results were not split by age, but the treatment led to a 69% response rate; 7 of 8 responding patients with chronic ITP later relapsed (evidence level III).67 However, treatment was reported as largely well tolerated.
IVIg.
The most common treatment schedule for IVIg in children with newly diagnosed ITP is 0.8 to 1 g/kg in a single dose,79,84,256,258-260 but some studies continue to use 0.4 g/kg per day over 5 days.244,261 It is often combined with steroids, antihistamines, and paracetamol to reduce side effects, especially headaches. Treatment is usually discontinued once a safe platelet count is reached, although it can be repeated as/if needed. In severe bleeding, a second dose of IVIg (0.8 to 1 g/kg) is often given if there has been no initial response to the initial dose.
Increased platelet count with IVIg has been reported in >80% of children with ITP (evidence level Ib-IIb).79,256,260 Common AEs include headache, nausea/vomiting, and fever/chills.79,256,259-261
As described above, 4 studies have compared IVIg with anti-D in children newly diagnosed with ITP; 3 studies reported no significant difference, and 1 study reported improved efficacy with IVIg (evidence level Ib-IIb).244,258,259,261
The phase 3 TIKI trial compared single-dose IVIg (0.8 g/kg) with observation in children with newly diagnosed ITP, platelet count ≤20 × 109/L, and grade 1-3 bleeding (evidence level Ib).256 Overall and complete response rates were significantly higher with IVIg vs observation at 1 week and 1 month, at 3 months only the complete response rate was significantly higher, and at 6 and 12 months there was no significant difference in either measure. The rate of chronic ITP development did not differ significantly between the 2 groups (∼10% in each group).
IV anti-D.
IV anti-D can be given to Rh(D)-positive children as a short infusion, and it is usually effective in transiently increasing platelet counts.90,244,258,259,261,262
Four studies since 2010 have evaluated the treatment efficacy of anti-D in newly diagnosed children with ITP and compared with IVIg, methylpredniso(lo)ne, or both.244,258,259,261 Three studies reported no significant difference between single-dose anti-D (50 or 75 µg/kg) and IVIg (single-dose 0.8-1 g/kg or 5 × 0.4 g/kg per day) in children newly diagnosed with ITP (evidence level Ib-IIb); 1 study also reported no difference compared with methylpredniso(lo)ne (2 mg/kg per day) (evidence level Ib).244,258,259 One study reported higher efficacy with IVIg vs anti-D (50 µg/kg) in newly diagnosed treatment-naive acute ITP patients, although a higher dose of IVIg was used (2 g/kg total dose) (evidence level Ib).261 Subcutaneous anti-D has also led to platelet count doubling in 74% of children with acute or chronic ITP,262 but it may induce a slower platelet increase than the IV formulation.
The most common adverse drug reactions observed in trials of children with ITP were headache, fever, chills, and infection.90 Anti-D is not available in Europe.
Emergency treatment in children
Recommendations for emergency treatment in children at any stage of their ITP
Combination therapy, including platelet transfusions, IV corticosteroids, and IVIg, with or without anti-D, is recommended. Platelet transfusions should be administered as a bolus, followed by continuous infusion in combination with IV high-dose steroids (eg, IV methylpredniso(lo)ne, 30 mg/kg per day). IVIg (0.8-1.0 g/kg per day, with or without single-dose IV anti-D (75 µg/kg), should be given for ICH or other life-threatening or serious bleeding. A second dose of IVIg and IV steroids may be required if a platelet response is not seen within 24 hours of the initial dose (Grade C recommendation).
IVIg, steroids, and IV anti-D (if available) can be used to attempt to ensure the most likely and fastest platelet increase. Antifibrinolytics may be given if bleeding continues despite therapy (Grade C recommendation).
If there is an ICH, emergency splenectomy and/or neurosurgical control of bleeding should be considered in conjunction with emergency platelet-raising therapy, but medical treatment should never be delayed because of surgical or radiologic intervention if at all possible (Grade C recommendation).
TPO-RAs should be considered; they may aid the acute response in patients and prevent a decrease in platelet count if initial response to emergency therapy is lost (Grade C recommendation).
In organ- or life-threatening bleeding, a two- to threefold higher dose of platelets should be infused, together with IV high-dose corticosteroids, IVIg, and/or IV anti-D, and possibly vincristine. The goal is to increase the platelet count as immediately as possible to minimize or eliminate severe bleeding and continue with platelet count maintenance until bleeding has stopped and the risk of rebleeding has resolved. The risk of bleeding following ICH may necessitate long-term treatment. There is no role for emergency splenectomy unless patients have failed multiple other treatments. Studies have only supported the use of recombinant factor VIIa in patients with massive bleeding after trauma.263
Treatment options for children with persistent or chronic ITP
Recommendations for treatment of persistent or chronic ITP in children
Most children can be managed with watchful waiting, as described (see “Expectant watch-and-wait policy”; Grade C recommendation).
Rescue therapy with corticosteroids, IVIg, and/or IV anti-D can be used in children on watchful monitoring to treat acute bleeding episodes if/when they occur (Grade C recommendation).
Children who are having frequent or severe bleeding episodes or impaired HRQoL (including reduction in important activities) require referral to a hematologist experienced in treating pediatric ITP for further treatment (Grade C recommendation).
Multiple pediatric studies support the use of TPO-RAs in children with persistent/chronic ITP, demonstrating good response and reduction in bleeding frequency with an absence of side effects in the majority of patients (evidence level Ib; Grade A recommendation).
If there is no response to 1 TPO-RA or there is a response that is lost, switch to an alternative TPO-RA and/or consider combining with MMF or another immunosuppressant (Grade C recommendation).
In those who fail TPO-RAs, especially adolescent females, rituximab and dexamethasone should be considered (evidence level III; Grade C recommendation).
Studies have indicated that older age,5,245,246 higher platelet counts,5,245,246 and absence of preceding infection5,246 are risk factors for chronic ITP (evidence levels IIb, III). Less established risk factors for chronicity include female sex245 and decrease in Ig (evidence level III).246 A recent registry analysis found no association between platelet count at diagnosis and ITP remission at 12 or 24 months.251 Spontaneous remission may occur at any time, even >3 years from diagnosis.264
Many children stabilize with an adequate platelet count (>20-30 × 109/L), have no symptoms unless injured, and do not benefit from restrictions in their activity.264 There may be ongoing vulnerability to excessive bleeding following trauma; it is advisable for the family to carry a card or letter or for the child to wear a bracelet with details of the disorder and contact information in case of emergency.
The role of puberty in this process remains uncertain. The onset of menstruation may be problematic and can be managed with antifibrinolytic agents and/or hormonal medication (see “ITP in adults” > “Emergency treatment”). Adolescents, in particular, may become very conscious of their appearance, in addition to other HRQoL issues, and need sympathetic support.
Treatment of children with persistent/chronic ITP consists primarily of TPO-RAs, rituximab, and MMF (supplemental Table 6).
Dexamethasone, high-dose methylpredniso(lo)ne, IVIg/anti-D.
There are few new data on these treatments in this population. For further details, see “Initial treatment options to increase platelet counts in children.”
TPO-RAs.
Eltrombopag and romiplostim have been studied extensively in children, leading to FDA and European Medicines Agency licenses in children ≥1 year old with ITP for >6 months’ duration with insufficient response to corticosteroids, Igs, or splenectomy.120,121 In general, TPO-RAs should be used as preferred treatment in patients with ITP in whom alleviating the thrombocytopenia is likely to provide a clear clinical benefit, including reducing the risk of bleeding and/or improving the HRQoL.120,121 The lowest dose required to achieve and maintain a platelet count ≥ 50 × 109/L should be used for eltrombopag and romiplostim.120,121
If there is no response to 1 TPO-RA or there is a response that is lost and a TPO-RA is the best option for treatment, switch to the alternative TPO-RA and/or consider combining it with MMF or another immunosuppressant (evidence level IV).
If a TPO-RA is initiated and effective, optimal duration of treatment is unknown. Consensus recommendation is to consider tapering the dose after 6 to 12 months of a stable response with a platelet count > 50 × 109/L to determine whether it can be discontinued. Tapering can be tried again if it fails initially. Switching to another treatment, such as rituximab, immunosuppressants, or splenectomy, requires extensive discussion with the patient and/or family.
A retrospective study of TPO-RAs in children with ITP (primary/secondary and newly diagnosed/chronic) reported a stable response rate of 40% and no significant difference in response pattern between romiplostim and eltrombopag (evidence level IIb).265 Thrombocytosis was reported in more patients treated with eltrombopag than romiplostim (7 vs 2). TPO-RA treatment was discontinued in 54% of patients, with treatment being weaned in 32% and abruptly discontinued in 66%.265
Eltrombopag.
The dosing of eltrombopag depends upon the age of the patient (lower dose for age 1-5 years), presence of hepatic impairment (reduced dose with impairment), or East Asian ancestry (reduced dose).121,141 The dose should be adjusted according to platelet count, up to a maximum of 75 mg/d. See the prescribing information for full details.121,141
The PETIT and PETIT2 studies of eltrombopag in children with chronic ITP reported a platelet count ≥50 × 109/L at least once in ∼80% of patients (evidence level Ib)266,267 ; in PETIT2, 40% of patients had a platelet count ≥50 × 109/L for ≥6 weeks without rescue.267 No World Health Organization grade 3 or 4 bleeding in patients on eltrombopag was reported in either trial.266,267 In the PETIT trial, 30% of children received the maximum dose of eltrombopag (75 mg/d); 89% of patients required ≥1 dose modification.266 Median time to response was 12 to 20 days, depending on the age of the patients.266
There are few data on the administration of eltrombopag for >1 year in children.
Romiplostim.
In children, the recommended initial dose is 1 µg/kg per week, administered subcutaneously; the dose can be adjusted depending on platelet count (see prescribing information for full details).119,120 A 24-week phase 3 study reported a median average dose of 3.9 µg/kg (interquartile range, 2.4-7.3) and a median most frequent dose during the final 8 weeks of 5.5 µg/kg (interquartile range, 3-10).268 In the absence of thrombotic risk, the authors use an initial dose of 3 µg/kg per week.
Studies of romiplostim in children with ITP (newly diagnosed and chronic) have reported overall response rates of 71% to 88%,268-270 and a median time to response of 4 to 5 weeks (evidence levels Ib-III).268,270 A study of long-term romiplostim treatment in children found AEs and platelet responses similar to those seen in adult patients and maintenance of effect over 4 years.271 However, another study with 24 weeks of romiplostim treatment reported no sustained response (platelet count ≥50 × 109/L for ≥6 months with no ITP medication) following romiplostim discontinuation (evidence level Ib).268
Rituximab.
Rituximab is not approved for the treatment of ITP.175,176 However, studies report that it may induce responses in children with chronic ITP. Overall response rates to rituximab in this population are reported at 23% to 69% (evidence levels IIb-III).272-274 Relapse rates vary from 14% to 42% of responding patients, within ∼1 year (evidence levels IIb-III).161,273-275 In 1 study, 66 children with ITP were treated with rituximab; 57% had a response, but by 5 years only 26% maintained that response.161 These results are identical to those in adults, with the exception that 2 years after treatment, no children, but some adults, relapsed.
One study combined rituximab with dexamethasone and demonstrated an initial response rate of 45% and a relapse rate of 40% after 60 months (evidence level III).276 In adolescent female patients with ITP duration < 24 months, 47% maintained long-term remission without further therapy.276
The studies of rituximab in children with ITP report that the treatment is generally well tolerated, with low levels of urticarial rash, headache, chills, fever, and abnormal Ig levels. No cases of long-term toxicity due to B-cell depletion have been reported.161,273,274
Two studies have indicated that response to rituximab is correlated with steroid response (P = .002) (evidence level IIb)272 or response to previous therapy in general (P = .04) (evidence level IIb).275 One study found that patients responding to rituximab were older than nonresponders (P = .027) (evidence level III),273 whereas another found no significant difference in age between responders and nonresponders (P = .2) (evidence level IIb).275 Sex, number of infusions, number of previous treatments, splenectomy, and platelet count did not influence the probability of rituximab response (evidence level IIb, III).273,275
MMF.
In 30 children with primary ITP (newly diagnosed, persistent, or chronic), treatment with MMF achieved a response rate of 56%, and 29% of patients relapsed (evidence level IIb).277 Limited toxicity (asthenia, vomiting, and skin effects in 1 patient each) was observed. Doses were not specified.
Fostamatinib.
Fostamatinib is not licensed for the treatment of children with ITP and there are no data on its use in children.180 For more information, see “ITP in adults” > “Subsequent therapy: medical” > “Fostamatinib.”
Surgical options for children with chronic ITP failing prior therapies
Recommendations for splenectomy in children with chronic ITP
Splenectomy is very rarely indicated in childhood ITP (Grade C recommendation) and should be undertaken in consultation with a hematologist experienced in the management of children with ITP. It should only be considered in children who have failed all available medical therapies, are having thrombocytopenia-related bleeding, and whose life is at risk or whose HRQoL is substantially impaired.
Splenectomy should be avoided if at all possible before 5 years of age and within 1 year of disease onset (Grade C recommendation).
Before considering splenectomy, reassess the diagnosis of ITP by excluding alternative diagnoses, including inherited thrombocytopenia, bone marrow failure, drug-induced thrombocytopenia, subclinical viral infections, immunodeficiency syndromes (eg, CVID, autoimmune lymphoproliferative syndrome), and myelodysplastic syndrome (Grade C recommendation).
Prior to splenectomy, ensure that vaccinations are up to date according to national policy. Vaccination, as a minimum, should include pneumococcal 13-valent conjugate vaccine, followed by pneumococcal 23-valent vaccine 4 weeks later; H influenzae type B; and both meningococcal vaccines to cover all 5 species subtypes (Grade C recommendation).
If there is any concern for an immunodeficiency-related ITP, even if undocumented, reducing the risk for postsplenectomy sepsis by assessing response to pneumococcal vaccines preprocedure is advisable (Grade C recommendation).
Splenectomy in ITP can achieve high and durable response rates. Since 2010, studies that included adults, as well as children/adolescents, reported initial response rates of 83% to 97% (evidence levels IIb-III),37,191 decreasing to 59% after a median 20-year (range, 10-43) follow-up.37 Firm conclusions on splenectomy in children cannot be drawn from the current literature, because studies do not separate results by age or disease duration.
Perisplenectomy management.
Prophylactic anticoagulation and/or antibiotics may be considered for splenectomy in children. Portal vein ultrasound to assess flow may be useful to predict the risk of portal vein thrombosis. The platelet count should be increased to hemostatic levels preoperatively if possible. Splenectomy should be performed laparoscopically. (All evidence level IV.)
Postsplenectomy management.
As for splenectomy in adults, long-term antibiotic prophylaxis and repeat vaccinations should be given according to national guidelines. The risk of infection in later life should be considered for polysaccharide-encapsulated organisms (eg, pneumococcus), as well as, for example, if the child later works in a school or as a veterinarian or travels to areas with endemic and/or epidemic Babesia, malaria, or dengue. The patient should be advised of the risks of postsplenectomy sepsis and thrombosis (and their appropriate management), to obtain yearly blood counts, and to keep vaccinations up to date.
Patient-reported outcomes and support of patients with ITP
The impact of ITP, especially chronic ITP, on patient HRQoL is substantial (evidence level III).278 The most difficult ITP symptom to treat is severe fatigue, reported in 39% to 59% of adult patients with ITP,9,279,280 yet it is underrecognized by health care practitioners.280 ITP leads to impaired HRQoL across emotional, functional, reproductive, and health domains, and it impacts daily living, in turn affecting mental health.
The ITP IWG recommends a treatment goal of a safe platelet count in the absence of bleeding, not a normal platelet count.48 Nevertheless, platelet counts remain a focus for health care practitioners, whereas fatigue and mental health aspects are the major concerns of patients. ITP patient support services are provided in numerous countries,281 but continued increased awareness of the impact of ITP on HRQoL among health care professionals is required.
Quality of life of adults with ITP
Recommendations for assessment and management of HRQoL outcomes in adults with ITP
A number of scales have been used successfully to assess HRQoL in adults. The ITP-specific 10-scale ITP-patient assessment questionnaire (ITP-PAQ) is a disease-specific questionnaire available as a tool that can be used to measure HRQoL, with estimated minimally important differences (MIDs) aiding interpretation. Additional measures of patient-reported outcomes (PROs) that have been studied in adult patients with ITP include the 36-item Short Form Health Survey (SF-36), EuroQol tool (EQ-5D), Hamilton anxiety and expression rating scales, Motivation and energy inventory-short form (MEI-SF), Fatigue subscale of the functional assessment of chronic illness therapy (FACIT-Fatigue), and Functional assessment of cancer therapy–thrombocytopenia (FACT-Th6) (Grade C recommendation).
Impaired HRQoL is multifactorial and includes (but is not limited to) issues around actual bleeding, fear of bleeding, reduced energy, depression, treatment side effects, and additive influences of underlying or comorbid diseases (Grade C recommendation).
Patients responding to treatment have improved HRQoL, with responders to TPO-RA improving more than responders to other therapies (evidence levels Ib, III); romiplostim may improve fatigue in responders (evidence level Ib) (Grade A recommendation).
PROs are essential outcome measures for clinical trials and should be considered in the routine management of children and adults with ITP.
Different tools to assess HRQoL and PROs in adults with ITP are available.282 These include ITP-PAQ,10 developed specifically for assessing HRQoL in ITP patients, EQ-5D index score,283 SF-36,284 Hamilton anxiety and depression rating scales,278 MEI-SF,285 FACIT-Fatigue,285 and FACT-Th6, a 6-item subset of FACT-Th.285
PRO tools have been developed and validated using different methodologies. The domains measured by each PRO and the time period over which a change can be detected vary. When using a PRO outside of the geographical region in which it was developed, a validation of cultural and language changes are desirable. Consideration of which PRO tool to use should reflect the population being assessed and the time period in which to expect changes; ideally, >1 validated tool should be used.
To understand the clinical significance of changes in the ITP-PAQ score, MIDs have been calculated.286 MID values of 8 to 12 are considered clinically significant for the following scales: Symptoms, Bother, Psychological, Overall HRQoL, Social Activity, Menstrual Symptoms, and Fertility. For Fatigue and Activity, MID values of 10 to 15 were considered clinically significant. These estimates were consistent with moderate effect sizes. However, MIDs were not estimable for the scales Fear and Work.286
Patients with ITP have been shown to have statistically significantly worse outcomes than the general population with regard to general fatigue, physical fatigue, reduced activity, and mental fatigue, as assessed by SF-36 (evidence level IIb).284 Higher fatigue severity was associated with poorer physical and mental HRQoL outcomes. Patients with persistent ITP were shown to have clinically meaningful impairments in physical functioning, social functioning, role physical, role emotional, and mental health scales (evidence level IIb).284 In 1 study, patients with chronic ITP appeared to be most affected by reduced work ability, fear, bleeding, and fatigue (evidence level III), 72% expressed some degree of depression, and 21% reported some degree of anxiety.278
Treatment and HRQoL.
In 1 study of adults with chronic ITP, the use of SF-36 found that treatment did not generally impact HRQoL (evidence level III).278 Platelet and red blood cell transfusions, splenectomy, and corticosteroid side effects, in particular, did not influence HRQoL, whereas treatment with IVIg increased bodily pain.278 However, a study of patients’ and hematologists’ perspectives of corticosteroid side effects in ITP found that patients reported severe side effect symptoms significantly more frequently than did hematologists (evidence level III).287 Patients also frequently responded that the corticosteroids side effects resulted in “a lot of bother,” the highest level of severity.287
Romiplostim.
The effect of romiplostim on HRQoL has been evaluated in 3 studies: 2 using ITP-PAQ and 1 using EQ-5D (evidence level Ib).10,283,288 All studies reported improvement in HRQoL for patients responding to romiplostim vs nonresponders, although differences were not always significant or greater than MIDs, and response criteria varied.10,283,288 Responders to romiplostim and standard of care showed an improvement in fatigue above MIDs.10
Compared with standard of care, patients receiving romiplostim showed a significant (and greater than MID) improvement over baseline in symptoms, bother, psychological health, and overall HRQoL on the ITP-PAQ; however, there was no significant difference in fatigue.10 Splenectomized patients receiving romiplostim showed significantly greater improvements in HRQoL vs placebo on the ITP-PAQ for symptoms, bother, social activity, and women’s reproductive health. Nonsplenectomized patients treated with romiplostim showed only a significant improvement in activity vs placebo.288
Eltrombopag.
The effect of eltrombopag on HRQoL in adults with ITP lasting ≥6 months has been evaluated in the RAISE and EXTEND studies using 4 instruments: SF-36v2, MEI-SF, FACIT-Fatigue, and FACT-Th6.149,285 In the RAISE study, at week 26 several scores were significantly improved from baseline in patients receiving eltrombopag (5 of 8 SF-36v2 scores, both SF-36v2 summary scores, and FACT-Th6) (evidence level Ib).149 Improvements were significantly associated with eltrombopag-mediated increases in platelet count and decreases in bleeding (World Health Organization scale).149 In the EXTEND study (all patients receiving eltrombopag), depending on the instrument used, 80% to 85% of patients experienced an improvement in HRQoL vs baseline; clinically meaningful improvement vs baseline was observed at least once during eltrombopag treatment in 48% to 64% of patients (evidence level III).285 Improvement from baseline in some measures of HRQoL (FACIT-Fatigue and FACT-Th6) persisted for up to 5 years, although the improvement was not significant at all time points.285
Quality of life of children with ITP
Recommendations for assessment and management of HRQoL outcomes in children with ITP
HRQoL should be reported using the Kid’s ITP Tool (KIT; or another validated scale) before and after treatment, to assess the effect of treatment beyond the platelet count (Grade C recommendation).
HRQoL of children with newly diagnosed ITP improves on disease resolution (evidence level IIa; Grade B recommendation).
Corticosteroids may worsen HRQoL in children (evidence level IIa; Grade B recommendation).
TPO-RAs may improve HRQoL of children with ITP, and romiplostim especially appears to improve parental burden (evidence level Ib, IIa; Grade B recommendation).
KIT, developed in 2007,289 is ITP specific and consists of child self-reporting (for children aged ≥7 years), parent proxy reporting, and assessment of parent impact.290 The established, but non-ITP specific, Pediatric Quality of Life Inventory (PedsQL) questionnaire may also be used. HRQoL is harder to assess in children than in adults; irritability or depression may be observed instead of fatigue.
In a study of children with newly diagnosed ITP, KIT and PedsQL were used at diagnosis and at follow-up appointments (evidence level IIa).291 Results of PedsQL were comparable with healthy children. KIT proxy reports and parent self-reports showed significantly higher HRQoL scores in children who recovered than in those with persistent ITP at 3 or 6 months. There was no significant difference in HRQoL by KIT or PedsQL between children with mild vs moderate bleeding (evidence level IIa).291
Treatment and HRQoL.
In 1 study, HRQoL was compared in children receiving treatment and those managed through observation (evidence level IIa).292 HRQoL (assessed by KIT) was not improved through treatment (IVIg, prednisone, IVIg plus steroids, anti-D, rituximab, or splenectomy); the lowest KIT scores were observed after treatment with prednisone. The parent-proxy KIT scores were significantly worse for newly diagnosed children receiving treatment, particularly prednisone. Thus, the side effects of treatment appear to negatively impact HRQoL and outweigh the benefits of an increased platelet count (evidence level IIa).292
Another study showed that HRQoL (by KIT and PedsQL) of newly diagnosed children with ITP was not significantly different between those randomized to observation vs IVIg (evidence level IIa).291
Romiplostim.
Two studies have shown that romiplostim may improve HRQoL (as assessed by KIT) in children with ITP lasting ≥6 months.290,293 One reported no significant difference, and the other reported numerically greater and occasionally significant improvements, in child self-reported HRQoL over baseline with romiplostim vs placebo (evidence levels Ib, IIa).290,293 Romiplostim significantly reduced parental burden vs placebo in both studies.
Eltrombopag.
The impact of eltrombopag on HRQoL in children with persistent or chronic ITP was evaluated in the PETIT trial (evidence level Ib).294 There was a wide variability in KIT scores at baseline. Small improvements in HRQoL were noted in responders to eltrombopag aged 1 to 5 years and 12 to 17 years but not those in who were 6 to 11 years old. Therefore, eltrombopag did not appear to substantially affect HRQoL of children, as assessed by KIT.
Support for children with ITP.
A survey of 278 pediatric hematologists or oncologists revealed a large variation in the perceived contact risk for different sports and in the advice offered about restricting sporting activities in children with ITP (evidence level III).295 Most physicians recommended restricting sport activity at platelet counts < 50 × 109/L, and only one third would encourage perceived low-risk sport (eg, golf, dancing, swimming). The current perceptions and advice given could negatively impact HRQoL in a generally benign disease; thus, decision making should be patient focused (evidence level III).295
A recent review article on sports participation for patients with bleeding disorders recommends maintaining a healthy level of safe participation in different activities.296 The recommendations below are based on national and organization guidelines.
Recommendations for school and participation in sporting activities for children with ITP
Children and adolescents 5 to 18 years old need ≥60 minutes of physical activity per day, ≥3 d/wk. This should include exercises or sports to promote strong muscles and bones (Grade C recommendation).
Normal attendance and play at kindergarten, school, or college, depending on age, is essential. The risk of bleeding and information about ITP should be provided to the school in a way that facilitates inclusion, not isolation (Grade C recommendation).
Active participation in low-risk activities should be maintained, irrespective of platelet count and treatment (Grade C recommendation).
Participation in nonlow-risk activities must be discussed with the family, school, and coach. A number of factors must be considered prior to participation, including age of the child, platelet count, bleeding history, and physical nature of the activity (Grade C recommendation).
Participation in high-risk activities (including BMX racing, boxing, American football, ice hockey, lacrosse, motorcycle riding, motocross racing, power lifting, outdoor rock climbing, rodeo, rugby, snowmobiling, trampoline, and wrestling) should be discouraged unless the patient has a near-normal platelet count on a consistent and stable basis. Alternatively, treatment should be administered to provide a safe platelet count during the activity (Grade C recommendation).
Intermittent or continuous treatment may be given to cover activities with appropriate discussion of risks vs benefits of the activity and treatment, with emphasis on psychological well-being and risks for injury, despite treatment (Grade C recommendation).
Choice of treatment and target platelet count must be carefully evaluated based on extensive consultation with the family and consideration of the specific activity desired and the bleeding tendency of the child (Grade C recommendation).
Conclusions
This consensus report provides a timely update to the previous report published in 2010. Based on the new evidence available, and the authors’ expert opinion, updates to the recommendations and the supporting evidence are presented. Should it become apparent that this consensus report requires further update, the panel will reconvene to discuss how best to do this.
Although there are numerous publications on the diagnosis and treatment of ITP, knowledge gaps remain, and up-to-date expert opinion and experience are key.
Acknowledgments
Professional medical writing support was provided by Lyndsey Kostadinov and Anne Carter of Medicalwriters.com.
This work was supported by unrestricted educational grants from Amgen, Novartis, and Rigel.
Authorship
Contribution: All authors provided input on each draft of the manuscript and approved the final version for submission.
Conflict-of-interest disclosure: D.P. has received research support and honoraria from Amgen and Novartis and has acted as a consultant for UCB, MedImmune, and Ono. D.M.A. has received research funding from Novartis, Bristol-Myers Squibb, and Rigel and has acted as a consultant for Novartis, Principia, and Rigel. J.B.B. has acted as a consultant (including serving on advisory boards) for Amgen, Novartis, Dova Pharmaceuticals, UCB, argenx, Momenta, Rigel, Tranquil, Regeneron Pharmaceuticals, Octapharma, Kezar, and the PDSA. B.H.C. has received honorarium from Novartis for participating in the speaker’s forum. N.C. has received honoraria for educational speaking events and for serving on advisory boards for Novartis, Amgen, Rigel, and Principia and has acted as the chief investigator or principal investigator of clinical trials for Amgen, Novartis, Rigel, Principia, and UCB. T.G. has received honoraria from Amgen; has acted as a consultant for Amgen, Dova Pharmaceuticals, Biogen, Cellphire, Fujifilm, Rigel, Shionogi, and Principia; and has received research support from Principia. W.G. has received research grants from Novartis, Bayer, and BMS/Pfizer; has received lecture honoraria from Novartis, Amgen, and Bayer; and has acted as a consultant for Amgen, Novartis, and MSD. B.G. has served as an expert for Amgen and Novartis and has received research funds from Amgen and Roche. T.J.G.-L. has received research grants and speaker honoraria from Amgen and Novartis. J.G. has received honoraria (speaker fees, travel grants) from Amgen and Novartis and has acted as a consultant for Amgen, Novartis, Ono, Alexion, and Biotest. C.K. has acted as a consultant for Novartis and UCB; has received honoraria from Amgen (paid to PDSA); PDSA funding from Amgen, Argenx, Dova, Novartis, Momenta, Principia, Octapharma, CSL Behring, Rigel, and UCB; and is a board member of the Thrombosis & Hemostasis Societies of North America. V.M. has acted as a consultant for AbbVie and has received honoraria from Bayer, Novartis, and Amgen. M.M. has received consultancy fees/honoraria (for serving on advisory boards and/or for being a member of the speakers bureau) from Amgen, Novartis, and Rigel in the last 2 years. A.C.N. has acted as a consultant for and has received honoraria directly from Amgen, Angle, argenx, Dova Pharmaceuticals, Novartis, Ono, Rigel, and Shionogi; has received funding from Amgen, Novartis, and Rigel; and has provided paid expert testimony for argenx and Rigel. S.P. has received fees for lectures, chairing sessions, and serving on advisory boards. F.R. has been a member of the speakers bureau/advisory board for Amgen, Novartis, and argenx. M.S. has received honoraria from Novartis for serving on its advisory board and for being a member of its speakers bureau. Y.T. has acted as a consultant for Sysmex and has received honoraria from Novartis, Kyowa Kirin, and Chugai. R.S.W. has received research funding from Amgen, Apellis Pharmaceuticals, Bayer, Boehringer-Ingelheim, Biogen-Idec, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Roche, and Sanofi and has acted as a consultant for Alexion, Amgen, Bayer, Boehringer-Ingelheim, Kyowa Kirin, and Novartis. F.Z. has received research funding from Novartis, Celgene, AbbVie, Amgen, and Janssen Cilag and has received honoraria for participating on advisory board and/or for presenting talks at meetings from Novartis, Celgene, AbbVie, Amgen, Janssen Cilag, Sandoz, Takeda, and Roche. D.J.K. has received research funding from Protalex, Bristol-Myers Squibb, Rigel, Bioverativ, Agios Pharmaceuticals, Syntimmune, Principia, and Alnylam Pharmaceuticals and has acted as a consultant for Ono Pharmaceutical, Pfizer, 3SBio, Eisai, GlaxoSmithKline, Genzyme, Shire, Amgen, Shionogi, Rigel, Syntimmune, MedImmune, Novartis, Alexion, Bioverativ, Argenx, Zafgen, Fujifilm, Principia, Kyowa Kirin, Takeda, and the Platelet Disorders Support Group. M.H. declares no competing financial interests.
Correspondence: Drew Provan, Department of Haematology, The Royal London Hospital, Pathology and Pharmacy Building, London E1 2ES, United Kingdom; e-mail: a.b.provan@qmul.ac.uk.
References
Author notes
The full-text version of this article contains a data supplement.