Key Points
Infectious diseases are the second most common cause of death in HCT recipients, but some are first identified only by autopsy.
Autopsy is underutilized and should be performed regularly to help improve infection-related morbidity and mortality.
Abstract
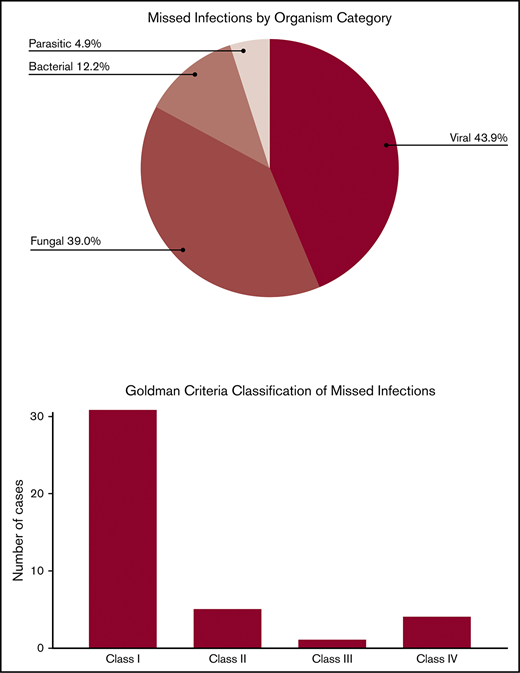
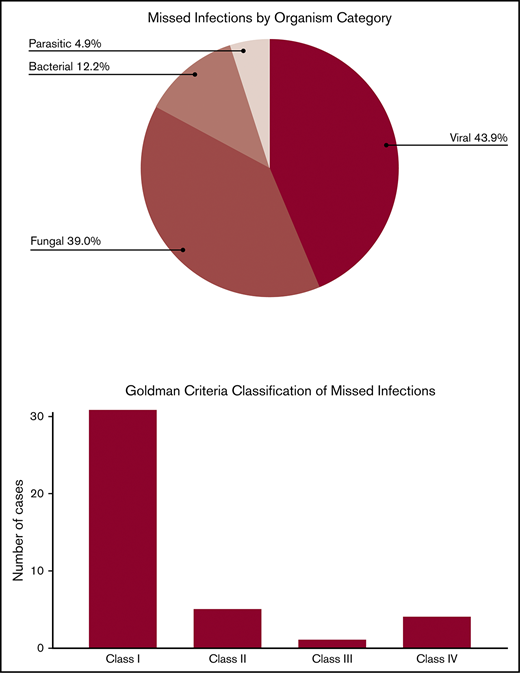
Hematopoietic cell transplantation (HCT) is potentially curative for patients with hematologic disorders, but carries significant risks of infection-related morbidity and mortality. Infectious diseases are the second most common cause of death in HCT recipients, surpassed only by progression of underlying disease. Many infectious diseases are difficult to diagnose and treat, and may only be first identified by autopsy. However, autopsy rates are decreasing despite their value. The clinical and autopsy records of adult HCT recipients at our center who underwent autopsy between 1 January 2000 and 31 December 2017 were reviewed. Discrepancies between premortem clinical diagnoses and postmortem autopsy diagnoses were evaluated. Of 185 patients who underwent autopsy, 35 patients (18.8%) had a total of 41 missed infections. Five patients (2.7%) had >1 missed infection. Of the 41 missed infections, 18 (43.9%) were viral, 16 (39.0%) were fungal, 5 (12.2%) were bacterial, and 2 (4.9%) were parasitic. According to the Goldman criteria, 31 discrepancies (75.6%) were class I, 5 (12.2%) were class II, 1 (2.4%) was class III, and 4 (9.8%) were class IV. Autopsies of HCT recipients frequently identify clinically significant infectious diseases that were not suspected premortem. Had these infections been suspected, a change in management might have improved patient survival in many of these cases. Autopsy is underutilized and should be performed regularly to help improve infection-related morbidity and mortality. Illustrative cases are presented and the lessons learned from them are also discussed.
Introduction
Hematopoietic cell transplantation (HCT) is a potentially curative therapy for patients with hematologic disorders, but carries significant risks of infection-related morbidity and mortality.1 Infectious diseases are recognized as the second most common cause of death in HCT recipients, surpassed only by relapse or progression of the underlying disease. Many infections are difficult to diagnose and treat, and HCT recipients may die of infectious complications despite extensive investigations and broad-spectrum antimicrobial therapy. Autopsy is the gold standard for establishing the cause of death but autopsy rates are decreasing despite their value.
Methods
The autopsy reports of HCT recipients at Stanford University Medical Center who died and had a limited or complete autopsy performed between 1 January 2000 and 31 December 2017 were reviewed. Any infection diagnosed on the autopsy record prompted an in-depth review of the clinical record. Patients were excluded if they were <18 years old, if their autopsy report was missing or incomplete, or if their medical record had insufficient clinical data available for review. Data collected included patient demographics, underlying disease, donor and HCT type, graft source, preparative regimen, development of graft-versus-host disease (GVHD), date of death, premortem clinical information, and postmortem autopsy diagnoses. The premortem clinical diagnoses as documented by the clinical teams were compared with postmortem autopsy diagnoses. Missed infections were defined as those that were not suspected or diagnosed premortem and were not being treated incidentally. Infections were classified by organism and by anatomic location of involvement. Disseminated infections were defined as those that involved >1 organ system. Discrepancies between premortem clinical diagnoses and postmortem autopsy diagnoses were classified according to the Goldman criteria (Table 1).2 All included cases were reviewed independently by 2 immunocompromised host infectious diseases–trained physicians; any discordances would be reevaluated and reconciled, with adjudication by a third immunocompromised host infectious diseases–trained physician as needed. This study was exempt from review and approval by the Stanford University Institutional Review Board because chart review of deceased subjects does not meet the definition of human subjects research.
Results
Between 1 January 2000 and 31 December 2017, a total of 4646 patients underwent 5024 HCTs at Stanford University Medical Center. Of these 5024 transplants, 2337 (47%) were allogeneic and 2687 (53%) were autologous. GVHD developed in 1409 patients. At the time of analysis, 2119 HCT recipients (45.6%) had died, of whom 211 (10.0%) had an autopsy performed. Of these, 185 met inclusion criteria; 182 were performed at Stanford and 3 were performed at another institution. Twenty-six patients were excluded from the analysis because of missing or incomplete autopsy report, insufficient clinical data, or age <18 years.
Baseline characteristics of 185 patients who underwent autopsy and met inclusion criteria are presented in Table 2. Most patients were male (62.2%). Acute myeloid leukemia (35.1%) was the leading indication for HCT, followed by non-Hodgkin lymphoma (28.6%) and acute lymphoblastic leukemia (13.0%). The vast majority of patients had undergone allogeneic HCT (83.2%), among whom HLA-identical siblings (36.2%) were the most common donor type. Mobilized peripheral blood allografts (89.2%) and myeloablative preparative regimens (64.3%) were most frequently used. GVHD developed in 108 patients (59.7%). There were no substantial demographic or transplant-related differences between those who underwent autopsy and those who did not.
Annual mortality and autopsy rates over time are presented in Figure 1. Mortality rates fluctuated but trended toward an overall decline over time, from 61.0% in 2000% to 43.9% in 2017. Autopsy rates also varied but remained low throughout the study period. The lowest autopsy rate (0.9%) occurred in 2000 and the highest autopsy rate (14.1%) occurred in 2004.
Annual mortality and autopsy rates of HCT recipients between 1 January 2000 and 31 December 2017, irrespective of the year of transplant.
Annual mortality and autopsy rates of HCT recipients between 1 January 2000 and 31 December 2017, irrespective of the year of transplant.
Details of missed infectious diseases and their anatomic location are presented in Table 3. Of the 185 patients who underwent autopsy, 35 patients (18.9%) had 41 missed infections. Five patients had >1 missed infection, including 4 patients with 2 infections and 1 patient with 3 infections. Of the 41 infections, 18 (43.9%) were viral, 16 (39.0%) were fungal, 5 (12.2%) were bacterial, and 2 (4.9%) were parasitic. Disseminated infections (41.5%) were the most common followed by pneumonia (24.4%) and gastrointestinal disease (17.1%). Of the 150 patients who did not have a missed or misdiagnosed infection on autopsy, 96 were diagnosed with at least 1 infection premortem, 17 had presentations that were suspicious for infection but did not have confirmatory diagnostic tests, and 37 did not have any confirmed or suspected infectious complications.
Cytomegalovirus (CMV; 14.6%) was the most common missed viral disease, including 4 (9.8%) involving the gastrointestinal tract. There were 4 cases of adenovirus, of which 3 were disseminated. There were also 4 cases of BK virus (all in the urinary tract), 2 cases of herpes simplex virus (HSV; including 1 case that was disseminated and 1 that involved the liver), 1 case of disseminated varicella-zoster virus (VZV), and 1 case of respiratory syncytial virus (RSV) pneumonia.
Candida spp. (12.2%) and Aspergillus spp. (12.2%) were the most common missed fungal diseases. There were also 4 cases of mucormycosis, 1 case of disseminated infection with Scedosporium/Pseudallescheria spp., and 1 case of disseminated coccidioidomycosis. Most fungal diseases were disseminated. Notably, 4 of the 5 Candida cases had autopsy findings consistent with pneumonia.
There were 5 missed bacterial diseases, including 3 cases of pneumonia (2 by Enterococcus spp. and 1 by Staphylococcus aureus). For the cases of endocarditis and peritonitis, the pathogens were detected by Gram staining but cultures did not yield any growth to identify the offending organisms.
The 2 missed parasitic diseases included 1 case each of disseminated Strongyloides stercoralis and disseminated Toxoplasma gondii.
The classification of missed infectious diseases by Goldman criteria is presented in Table 4. Thirty-one infectious diseases (75.6%) were classified as class I and 5 (12.2%) were class II. Class I discrepancies were most commonly due to fungal (34.2%) or viral (29.3%) diseases.
Of the 35 patients with missed infectious diseases, death occurred at a median of 93 days post-HCT (mean, 213 days; range, 22-1506 days). Eighteen patients died between day 0 and day +100, 10 died between day +100 and 1 year, and 7 died after 1 year.
Illustrative cases
Case 1: missed viral disease.
A 52-year-old man with acute lymphoblastic leukemia underwent myeloablative allogeneic HCT from an HLA-identical sibling using a peripheral blood allograft in 2012. Nineteen months post-HCT, he was admitted (hereto regarded as hospital day 0 [HD0]) with 1 week of low-grade fever and respiratory tract symptoms. His only prophylaxis was trimethoprim-sulfamethoxazole (TMP-SMX). At presentation, his absolute lymphocyte count was 350 cells per microliter, total bilirubin was 2.4 mg/dL, aspartate transaminase was 70 U/L, alanine aminotransferase was 79 U/L, and alkaline phosphatase was 328 U/L. A nasopharyngeal swab was positive for RSV by direct fluorescent antibody (DFA), and a computed tomography (CT) scan of the chest demonstrated bilateral centrilobular nodules and ground-glass opacities with a bibasilar predominance. Broad-spectrum antibiotics started at admission were changed to levofloxacin and oral ribavirin; the latter was stopped on HD9 after a repeat nasopharyngeal swab resulted negative for RSV. However, his liver chemistries continued to worsen, with total bilirubin at 10.9 mg/dL, aspartate transaminase at 2277 U/L, alanine aminotransferase at 1425 U/L, and alkaline phosphatase at 395 U/L on HD10. On the same day, he developed abdominal pain, oliguria, atrial fibrillation with rapid ventricular response, and hypotension. Serum lipase was >3000 U/L. He was transferred to the intensive care unit and started on IV acyclovir, piperacillin-tazobactam, and vancomycin. Repeat chest CT scanning demonstrated extensive ground-glass and consolidative opacities. He developed multiorgan failure and the patient died the following day (HD23) after being transitioned to comfort care.
At autopsy, widespread VZV-induced cytopathic changes and hemorrhagic necrosis were seen in the liver (Figure 2), pancreas, peripancreatic tissue, kidneys, colon, lungs, hilar lymph nodes, and bone marrow. Characteristic histologic findings of RSV in the lungs were not seen and postmortem lung RSV polymerase chain reaction (PCR) was negative. In addition to disseminated VZV infection, autopsy revealed 2 separate focal fungal diseases, with esophageal ulceration with involvement by fungal elements morphologically consistent with Mucorales spp., and a multifocal mild pneumonia with fungal elements morphologically resembling Candida spp.
Images corresponding to illustrative case 1 (missed viral disease). (A) Low-power view (original magnification ×4) of the liver with hematoxylin and eosin stain showing extensive hemorrhagic necrosis (arrows) in a background of micronodular cirrhosis. (B) High-power view (original magnification ×60) of the liver with hematoxylin and eosin stain demonstrating prominent viral cytopathic changes with eosinophilic nuclear inclusions (arrows). (C) High-power view (original magnification ×60) of the liver with VZV immunostain demonstrating strongly positive staining of atypical multinucleated hepatic cells (arrows).
Images corresponding to illustrative case 1 (missed viral disease). (A) Low-power view (original magnification ×4) of the liver with hematoxylin and eosin stain showing extensive hemorrhagic necrosis (arrows) in a background of micronodular cirrhosis. (B) High-power view (original magnification ×60) of the liver with hematoxylin and eosin stain demonstrating prominent viral cytopathic changes with eosinophilic nuclear inclusions (arrows). (C) High-power view (original magnification ×60) of the liver with VZV immunostain demonstrating strongly positive staining of atypical multinucleated hepatic cells (arrows).
Case 1: lessons learned
Multiorgan involvement should raise suspicion for disseminated infections, including bacterial, fungal, viral, and parasitic etiologies.
The patient’s pre-HCT infectious disease serologies and post-HCT antimicrobial regimen should be reviewed when generating the differential diagnosis. This patient was not on antiviral or antifungal prophylaxis at the time of symptom onset, increasing the likelihood of these etiologies. Adherence with appropriately dosed TMP-SMX prophylaxis renders certain infections (eg, Pneumocystis jirovecii, T gondii, Nocardia, and Listeria) less likely.
Immunocompromised patients, especially those who have undergone HCT, can have multiple concomitant infections. Continued investigations should be pursued in such scenarios because RSV would be unlikely to explain this patient’s septic shock, hepatitis, or pancreatitis.
Albeit quite rare, Candida can cause pneumonia in immunocompromised patients.3,4
Case 2: missed fungal disease.
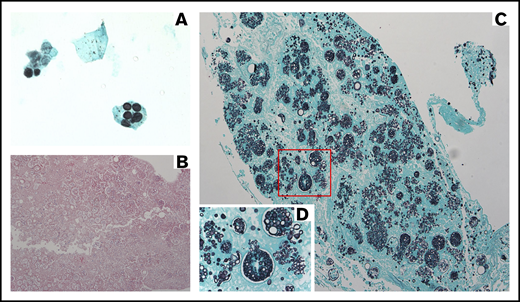
A 73-year-old man with acute myeloid leukemia underwent nonmyeloablative conditioning and allogeneic HCT with a peripheral blood allograft from an HLA-mismatched unrelated donor in 2015. Nine months pre-HCT, a chest CT scan demonstrated pulmonary nodules concerning for possible invasive fungal infection, prompting a limited negative noninvasive fungal workup and empirical treatment with voriconazole. The patient’s post-HCT course was significant for recurrent Epstein-Barr virus viremia without evidence of posttransplant lymphoproliferative disease in the first and third months post-HCT, for which he received a total of 8 doses of rituximab with clearance of Epstein-Barr virus viremia. Eight months post-HCT, a repeat chest CT scan demonstrated radiographic improvement of the lung nodules, and all immunosuppressive therapy was discontinued without evidence of GVHD; voriconazole was therefore discontinued. Two weeks later, he developed fevers, fatigue, and cough and was hospitalized (HD0). Laboratory results were notable for transaminitis and lack of peripheral eosinophilia. A chest CT scan demonstrated innumerable scattered noncalcified small nodules and several cavitary nodules measuring up to 15 mm bilaterally. An extensive infectious workup was performed and he was started on broad-spectrum antibacterial agents. On HD6, his clinical status continued to decline, requiring transfer to the intensive care unit for ventilator and vasopressor support. Liposomal amphotericin B (AmBisome) 3 mg/kg IV every 24 hours, TMP-SMX 5 mg/kg IV every 8 hours, and methylprednisolone 40 mg IV every 12 hours were started empirically. Liposomal amphotericin B was changed to voriconazole on HD8 due to renal failure. On HD10, bronchoscopy and bronchoalveolar lavage (BAL) were performed. BAL fluid was negative for Pneumocystis by DFA, but Gomori methenamine silver (GMS) staining demonstrated microorganisms morphologically suggestive of P jirovecii (Figure 3A). The patient continued to decline clinically and died on HD12. Both blood and BAL fluid cultures subsequently grew Coccidioides immitis. Coccidioides serologies obtained on HD8 resulted positive for immunoglobulin G by immunodiffusion and negative by complement fixation postmortem, likely indicating reactivation of previously acquired infection.
Images corresponding to illustrative case 2 (missed fungal disease). (A) BAL specimen (original magnification ×100, oil immersion) with GMS stain demonstrating dark spherical structures, originally misdiagnosed on cytopathology as P jirovecii but subsequently determined to be Coccidioides endospores. (B) Section of lung with hematoxylin and eosin stain (original magnification ×10) demonstrating a mixture of large thick-walled spherules containing variably sized endospores, diagnosed as invasive C immitis. (C) Section of lung (original magnification ×20) showing invasive C immitis as highlighted by GMS stain. (D) Magnification of area outlined in red (original magnification ×60) illustrating multiple ruptured Coccidioides spherules releasing endospores into surrounding lung tissue.
Images corresponding to illustrative case 2 (missed fungal disease). (A) BAL specimen (original magnification ×100, oil immersion) with GMS stain demonstrating dark spherical structures, originally misdiagnosed on cytopathology as P jirovecii but subsequently determined to be Coccidioides endospores. (B) Section of lung with hematoxylin and eosin stain (original magnification ×10) demonstrating a mixture of large thick-walled spherules containing variably sized endospores, diagnosed as invasive C immitis. (C) Section of lung (original magnification ×20) showing invasive C immitis as highlighted by GMS stain. (D) Magnification of area outlined in red (original magnification ×60) illustrating multiple ruptured Coccidioides spherules releasing endospores into surrounding lung tissue.
Autopsy findings were consistent with disseminated coccidioidomycosis, involving the lungs (Figure 3B-D), liver, spleen, and multiple lymph nodes. Premortem, the Coccidioides endospores visualized on GMS stain of BAL fluid were misdiagnosed as P jirovecii.
Case 2: lessons learned
Because of the nonspecific nature of pulmonary nodules and the high risk for infectious complications with hematologic malignancy and HCT, every possible effort should be pursued to establish an etiologic diagnosis (acknowledging that diagnostic yield of tests and procedures is variable and depends upon multiple factors).5-9
Had the patient undergone additional investigations based upon epidemiologic risk factors and been diagnosed with coccidioidomycosis pre-HCT, he would have likely remained on lifelong secondary antifungal prophylaxis, thereby potentially averting this fatal outcome.10
Because of the lower sensitivity of DFA to detect P jirovecii in non-HIV–immunocompromised hosts, this negative test result could not reliably exclude the diagnosis in this patient, highlighting the potential role of newer diagnostic assays such as PCR.11,12
Case 3: missed parasitic disease.
A 71-year-old man with high-grade myelodysplastic syndrome underwent nonmyeloablative allogeneic HCT with a peripheral blood allograft from an HLA-matched unrelated donor in 2016. Two months pre-HCT, a chest CT scan demonstrated multiple bilateral pulmonary nodules. The largest nodule (2.3 × 1.7 × 2 cm) in the right upper lobe was biopsied. Pathology demonstrated diffuse alveolar damage; all infectious workup including cultures and fungal serologies and antigens were negative. He was treated empirically with voriconazole and a repeat chest CT scan 1 month pre-HCT showed near complete resolution of the nodules. Voriconazole was continued post-HCT as secondary prophylaxis. Two months post-HCT, he was diagnosed with acute gut GVHD for which he was started on prednisone 2 mg/kg daily with subsequent taper. Three months post-HCT, while on prednisone 20 mg daily, he developed progressive dyspnea on exertion and a petechial rash on his abdomen and back. He was admitted (HD0) and found to be tachycardic, hypotensive, and hypoxic, but afebrile. Laboratory results were pertinent for worsening anemia. He rapidly decompensated from hypoxic respiratory failure. Chest CT scan demonstrated new, bilateral diffuse ground-glass opacities and a superimposed cavitary lesion/abscess in the left lower lobe (Figure 4A). Voriconazole was changed to liposomal amphotericin B, and he was started on TMP-SMX in conjunction with high-dose steroids. On HD1, he was intubated electively for bronchoscopy, which revealed evidence of diffuse alveolar hemorrhage (DAH). His clinical status continued to decline despite maximal supportive measures. He was transitioned to comfort care and died on HD12.
Images corresponding to illustrative case 3 (missed parasitic disease). (A) Chest CT scan demonstrating bilateral diffuse ground-glass opacities and a superimposed cavitary lesion/abscess in the left lower lobe. (B) Sections of the duodenum (original magnification ×10) with hematoxylin and eosin stain showing obliteration of the inner mucosa by S stercoralis larvae (arrows).
Images corresponding to illustrative case 3 (missed parasitic disease). (A) Chest CT scan demonstrating bilateral diffuse ground-glass opacities and a superimposed cavitary lesion/abscess in the left lower lobe. (B) Sections of the duodenum (original magnification ×10) with hematoxylin and eosin stain showing obliteration of the inner mucosa by S stercoralis larvae (arrows).
Autopsy revealed diffuse bilateral acute pulmonary S stercoralis disease, invasive S stercoralis duodenitis (Figure 4B), and an isolated left lower lobe 3.5 cm cavity with fungal elements due to Mucorales. Postmortem review of his chart for Strongyloides risk factors revealed that he was an Army veteran who had served in the Vietnam War for 11 months at least 40 years prior to his death.
Case 3: lessons learned
Risk factors for S stercoralis infection and the parasite’s ability to remain latent and undetected for decades are frequently underrecognized.13-15 Any lifetime exposure is a potential risk factor for reactivation in the setting of immunosuppression, especially corticosteroids. Pre-HCT screening for this patient was indicated because of his prior military service in an endemic area. Had he been screened, he could have been treated pre-HCT, potentially avoiding the Strongyloides hyperinfection syndrome and his demise.
Clinicians should have strong clinical suspicion for Strongyloides hyperinfection syndrome in the setting of DAH after the introduction of corticosteroids in a patient with epidemiologic risk.13,14,16
Careful attention should be paid to radiographic patterns when generating a differential diagnosis. This patient had 2 distinct radiographic patterns on chest CT scan, with the bilateral ground-glass opacities corresponding to DAH due to S stercoralis and the left lower lobe cavitary lesion/abscess corresponding to mucormycosis. This case again illustrates that HCT patients can have multiple concomitant infections with major clinical significance.
Discussion
This autopsy-driven study describes our experience with the missed diagnosis and misdiagnosis of infectious diseases and appraises the value of autopsy in HCT recipients at a high-volume academic transplant center over a 17-year period. HCT carries a significantly increased risk of infection-related morbidity and mortality. A prospective multicenter cohort study found that 93% of transplants are complicated by infection and 21% of deaths are attributed to infection, representing the second most common cause of death after disease relapse (44%).1 More than one-half of patients with missed infectious diseases in our study died within the preengraftment and early postengraftment (until day +100) periods.17 Mirroring the Center for International Blood and Marrow Transplant Research outcomes database and a single-center study by Gooley et al, our center’s HCT mortality rates demonstrated an overall decline over time.18,19 Annual autopsy rates at our center varied unpredictably but remained disappointingly low throughout the study period. Other studies have reported that autopsy rates steadily declined but maintained their utility over time.2,20-23
Viral diseases were the most commonly missed infection in our study. Although CMV was the most common missed viral disease, only 6 cases were found, including 4 involving the gastrointestinal tract. Many of these cases were clinically suspected to be worsening steroid-refractory gut GVHD and therefore treated with intensified immunosuppression. CMV has historically been 1 of the most common post-HCT infectious complications, and tissue-invasive disease has been associated with high mortality rates.1,24-30 CMV enteritis is notoriously challenging to diagnose because it is clinically indistinguishable from gut GVHD, viremia does not correlate with gastrointestinal disease, mucosal biopsies are subject to sampling error, and CMV-specific tissue testing (including immunohistochemistry and PCR) has limited sensitivity and specificity.31-33 Clinicians should also be aware of the possible coexistence of CMV and GVHD in the gastrointestinal tract.31 Notably, our study found only 1 missed case of CMV pneumonia. With the advent of preemptive approaches to CMV, in conjunction with the development of improved diagnostic assays and treatment options, the incidences of CMV tissue-invasive disease and associated mortality have decreased over time.1,24-30,34 There were also 4 missed cases of adenoviral disease, including 3 that were disseminated, likely due to low clinical suspicion and issues related to its diagnosis and treatment. In the pre-PCR era, failure to diagnose adenovirus premortem was due to the poor operating characteristics of diagnostic assays such as viral culture and DFA.35 In the PCR era, diagnostic sensitivity for adenovirus has increased. However, clinicians should be familiar with the multiplex syndromic PCRs used at their institution because some of these assays may not detect all adenovirus serotypes known to cause disease in HCT recipients.35-38 In the setting of high clinical suspicion for adenoviral disease, clinicians may hesitate to administer cidofovir empirically due to its prohibitive nephrotoxicity.39 Safer treatment options allowing for empirical treatment may improve outcomes in similar settings.40
Invasive mold infections are well recognized to be a significant cause of mortality in HCT recipients due to challenges in diagnosis and treatment.1,23,26,41-43 Several new triazoles, echinocandins, and lipid formulations of amphotericin B were introduced during the study period, which undoubtedly influenced patient outcomes. However, the specific effects of these agents were unable to be assessed in this retrospective study. Although the overall incidence of invasive mold infections in HCT recipients has been decreasing over time, an epidemiological shift from Aspergillus to non-Aspergillus molds has been increasingly reported, a phenomenon that is suspected to be at least partly related to evolving antifungal prophylaxis regimens.1,23,26,42-44 In our study, fungal diseases were the second most common missed infection, including 5 cases of Candida, 5 Aspergillus, 5 non-Aspergillus molds, and 1 Coccidioides. Four of the 5 Candida cases had postmortem evidence of pneumonia, a clinical entity that is very rare and therefore underappreciated but which warrants increased attention.3,4,41,45 Our study found 1 case of disseminated coccidioidomycosis, which was likely related to reactivation of latent infection. Any prior history of coccidioidomycosis warrants antifungal prophylaxis post-HCT and clinicians should remain cognizant of the possibility of recrudescence despite prophylaxis.46-48
Our study found only 2 cases of missed parasitic diseases, including 1 case of disseminated S stercoralis and 1 case of disseminated T gondii. Risk factors for S stercoralis infection, such as prior military service or residence in endemic areas, and the parasite’s ability to remain undetected for decades are frequently underrecognized by clinicians; consequently, pre-HCT screening is not performed as frequently as indicated.13-15,49 Immunosuppressive medications, especially corticosteroids, can accelerate the autoinfection cycle of S stercoralis and increase the risk of hyperinfection syndrome.13 The clinical manifestations of hyperinfection syndrome can be variable but the development of respiratory failure, acute respiratory distress syndrome, DAH, and/or diffuse bilateral ground-glass opacities on chest CT scan in a patient with epidemiologic risk factor(s) for S stercoralis and who is receiving corticosteroids should heighten clinical suspicion for this clinical entity. Hyperinfection syndrome is almost universally fatal but is potentially preventable by appropriate screening and treatment.13,50,51 T gondii can also remain latent for years and subsequently reactivate post-HCT, potentially involving multiple organs including the lungs, heart, liver, adrenal glands, eyes, and brain.52-56 Therefore, pre-HCT recipient screening for T gondii immunoglobulin G should be performed routinely. Transmission of T gondii from an acutely infected donor to a seronegative HCT recipient is theoretically possible but has not been reported.57 Post-HCT reactivation of T gondii is potentially preventable with appropriately dosed TMP-SMX prophylaxis, whereas atovaquone should not be considered fully protective in this patient population because breakthrough infections have been previously reported.53-56
There were only 5 cases of missed bacterial diseases, most likely related to the fact that bacterial infections are relatively easier to diagnose and many of these HCT recipients received empirical or targeted broad-spectrum antibacterial therapy, especially if they were critically ill. Interestingly, there were 2 missed cases of Enterococcus pneumonia. Similar to Candida pneumonia, this is also a very rare cause of pneumonia that warrants increased attention.58-60 A positive respiratory culture for Enterococcus should not be disregarded in an HCT recipient whose respiratory status is declining while on antimicrobial therapy lacking antienterococcal activity. There were also 2 cases of missed bacterial infections without microbiological species identification (1 case of endocarditis due to gram-positive cocci and 1 case of peritonitis due to gram-negative bacilli) but neither patient received antibacterial therapy that would be considered appropriate for that indication.
Discrepancies between premortem clinical diagnoses and postmortem autopsy diagnoses were classified using the Goldman criteria.2 Previous studies similar to ours used the same classification system that has remained the standard since its development.61-64 Of the 4 classes, class I discrepancies are the most valuable because these were potentially preventable and present opportunities to optimize clinical judgment, decision-making, and patient outcomes. In our study, class I discrepancies (31 of 41; 75.6%) comprised a large majority and were almost exclusively fungal (14 of 41; 34.2%) and viral (12 of 41; 29.3%). This adds further evidence to a robust body of literature highlighting the relative frequency and importance of fungal and viral diseases in HCT recipients, especially with regard to their diagnostic and therapeutic challenges and their associated mortality.1,21,23,25,26,42-44,61-63,65,66 Our study strongly challenges another study’s conclusion that autopsies rarely provided missed diagnoses that would alter the management of subsequent patients.64 In our experience, many valuable lessons can be learned from autopsies and suggest that improved autopsy rates would likely yield important information that could help advance the field and improve patient outcomes.
Our study has several identifiable limitations. First, our reliance on clinician documentation is subject to error. Second, our center is a high-volume academic HCT center and so our study’s patient population and single-center design limit generalization of our findings to other patient populations. Third, patients in the earlier part of the study period were more likely to be excluded because their medical record was more likely to have insufficient clinical data available for review. Therefore, missed infections in the early study period and trends in infections over time could not be reliably evaluated. Fourth, our study did not account for the changes in the practice of HCT over time (including preparative regimens and graft types, the increasing use of HCT in older and frailer patients, and advances in GVHD prevention and treatment), developments of newer diagnostic assays, as well as improvements in antimicrobial prophylaxis and treatment strategies, which may have influenced premortem and postmortem diagnoses. Fifth, the overall low autopsy rate may have also introduced bias, as patients who underwent autopsy may not be fully representative of the patient population as a whole. Sixth, the Goldman criteria introduced an unavoidable element of subjectivity, which we sought to address with 2 independent physician reviews.
In conclusion, autopsies of HCT recipients frequently identify clinically significant infectious diseases that were not suspected premortem. Had these infections been suspected, a change in management might have improved patient survival in many of these cases. Our study reinforces the immense educational value of the autopsy, which is underutilized and should be performed regularly to help improve infection-related morbidity and mortality.
Acknowledgments
The authors extend their deepest gratitude to Linda Elder, Meredith West, and D. Kathryn Tierney for their contributions to this study and to Janice Brown for critical review of this manuscript.
Authorship
Contribution: A.M., L.S.A., T.W., R.A.S., D.J.E., A.R.R., and D.Y.H. conceived and designed the work and approved the final version of the manuscript to be published; A.M., L.S.A., T.W., R.A.S., and D.J.E. collected the data; A.M., L.S.A., T.W., R.A.S., A.R.R., and D.Y.H. analyzed the data; A.M. and L.S.A. drafted the manuscript; and T.W., R.A.S., D.J.E., A.R.R., and D.Y.H. critically reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dora Y. Ho, Division of Infectious Diseases and Geographic Medicine, Department of Medicine, School of Medicine, Stanford University, 300 Pasteur Dr, Lane Building L-135, Stanford, CA 94305; e-mail: doraywho@stanford.edu.
References
Author notes
For original data, please contact doraywho@stanford.edu.