Key Points
There is a high prevalence of autoimmune markers in patients with ITP.
An increased risk of thrombosis was observed in patients with ITP with a positive lupus anticoagulant or antinuclear antibody.
Abstract
Prior studies have demonstrated an increased prevalence of autoimmune markers in patients with immune thrombocytopenia (ITP). Clinical experience has suggested that there may be an association between autoimmune markers and poor outcomes in ITP, but current guidelines do not encourage routine testing in these patients. We retrospectively assessed the prevalence of autoimmune markers in adult patients with ITP from our institutional database and used multiple logistic regression analyses to test for an association between autoimmune marker positivity and thrombotic events or clinical remission. We also assessed whether positivity for common autoimmune markers was associated with positivity for platelet autoantibodies. There was a high rate of autoimmune marker positivity in this population, with antinuclear antibody (65%), antithyroid peroxidase antibody (31%), and direct antiglobulin (29%) the most commonly found. Antithyroid peroxidase antibody positivity was associated with a lower probability of remission (odds ratio [OR], 0.26; 95% confidence interval [CI], 0.09-0.79; P = .017). Lupus anticoagulant positivity was associated with a higher rate of thrombosis (OR, 8.92; 95% CI, 1.94-40.95; P = .005), and antinuclear antibody was strongly associated with thrombosis (P = .001). There was no relation between platelet autoantibody positivity and the presence of autoimmune markers. These results suggest that many patients with ITP have a state of immune dysregulation that extends beyond platelet autoantibodies and that certain autoimmune markers may be prognostically useful in this disorder.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disorder resulting from decreased platelet production and increased platelet destruction. Platelet autoantibodies in ITP lead to both acceleration of platelet destruction in the spleen and inhibition of platelet production by bone marrow megakaryocytes.1 Over recent years, there has been increasing recognition that the pathogenesis of this disease involves complex and global immune dysregulation. There is mounting evidence that an imbalance between autoreactive and protective T-cell subsets is an important driver of ITP pathogenesis.2 In addition to bleeding risks, patients with ITP are at increased risk of thrombosis, and efforts are ongoing to understand the pathophysiology of this thrombotic risk.3
Although current guidelines recommend against routine testing of patients with ITP for autoimmune markers in the absence of disease-specific symptoms, there is general recognition that they frequently have autoantibodies associated with other autoimmune disorders in the absence of any clinical evidence of these disorders.4,5 A number of other studies have suggested that patients with ITP have a higher prevalence than the general population of positive autoimmune markers, including antinuclear antibody (ANA), rheumatoid factor (RF), anticardiolipin antibodies (ACL) immunoglobulin G (IgG) and immunoglobulin G (IgM), red blood cell direct antiglobulin test (DAT), antithyroid peroxidase antibodies (anti-ThyPeroxAb), and lupus anticoagulant (LAC).6-13
Since the early 1990s, patients with ITP presenting to our ITP Center have been tested for autoimmune markers for diagnostic purposes and to evaluate the autoimmune phenotype of the patient and any potential relationship with clinical ITP outcomes. In this study, we examined the frequency of autoimmune marker positivity for a wide variety of autoantibodies in these patients with ITP and evaluated for a relation between autoimmune marker positivity and rates of remission and thrombosis. We also assessed for a relation between the presence of autoimmune markers and platelet autoantibodies.
Methods
Patients and data collection
This study was approved by the Institutional Review Board (approval 2015P000152) of Massachusetts General Hospital (MGH). The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All of the authors contributed to the data analysis. A retrospective review was performed of patients with ITP presenting to our center from 1 January 1992 to 1 December 2015 who had at least 1 of the following autoimmune markers measured: ANA, red blood cell DAT, antiThyPeroxAb, anticardiolipin IgM, ACL IgG, RF, and LAC. These patients with ITP had been tested for autoimmune markers, given the hypothesis that there may be an autoimmune phenotype associated with ITP, not for suspicion of other underlying diseases. Results of platelet autoantibody assays were also collected if available. In addition to laboratory information, demographics, and clinical information, including age, sex, and date of initial clinic evaluation were collected. The diagnosis of ITP was made on the basis of the 2011 American Society of Hematology (ASH) Clinical Practice Guidelines. Evaluation included, at a minimum, a detailed history and physical examination, a complete blood count with differential, and hepatitis C virus and HIV testing. Patients were required to have primary ITP and to satisfy the 2011 ASH Clinical Practice Guidelines for diagnosis for inclusion in the study.5 Patients were excluded from the analysis if they carried a diagnosis of systemic lupus erythematosus, rheumatoid arthritis, or antiphospholipid antibody syndrome at the time of ITP diagnosis or at any point during follow-up.
The start date of the follow-up period was defined as the date of the first hematology clinic visit to MGH. The end date of the follow-up period was defined as the date of the most recent platelet count, hematology clinic visit, or primary care clinic visit at MGH. A panel of autoimmune markers was commonly ordered for each ITP patient at the time of the initial clinic visit, unless the tests had been performed previously in our system.
Remission status was assessed at the most recent follow-up visit. Remission was defined as a platelet count greater than 30 × 109/L in a patient who was off all ITP treatment and who had not undergone splenectomy.14 Patients not fulfilling these criteria at the most recent follow-up visit were considered to have ongoing disease. Thrombotic events included deep venous thrombosis (DVT), pulmonary embolism (PE), embolic stroke, myocardial infarction (MI), or lower extremity (LE) arterial thromboembolism. A thrombotic event was considered to have occurred if an event was documented in a physician note and/or if there was radiographic evidence on a relevant radiographic study in the MGH electronic medical record system.
Laboratory studies
Autoimmune markers were measured according to standard methods at MGH, unless otherwise indicated in the next paragraph. If a marker was measured more than once during a patient’s course, the result was considered positive for our analysis if the result was ever positive.
ANA assays were performed by indirect immunofluorescence assay, using Hep-2 cells (Zeus Scientific, Inc, Branchburg, NJ). All samples were screened at 1:40 and 1:160 dilutions. Samples that were positive on screening were titrated at 1:320, 1:640, 1:1280, 1:2560, and 1:5120, and the end titer was reported. Positive results for ANA were defined as a titer of ≥1:40. If the ANA titer was measured more than once, the highest titer was included in this analysis. Anti-ThyPeroxAb assays were performed at Quest Diagnostics (Secaucus, NJ) using an immunoassay method. RF markers were measured with an immunoturbidimetric assay on a Cobas C502 platform (Roche, Basel, Switzerland). Positive results for ACL were defined as >15 for IgG phospholipid units or IgM phospholipid (MPL) units. Glycoprotein-specific direct platelet autoantibody testing was performed with the PakAuto assay (Immucor, Brookfield, WI).
Data analysis and statistical testing
To assess for a relation between immune markers and thrombosis or remission, multiple logistic regression analyses were performed, with age, sex, splenectomy status, and immune marker (ANA, LAC, ACL, anti-ThyPeroxAb, RF, or DAT) as independent variables and thrombosis or remission status as the dependent variable. An additional logistic regression analysis was performed to assess for a relation between autoimmune markers and presence of platelet autoantibodies. Splenectomized patients were excluded from remission analysis. Because of the limitations of regression analysis, a χ2 test of independence was also performed to assess the relationship between ANA and thrombosis. Kaplan-Meier (KM) survival analyses using log-rank testing were performed to evaluate thrombosis-free survival to control for the various durations of patient follow-up. Regression analyses were performed with Stata version 14.2 (StataCorp, LLC, College Station, TX), and KM survival curves were generated and analyzed with Prism version 8.0.2 (Graph Pad Software, Inc, La Jolla, CA).
Results
Patient characteristics
Four hundred fifty unique patients had clinic visits for ITP between 1992 and 2015. Of those, 157 patients met ASH criteria for ITP,5 did not have secondary ITP, and had testing for at least one of the following autoimmune markers: ANA, DAT, anti-ThyPeroxAb, ACL IgM, ACL IgG, RF, and LAC. One hundred nineteen (76%) of the patients had autoimmune marker testing within 90 days of the initial ITP clinic visit, 38 (24%) had autoimmune marker testing >90 days before or >90 days after the initial ITP clinic visit, and 33 of them also had glycoprotein-specific direct platelet autoantibody testing performed. The mean ± standard deviation age at initial evaluation was 48 ± 18 years, and 95 (60%) were female. At the time of initial evaluation, 77 (49%) were on therapy for ITP, and 23 (15%) had already undergone splenectomy. The mean ± standard deviation duration of follow-up was 5.1 ± 4.0 years. Of the 157 patients, 35 had all 7 autoimmune markers measured, 46 had 6, 36 had 5, 10 had 4, 12 had 3, 12 had 2, and 6 had only 1.
Prevalence of autoimmune markers
There was a high rate of autoimmune marker positivity in this population (Table 1). Of all 157 patients, 121 (77%) were positive for ≥1 of the tested autoimmune markers, and 36 (23%) had negative results for all autoimmune markers tested. Of 35 patients who had all 7 autoimmune markers tested, only 4 (11%) had no positive marker. Twelve of those 35 patients (34%) were positive for only 1 marker, 54% were positive for ≥2 markers, and 23% were positive for ≥3 markers. One patient of 35 (3%) was positive for all 7 markers.
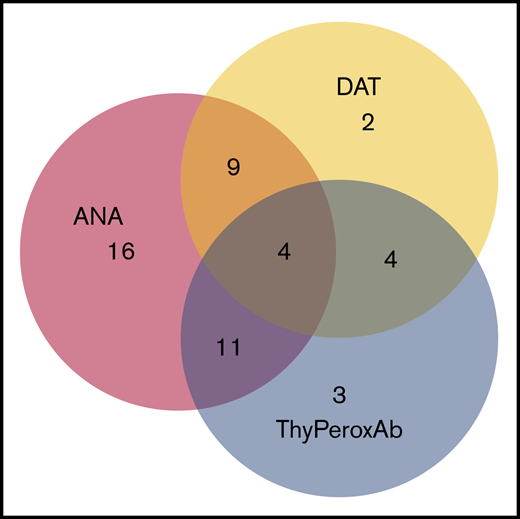
The most prevalent positive autoimmune markers were ANA (65%), anti-ThyPeroxAb (31%), and DAT (29%). Of the 67 subjects who had all 3 of those markers measured, 49 (73%) had ≥1 marker positive. There was significant overlap of the 3 positive markers: 28 of 49 (57%) had >1 positive autoimmune marker (Figure 1).
Overlap of autoantibody positivity. There were 67 patients in whom ANA, DAT, and ThyPeroxAb were all measured. Forty-nine of these patients had at least 1 positive autoantibody and are depicted in this figure; 18 were negative for all 3 autoantibodies.
Overlap of autoantibody positivity. There were 67 patients in whom ANA, DAT, and ThyPeroxAb were all measured. Forty-nine of these patients had at least 1 positive autoantibody and are depicted in this figure; 18 were negative for all 3 autoantibodies.
The sex of the ANA+ patients did not differ from that of the overall cohort; of the 94 patients who were ANA+, 58 (62%) were female. ANA titers were 1:40 in 28 of 94 (30%) ANA+ patients, 1:80 in 5 (5%), 1:160 in 34 (3%), 1:320 in 8 (9%), 1:640 in 8 (9%), 1:1280 in 5 (5%), 1:2560 in 2 (2%), and >1:5120 in 4 (4%). Of the 144 patients who were tested for ANA, 66 (45%) had a titer of ≥1:80, and 61 (41%) had a titer of ≥1:160.
None of the patients with a positive anti-ThyPeroxAb had clinically apparent thyroid disease at initial evaluation or during study follow-up. Of the 35 patients with a positive DAT, only 5 (14%) had clinically defined warm autoimmune hemolytic anemia.
ACL IgM was found in 16% of the patients and ACL IgG in 13%. RF was positive in 8% of the patients and LAC in 11%.
Platelet autoantibody assay results were available for 33 patients, 26 of whom had active ITP at the time of platelet autoantibody assay. (Seven patients were in full clinical remission with normalized platelet counts.) Of the patients with active ITP, 73% had a positive platelet autoantibody assay.
Association of autoimmune markers with thrombosis
Thrombosis occurred in 19 (12%) of the 157 patients evaluated in this study at any point in their course (Table 2). Twelve patients had venous events only, 3 had arterial events only, and 4 had both arterial and venous thrombotic events. Thirteen patients had a thrombosis during the period of study follow-up (a total of >806 patient-years of follow-up), making the event rate 1.6 thrombotic events per 100 patient-years of follow-up. Multiple logistic regression models including age, sex, splenectomy status, and autoimmune markers demonstrated a statistically significant predictive relation between LAC and thrombosis (odds ratio [OR] of positive LAC for thrombosis, 8.92; 95% confidence interval [CI], 1.94-40.95; P = .005). Logistic regression modeling was not possible for ANA testing because of the perfect prediction of this variable in the model (all patients with thrombosis were ANA+), and χ2 testing was therefore performed to assess the association between ANA positivity and thrombosis, which demonstrated a statistically significant association (χ2 = 10.25; P = .001). There was no statistically significant difference in the prevalence of thrombosis in patients with high-titer ANA (1:160 or higher) vs low-titer ANA (1:80 or 1:40; supplemental Table 1). There was also no statistically significant difference in the prevalence of thrombosis in patients with high-titer vs low-titer ACL (supplemental Tables 2 and 3). Modeling did not demonstrate a predictive relation between ACL, anti-ThyPeroxAb, DAT, RF, or platelet autoantibodies, and thrombosis. Modeling additionally demonstrated a statistically significant predictive relation between age and thrombosis (OR of thrombosis, 1.03; 95% CI, 1.01-1.07, per additional year of age, P = .02). Of note, in the patients who underwent splenectomy, the splenectomy nearly always occurred before measurement of the autoimmune markers.
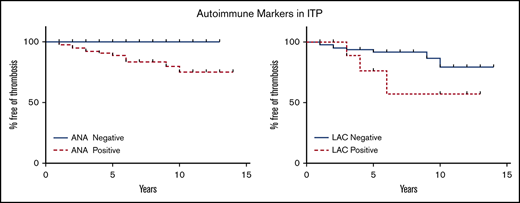
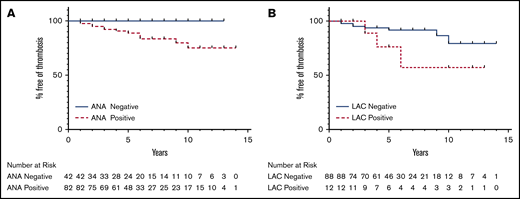
A KM survival analysis comparing ANA+ and ANA− patients showed a significant difference in the rates of thrombosis-free survival (P = .02; Figure 2A). A KM survival analysis comparing LAC+ patients with LAC− patients did not show a statistically significant improvement in thrombosis-free survival with negative LAC (P = .15; Figure 2B).
KM curves of thrombosis-free survival. (A) There is a statistically significant difference in thrombosis-free survival between ANA+ and ANA− patients (P = 0.02). (B) The difference in thrombosis-free survival between LAC+ and LAC– patients was not statistically significant (P = .15).
KM curves of thrombosis-free survival. (A) There is a statistically significant difference in thrombosis-free survival between ANA+ and ANA− patients (P = 0.02). (B) The difference in thrombosis-free survival between LAC+ and LAC– patients was not statistically significant (P = .15).
Association of autoimmune markers with remission
At the time of most recent follow-up, 78 (50%) of the 157 patients were in remission. Multiple logistic regression models including age, sex, and autoimmune markers demonstrated a statistically significant inverse predictive relation between anti-ThyPeroxAb positivity and remission (OR of positive anti-ThyPeroxAb for remission, 0.26; 95% CI, 0.09-0.79; P = .017). Modeling did not demonstrate a predictive relation between ANA, LAC, ACL, DAT, RF, or platelet autoantibodies and remission.
Association of autoimmune markers with platelet autoantibodies
Multiple logistic regression models including age, sex, splenectomy status, and autoimmune markers did not demonstrate a significant predictive relation between platelet autoantibody positivity and any of the autoimmune markers tested.
Discussion
These results demonstrate that most patients with ITP have additional autoantibodies present and suggest a wider immune dysregulation associated with the presence of an autoimmune phenotype in these patients. Our findings confirm other studies that have shown a higher prevalence of autoimmune markers in patients with ITP than in the general population,6-11 but have gone on to assess the association of these autoimmune markers with subsequent rates of thrombosis and remission.
The high rate of ANA positivity is particularly striking: 65% of the patients with ITP in this study had a positive ANA of any titer, and 44% had an ANA titer of ≥1:80. The percentage of ANA+ patients in our study population was higher than that observed in other ITP cohorts, where rates of ANA positivity have been reported to be ∼13% to 30%, similar to reported rates of ANA positivity in the general population.6-8,15,16 The differences in rate of ANA positivity across studies is most likely related to the, at times, low sample sizes, differences in ANA assay methodologies, and/or differences in the patient populations under study (especially considering use of the ASH Guidelines for characterizing the patients in our cohort). None of our patients had a diagnosis of lupus.
Of the patients with ITP in this cohort, 29% had a positive DAT, in line with a prior report that found a 20% rate of DAT positivity in a cohort of adult patients with ITP.7 In contrast, 0.1% of healthy blood donors and 1% to 15% of hospitalized patients have a positive DAT without evidence of hemolysis.7,17,18 Only 14% of DAT+ patients in our cohort showed evidence of overt hemolysis. Of note, none of the DAT+ patients in our cohort experienced a thrombotic event.
Anti-ThyPeroxAb was positive in 31% of the patients. This result is similar to the prevalence of anti-ThyPeroxAb in a study of pediatric patients with ITP: 38% of acute pediatric patients with ITP and 35% of chronic pediatric ITP patients were positive for this autoantibody.12 In that same study, the prevalence of anti-ThyPeroxAb in healthy pediatric controls was 2.8%; others have found the prevalence of anti-ThyPeroxAb in the healthy adult population to be as high as 12% to 15%.19
RF was positive in only 9% of patients in this cohort, compared with a 4% RF positivity rate in a prior adult ITP cohort.7 The prevalence of positive RF in healthy subjects has been found by in other studies to be ∼4%.20
Antiphospholipid antibodies have been reported to be present in 25% to 75% of primary patients with ITP.11 In our population, 16% of the patients were positive for ACL IgM, 13% for ACL IgG, and 11% for LAC. The threshold for a positive LAC or ACL antibody result at our institution is defined as above the 95th percentile of normal, so one would expect ∼5% of healthy patients to have a positive result. For comparison, 20% to 53% of patients with antiphospholipid antibody syndrome have thrombocytopenia.21
A key question that we had in undertaking this analysis was whether the presence of ≥1 of these autoimmune markers affects rates of remission or thrombosis. Modeling controlling for age and sex of subjects found that anti-ThyPeroxAb positivity was associated with a lower remission rate. A study examining anti-ThyPeroxAb positivity and autoimmune thyroiditis in pediatric patients with ITP did not find a relation between the presence of autoimmune thyroiditis and the likelihood of progressing to chronic ITP.22 Given our findings, however, the prognostic significance of anti-ThyPeroxAb in adult patients with ITP warrants further investigation. Our finding suggests that patients who have positive anti-ThyPeroxAb may have a unique and potentially more severe autoimmune phenotype than other patients with ITP. Further biological study will be important for delineating in what ways the autoimmune dysregulation may differ among this subset of ITP patients.
In consideration of thrombosis, prior reports have yielded conflicting results regarding the association of antiphospholipid antibodies with thrombotic risk in patients with ITP.23 A meta-analysis of the risk of thrombosis associated with antiphospholipid antibodies in primary ITP revealed a significant association between the presence of either LAC or ACL antibodies and the occurrence of thrombosis.11 The presence of LAC was more strongly associated with thrombosis than ACL, with an OR for thrombosis of 6.11.11 Multiple logistic regression analysis in our study similarly revealed a statistically significant increased probability of thrombosis in patients who are LAC+ (OR for thrombosis, 8.92), while controlling for age, sex, and splenectomy status, all highly relevant potential confounding variables for thrombotic risk. Most likely the reason that the KM analysis of LAC+ vs LAC− patients did not reveal a statistically significant difference in thrombosis-free survival in the 2 groups is that this analysis did not control for these 3 important potential confounding variables. Unlike the prior study, we did not find a predictive relation between ACL positivity and thrombosis.
We did, however, find a significant association between ANA positivity and thrombosis risk. To our knowledge this is the first such evidence for this association in patients with ITP. A recent study comparing ANA positivity in non-ITP patients with venous thromboembolism found a fivefold higher prevalence of ANA positivity in patients with venous thromboembolism than in controls.24 The thrombosis rate of 1.6 events per 100 patient-years in our study is in line with results from a recent national database study of patients with ITP that showed rates of 1.50 and 1.47 per 100 patient-years in France and Sweden, respectively.25 Of note, these results are lower than what was found in another recent study that reported 5.5 events per 100 ITP patient years.26
Of diagnostic interest is whether any of the common autoimmune markers was predictive of platelet autoantibody positivity. We did not find such a relation in this study, although our analysis was limited by the small number of patients who had platelet autoantibody testing performed.
This study has several notable strengths. The study population was a large cohort of patients with ITP who had been diagnosed by ASH criteria, had been followed at a single institution with a standard care algorithm, and had a relatively long follow-up. There are also several important limitations of our study. There was a lack of homogeneity in testing for autoimmune markers and platelet autoantibodies, such that all patients were not tested for every marker and for platelet autoantibodies. The retrospective nature of the analysis is an additional limitation, as it is possible that some thrombotic events occurred during the follow-up period, but were not reported. Variable lengths of follow-up could have affected the thrombosis analysis, which prompted us to perform KM survival analyses to reduce bias introduced by variable lengths of follow-up by censoring data from patients once they have been lost to follow-up.
Although we studied a large cohort of patients with ITP, especially given that this is a relatively rare disease, another limitation of this study is that it was potentially underpowered to detect some associations. For example, prior studies have revealed an association between thrombosis and splenectomy.27 We may not have detected this association, given the lack of power and the relatively few patients that our ITP Center recommends for splenectomy, and we therefore cannot draw conclusions about the lack of association here. However, the positive associations that we observed between certain markers and thrombosis (ANA and LAC) and remission (anti-ThyPeroxAb) were statistically robust, despite the potential limitations noted above.
The findings in our study suggest that testing for ANA, LAC, and anti-ThyPeroxAb may be prognostically relevant in patients with ITP. In agreement with current ASH and international guidelines, we do not advocate for routine testing for these autoantibodies.28 However, our results support consideration of targeted testing in certain clinical scenarios. Given the reproducible increased thrombotic risk for patients with LAC and our finding of a strong association between ANA and thrombosis, it would be prudent to test for these markers in patients being considered for thrombopoietin receptor agonist (TPO-RA) therapy. Other studies have suggested routine screening for antiphospholipid antibodies before initiation of TPO-RA therapy; our results support testing for LAC and ANA, as well, in this context.29 Although prior studies in patients with ITP receiving TPO-RAs have not reported any increased frequency of LAC or ANA in patients who have developed thrombosis, these studies are imperfect, in that testing was not performed in most individuals. In addition, many trials of TPO-RAs in ITP patients have excluded patients with known risk factors for thromboembolic disease, which may have influenced the prevalence of thrombotic events in these studies.30 If anti-ThyPeroxAb are confirmed to predict reduced remission rates in patients with ITP, this marker may be useful in adjudicating therapy selection in the second line and beyond, including when splenectomy may be more appropriate.
In summary, our data suggest that there is a high prevalence of autoimmune marker positivity in patients with ITP, that anti-ThyPeroxAb positivity may predict a lower probability of remission, and that testing for ANA and LAC may be useful in identifying patients with ITP at increased risk of thrombosis. The frequency of these antibodies suggests a broad immune dysregulation in ITP that may extend beyond simple platelet destruction. Additional study is necessary to confirm our findings and further examine the pathophysiology and significance of autoimmune markers in ITP.
Acknowledgments
This work was supported by a Stanford Chemistry, Engineering, and Medicine for Human Health Physician-Scientist Research Fellowship (M.A.H.). H.A.-S. is the recipient of the National Hemophilia Foundation-Shire Clinical Fellowship Award, which provides partial salary support.
Authorship
Contribution: M.A.H. collected and analyzed data, created the tables and figures, wrote and revised the manuscript, and designed the study; H.A.-S. collected and analyzed the data and wrote and revised the manuscript; and D.J.K. collected the data, critically revised the manuscript, and supervised and designed the study.
Conflict-of-interest disclosure: H.A.-S. is a consult to Agios; D.J.K. received research funding from Protalex, Bristol-Myers Squibb, Rigel, Novartis, Agios, Alexion, Principia, and Alnylam and was a consultant to ONO, Pfizer, 3SBios, Eisai, GlaxoSmithKline, Genzyme, Amgen, Shionogi, Rigel, Alexion, MedImmune, Alnylam, Shire, Novartis, Bioverativ, Argenx, and Zafgen. M.A.H. declares no competing financial interests.
Correspondence: Marie A. Hollenhorst, Division of Hematology, Stanford Hospital and Clinics, 875 Blake Wilbur Dr, Stanford, CA 94305-5820; e-mail: marie.hollenhorst@stanford.edu.
References
Author notes
For original data, please contact the corresponding author.
The full-text version of this article contains a data supplement.