Key Points
MRD response has value as a prognostic factor for blinatumomab treatment in R/R B-cell precursor ALL.
MRD response was associated with better outcomes in terms of OS and RFS in blinatumomab-treated R/R ALL.
Abstract
Minimal residual disease (MRD), where leukemic cell levels are lower than the morphologic detection threshold, is the most important prognostic factor for acute lymphoblastic leukemia (ALL) relapse during first-line chemotherapy treatment and is standard of care in treatment monitoring and decision making. Limited data are available on the prognostic value of MRD response after relapse. We evaluated the relationship between MRD response and outcomes in blinatumomab-treated adults with relapsed/refractory (R/R) B-cell precursor ALL. Of 90 patients with complete remission (CR) or CR with partial hematologic recovery (CRh), 64 (71.1%) achieved a complete MRD response (no detectable individual rearrangements of immunoglobulin/T-cell receptor genes by polymerase chain reaction [PCR] at a minimum sensitivity level of 10−4). Eleven patients had MRD <10−4. Therefore, overall, 75 (83.3%) experienced an MRD response (no detectable MRD or detectable MRD) measured by PCR within the first 2 treatment cycles. Overall survival (OS) and relapse-free survival (RFS) were significantly longer in patients who achieved CR/CRh and MRD response (median, 20.6 and 9.0 months, respectively) compared with CR/CRh patients without MRD response (median, 12.5 and 2.3 months, respectively). In conclusion, longer durations of OS and RFS associated with MRD response support the value of achieving MRD response and its use as a prognostic factor for blinatumomab treatment in R/R ALL. This trial was registered at www.clinicaltrials.gov as #NCT01466179.
Introduction
Current treatments for acute lymphoblastic leukemia (ALL) result in complete remission (CR) in 80% to 90% of newly diagnosed adults and considerable improvement in overall outcomes.1,-3 After relapse, outcomes are poor. Median survival time in first relapse after standard chemotherapy ranges from 4.5 to 6.3 months, and 5-year overall survival (OS) ranges from 7% to 10%.4,-6
In a phase 2 study of blinatumomab in patients with relapsed/refractory (R/R) B-cell precursor ALL (BCP-ALL), 43% of patients achieved CR or CR with partial hematologic recovery (CRh) after 2 cycles.7 These results were confirmed in a phase 3 study in which 44% of blinatumomab-treated patients attained CR/CRh within 12 weeks, compared with 25% treated with standard-of-care chemotherapy (P < .001).8 Nevertheless, with successive relapses, the percentage of patients who achieve CR decreases and OS becomes shorter.9,10
Minimal residual disease (MRD) has been shown to be 1 of the strongest predictors of outcome after first-line chemotherapy for ALL.3,11,12 However, the prognostic value of MRD may be different in patients with R/R ALL. Studies of pediatric patients with ALL in first relapse have shown a statistically significant association between complete MRD response and improved event-free survival,13,14 but the significance of MRD response with regard to outcomes in adult patients with R/R ALL has not been adequately studied. In the current report, MRD response in blinatumomab-treated adult patients with R/R BCP-ALL is analyzed. The relationships between MRD response and patient characteristics, hematologic response, and outcome are evaluated.
Methods
Eligibility requirements for this single-arm, open-label, phase 2 study have been described in detail previously (ClinicalTrials.gov: NCT01466179).7 Eligible patients had Philadelphia chromosome–negative BCP-ALL refractory to first salvage or recurring within 12 months of first remission or after allogeneic hematopoietic stem cell transplantation (HSCT) independent of first CR duration. Blinatumomab (9 μg for first 7 days followed by 28 μg per day in the first cycle, then 28 μg per day [target dose] thereafter, beginning from cycle 2) was administered via continuous intravenous infusion daily, with 1 cycle consisting of a 4-week treatment period followed by a 2-week treatment-free period. Patients with a response within the first 2 cycles could receive up to 3 additional cycles. Prespecified exploratory end points of rates of MRD response and complete MRD response within 2 cycles of treatment are reported here, using the full analysis set (defined as all patients who received any infusion of blinatumomab).
MRD (response and complete response) was measured by real-time quantitative polymerase chain reaction (PCR), with an assay sensitivity of ≤10−4, at a central reference laboratory (Labor für Hämatologische Spezialdiagnostik, University of Kiel, Kiel, Germany). The trial had 2 MRD response end points. Complete MRD response was defined as no detectable patient-specific rearrangements of immunoglobulin/T-cell receptor genes. MRD response also included patients with detectable leukemic cells <10−4 (ie, low positive MRD).7
Current analyses included patients who had documented CR (≤5% bone marrow blasts, no evidence of disease, platelets >100 × 109/L, and absolute neutrophil count >1000/µL) or CRh (≤5% bone marrow blasts, no evidence of disease, platelets >50 × 109/L, and absolute neutrophil count >500/µL) within the first 2 cycles and had evaluable MRD data (MRD-evaluable patients). Outcomes assessed were relapse-free survival (RFS; time from remission until documented hematologic relapse, extramedullary disease, or death resulting from any cause), OS (time from treatment start until death resulting from any cause or date of last follow-up), and duration of response (DoR; time from first onset of CR or CRh until documented relapse [hematologic or extramedullary] or death resulting from disease progression); patients with no such events were censored. RFS, OS, and DoR were calculated using Kaplan-Meier estimates with 95% confidence intervals (CIs). The influence of allogeneic HSCT on outcomes was evaluated by landmark methods.15
The protocol was approved by each center’s institutional review board or ethics committee. All patients provided written informed consent.
Results and discussion
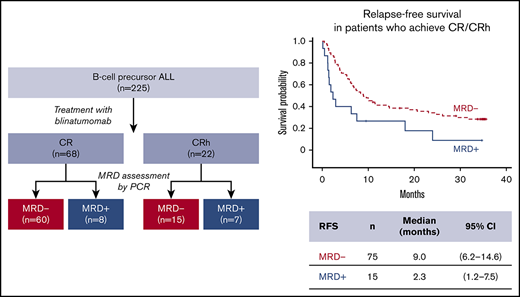
Of the full analysis set (N = 225) of blinatumomab-treated patients from the phase 2 study, 90 patients were MRD evaluable (achieved CR/CRh during the first 2 cycles and had evaluable MRD data). Among those, 24%, 46%, and 30% received blinatumomab as first, second, or later salvage treatment, respectively. All patients had either early relapse (≤12 months from diagnosis) or refractory relapse. For MRD-evaluable patients with prior allogeneic HSCT (n = 28; Table 1), 57% had experienced relapse within 12 months of HSCT.
MRD response was achieved in 75 patients (83.3%), with 64 (71.1%) having complete MRD response during the first 2 cycles. In patients with CR, the MRD response rate was 88.2% (60 of 68), vs 68.2% (15 of 22) in those with CRh. Among MRD responders with on-study HSCT (patients were allowed to undergo allogeneic HSCT at the investigator’s discretion postblinatumomab treatment; n = 39), 14 patients relapsed and 10 died. Among 64 complete MRD responders, 29 (45.3%) underwent on-study HSCT while in CR.
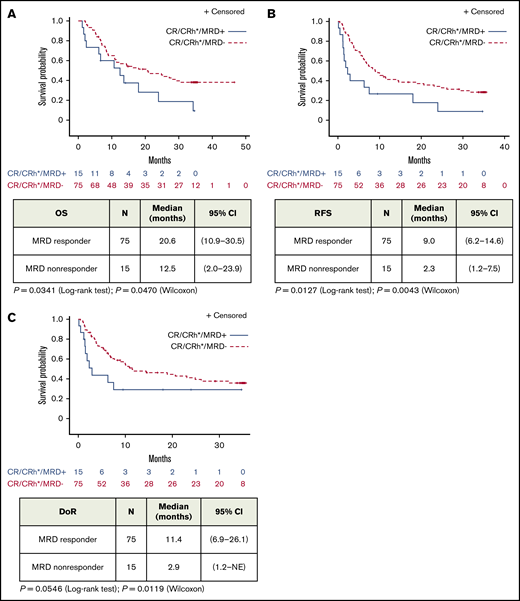
Median OS was 20.6 (95% CI, 10.9-30.5) months for MRD responders (n = 75) and 12.5 (95% CI, 2.0-23.9) months for MRD nonresponders (n = 15; Figure 1A). Median RFS was 9.0 (95% CI, 6.2-14.6) months for MRD responders and 2.3 (95% CI, 1.2-7.5) months for MRD nonresponders (Figure 1B). Similarly, MRD responders had a significantly longer median DoR than MRD nonresponders: 11.4 (95% CI, 6.9-26.1) months vs 2.9 (95% CI, 1.2 to not estimable) months, respectively (Figure 1C). MRD responders demonstrated longer OS with allogeneic HSCT, but this observation was not statistically significant (supplemental Figure 1). No significant differences in adverse events were observed (supplemental Table 1).
Association of MRD response with outcome in patients with BCP-ALL after treatment with blinatumomab (full analysis set). Kaplan-Meier plots of OS (A), RFS (B), and DoR (C) in patients with hematologic CR/CRh and evaluable MRD assessments by MRD response (MRD-evaluable patients, n = 90). NE, not estimable.
Association of MRD response with outcome in patients with BCP-ALL after treatment with blinatumomab (full analysis set). Kaplan-Meier plots of OS (A), RFS (B), and DoR (C) in patients with hematologic CR/CRh and evaluable MRD assessments by MRD response (MRD-evaluable patients, n = 90). NE, not estimable.
Among patients with CR with <50% bone marrow blasts at baseline, 91.1% (41 of 45; 95% CI, 78.8%-97.5%) experienced MRD response, as did 75.6% (34 of 45; 95% CI, 60.5%-87.1%) of patients with CR with ≥50% bone marrow blasts. A majority of MRD-evaluable patients with CR achieved MRD response regardless of the number of previous lines of therapy (Table 1), and the proportion of patients achieving MRD response was similar for patients with (78.6%) and without (85.5%) prior allogeneic HSCT.
This is the largest study to date using a central laboratory PCR–based MRD assessment in adult patients with R/R ALL. In the current study, 83% of patients with CR/CRh experienced MRD response, and 71% achieved complete MRD response. As expected, slightly smaller proportions of patients with CRh and blast-free bone marrow achieved MRD response compared with patients with CR. Notably, MRD response was associated with better outcomes within the group of patients with CR/CRh. Among patients who achieved CR/CRh, patients with MRD response had longer OS and RFS compared with patients without MRD response (Figure 1). This result was observed in a population of patients with overall poor prognosis.
In this study, MRD response was shown to be an important factor in maintaining remission in patients with R/R ALL, irrespective of prior HSCT or number of lines of prior therapy. Although the importance of early salvage status for achievement of MRD remission was recently demonstrated,16 blinatumomab was effective in this analysis in inducing MRD response in patients with multiple prior lines of therapy; 77% of patients who had ≥3 lines of prior therapy achieved MRD response, and 54% achieved complete MRD response (Table 1; supplemental Figure 2). More than 78% of patients with or without prior allogeneic HSCT achieved MRD response, which supports the concept that MRD is an independent prognostic factor that can identify "good-risk" patients.17 Although not statistically significant, we also observed that patients with ≥50% bone marrow blasts had a lower rate of MRD response than those with <50% bone marrow blasts, which is consistent with the observation that patients with higher bone marrow blasts show a poorer response to blinatumomab.8 MRD response was also observed in 7 patients with hypoplastic or aplastic bone marrow, patients who were not considered responsive to blinatumomab. Additional studies are needed to assess the significance of MRD response in these patients.
In conclusion, MRD response was associated with better outcomes in terms of OS and RFS, supporting the value of achieving MRD response in adults with R/R ALL treated with blinatumomab.
Acknowledgments
The authors thank Geoff Smith (Amgen, Inc., Thousand Oaks, CA) and Bryan Thibodeau (Fishawack Communications, Inc.) for medical writing assistance in the preparation of this manuscript, which was funded by Amgen, Inc.
This study was supported by research funding from Amgen, Inc.
Authorship
Contribution: N.G., M.B., R.C.B., G.Z., D.N., and M.S.T. conceived and designed the study; N.G., H.M.K., A.S.S., R.C.B., H.D., A.K.F., L.H., F.R.-H., M.L., S.O., S.J.F., and M.S.T. were involved in patient data collection and data acquisition; N.G., H.M.K., M.B., A.S.S., R.C.B., H.D., A.K.F., G.Z., S.G., D.N., and M.S.T. contributed to the analysis and interpretation of the data; S.G. performed statistical analysis; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: N.G. received research funding from Amgen, Pfizer, and Novartis; received advisory board and speaker fees from Amgen, Pfizer, and Novartis; received travel grants from Amgen, Pfizer, and Novartis; and received advisory board honoraria from Kite/Gilead and Celgene. H.M.K. received research funding from AbbVie, Agios, Amgen, Ariad, Astex, Bristol-Myers Squibb, Cyclacel, ImmunoGen, Jazz, and Pfizer and received honoraria from AbbVie, Actinium, Agios, Amgen, ImmunoGen, Orsinex, Pfizer, and Takeda. M.B. received research funding from Amgen; received reference diagnostics from Affimed, Amgen, and Regeneron; received consulting fees from PRMA Consulting, Ltd; and received speaker fees from Amgen, Hoffman-La Roche, Incyte, and Pfizer. A.S.S. received speaker fees from Amgen and Celgene. R.C.B. received fees for advisory activities from Amgen, AstraZeneca, Cellex, GEMoaB Monoclonals, GmbH, Molecular Partners, Novartis, and Pfizer; and has a blinatumomab patent with royalties paid. H.D. received research funding from Amgen, Incyte/Ariad, Novartis, Pfizer, and Servier; received consultancy fees from Amgen, Celgene, Cellectis, and Pfizer; received advisor honoraria from Amgen, Celgene, Cellectis, Incyte/Ariad, Novartis, Pfizer, Servier, and Shire/Baxalta; and received speaker fees from Amgen, Celgene, Incyte/Ariad, and Pfizer. L.H. received institutional research funding from AbbVie, ADC Therapeutics, Astex Pharmaceuticals, Genentech, and Pharmacyclics and received personal fees from Pharmacyclics. F.R.-H. received advisory board fees from Amgen, Bristol-Myers Squibb, Incyte, Jazz Pharmaceuticals, Novartis, and Pfizer. M.L. received research funding from Amgen. S.O. received consultancy fees from Amgen. G.Z. is an employee of Amgen and holds several pending patents. S.G. is a former employee of Amgen. D.N. is an employee and stockholder of Amgen and holds blinatumomab-related pending patents. M.S.T. received research funding and personal fees from Amgen. The remaining authors declare no competing financial interests.
Correspondence: Nicola Gökbuget, Universitätsklinikum, Medizinische Klinik II, Hämatologie/Onkologie, Universitäres Centrum für Tumorerkrankungen, 60590 Frankfurt/Main, Germany; e-mail: goekbuget@em.uni-frankfurt.de.
References
Author notes
Qualified researchers may request data from Amgen clinical studies. Complete details are available at: http://www.amgen.com/datasharing.
The full-text version of this article contains a data supplement.