Key Points
Bortezomib with dexamethasone and low-dose lenalidomide is an active therapy for previously untreated patients with AL amyloidosis.
VRD can induce MRD-negative responses, but nonhematologic toxicity may be significant in patients with advanced disease.
Abstract
Bortezomib and dexamethasone with cyclophosphamide (CyBorD) or melphalan (BMDex) are commonly used primary treatments for light-chain (AL) amyloidosis, but limited data exist on bortezomib with immunomodulatory drug combinations. We report our experience with primary therapy with a bortezomib, lenalidomide, and dexamethasone (VRD) “light” regimen in 34 consecutive patients with AL amyloidosis. The majority (79%) had cardiac involvement, 15% and 23% were Mayo stage 3A and 3B, respectively, and 54% had renal involvement. After the first VRD cycle, 71% of patients achieved a hematologic response (44% at least very good partial response [VGPR]). On intent to treat, 11 (32%) achieved a complete response (of whom 5 of 11 were minimal residual disease [MRD] negative at 10−5), 17 (50%) a VGPR, and 2 (7%) a partial response. The 12-month survival was 73%. Starting lenalidomide dose was 5 mg in 86% of patients. Hematologic toxicity was mild; nonhematologic toxicities included rash (grade 3/4 [16%]), infections (grade ≥3 [12%]), constipation (grade ≥3 [9%]), and peripheral neuropathy (grade 2 [20%]); 37.5% of patients required lenalidomide dose reduction, 27% discontinued lenalidomide, 38% required bortezomib dose reduction, and 12% discontinued bortezomib. We compared VRD to CyBorD in 68 patients matched for Mayo stage and baseline difference between involved minus uninvolved serum free light chain levels, and observed a trend for deeper response at 3 and 6 months with VRD. In conclusion, VRD can be an active regimen for newly diagnosed patients with AL amyloidosis able to induce very deep hematologic responses at the expense of increased toxicity.
Introduction
The aim of therapy in light-chain (AL) amyloidosis is to rapidly eliminate the production of the toxic amyloidogenic light chains by targeting the plasma cell clone.1,2 Especially for patients with advanced cardiac involvement, a rapid hematologic response may be critical, while the depth of hematologic response is important in order to maximize the probability of organ function improvement and organ response.3 Chemotherapy remains the most effective therapy for AL amyloidosis through the elimination of the light-chain–producing plasma cell clone and is based on the adaptation of regimens developed for patients with myeloma.3 Bortezomib, dexamethasone, and cyclophosphamide (CyBorD)4,-6 or bortezomib, dexamethasone, and melphalan (BMDex)7 remain the most commonly used first-line treatments for patients with AL amyloidosis. However, a small retrospective study from our center indicated that in patients with AL amyloidosis, the addition of cyclophosphamide to the bortezomib/dexamethasone (VD) backbone may not improve significantly the efficacy of the regimen,8 and a better partner for VD may be needed.
In multiple myeloma (MM) patients, the combinations of bortezomib with an immunomodulatory drug (IMiD) (thalidomide [bortezomib, thalidomide, and dexamethasone]9,-11 or lenalidomide [bortezomib, lenalidomide, and dexamethasone [VRD]12,-14 ) are widely used in newly diagnosed patients and have been associated with higher response rates than bortezomib combinations without an IMiD.9,-11,15 In AL amyloidosis, the tumor burden is usually low, without adverse prognostic cytogenetic features; thus, regimens combining bortezomib with an IMiD could be particularly effective. However, thalidomide has been associated with toxicity and poor tolerability in patients with AL amyloidosis,16,,,-20 especially considering the added neurotoxicity of the bortezomib, thalidomide, and dexamethasone regimen.9,11,21 Lenalidomide is significantly less neurotoxic than thalidomide, but its use has also been associated with significant toxicity in patients with AL amyloidosis,22,,,-26 and its tolerability is poorer than in MM patients. Typically, lower doses of IMiDs are used in AL patients, either in newly diagnosed or in relapsed/refractory patients, and thus, the use of VRD as primary therapy may be challenging.
While VRD is a common first-line regimen in myeloma patients, even in older ones,12 its use in patients with AL amyloidosis is far less common, and so far, there are no published data on the efficacy and toxicity of this combination in newly diagnosed AL patients. Here, we report our experience with a VRD “light” regimen as primary therapy in consecutive patients with AL amyloidosis.
Patients and methods
After March 2017, our institution switched to a combination of bortezomib, lenalidomide, and dexamethasone as the preferable first-line therapy for patients with AL amyloidosis. The regimen included subcutaneous bortezomib at a dose of 1.3 mg/m2 on days 1, 8, 15, with lenalidomide at low doses (starting at 5 to 15 mg, according to age, cardiac, and renal function) on days 1 to 21 and dexamethasone 20 mg weekly, every 28 days, for 8 cycles (VRD regimen). The dosing and schedule was based on the available data from our patients treated with lenalidomide-based regimens26 and data from elderly myeloma patients treated with a VRD light regimen.27,28 We started at 5 mg lenalidomide in patients with any of the following: estimated glomerular filtration rate (eGFR) <50 mL/min, heavy proteinuria (≥5 g/d), and/or low serum albumin (<2.5 g/dL), age >75 years, Mayo stage 2 with N-terminal pro-B-type natriuretic peptide (NTproBNP) >4000 pg/mL, or Mayo stage 3 disease.
Until June 2018, 34 consecutive patients were treated at the Department of Clinical Therapeutics, Athens, Greece, with VRD; during the same period, 13 patients were not given VRD for reasons unrelated to their disease (logistical issues, need for dialysis, or very poor condition). A control group of patients that had been treated with VCD/CyBorD in our department between January 2013 and February 2017 were matched for Mayo stage and NTproBNP levels and baseline difference between involved minus uninvolved serum free light chain (dFLC) levels (1:2 matching, for a total of 68 subjects treated with VCD/CyBorD).
The standard and updated criteria for organ involvement and response evaluation and for hematologic response were used to assess these patients.29,,-32 The declaration of complete response (CR) required a normal free light chain (FLC) ratio and a negative immunofixation in at least 2 consecutive measurements. A rigorous assessment protocol following our standard institutional protocol for efficacy and toxicity8,26,33,-35 is followed for all patients treated for AL amyloidosis, including monthly assessment of hematologic and organ response, during the course of active therapy. Toxicity grading is following Common Terminology Criteria for Adverse Events version 4.03 criteria, according to our institution’s policy. Minimal residual disease (MRD) was assessed according to the Euroflow protocol and standards, reaching at a sensitivity of at least 10−5, as previously reported.36 The collection and analysis of the data of the patients treated with VRD has been approved by our institution’s ethics committee/scientific council (Alexandra Hospital). All patients gave informed consent for data collection and analysis.
Statistical analysis
Descriptive statistics are reported as medians with range values. All efficacy analyses are on an intent-to-treat (ITT) basis, unless otherwise specified. For between-group comparisons, the χ2 test was used. Time to event was calculated from the date of first treatment until the date of death or other event or until the date of last follow-up, if the respective event has not occurred. Analyses were performed using SPSS (IBM SPSS Statistics for Windows, version 25; IBM, Armonk, NY)
Results
Between March 2017 and March 2018, 34 patients were treated with VRD; the median age of all patients was 66.5 years (range, 46-84 years), and 71% were male. The majority of patients (79%) had cardiac involvement; the median NTproBNP was 3649 pg/mL (81 to >30 000); 14%, 54%, 14%, and 18% of patients were rated as Mayo stage 1, 2, 3A, and 3B, respectively; 54% had renal involvement with a median eGFR of 59 mL/min per 1.73 m2 (range, 10-133 mL/min per 1.73 m2); and the renal stage distribution was 13%, 53%, and 33% for stages 1, 2, and 3, respectively. No patient required dialysis at the time of initiation of VRD. Measurable FLCs (ie, a dFLC ≥50 mg/L) were present in 29 patients (85%); in 4 patients, baseline dFLC was >20 mg/L, and these patients were also evaluable for response.37,38 In Table 1, the characteristics of the patients are depicted in more detail.
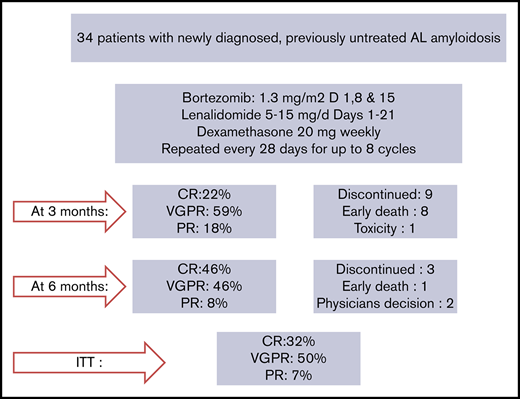
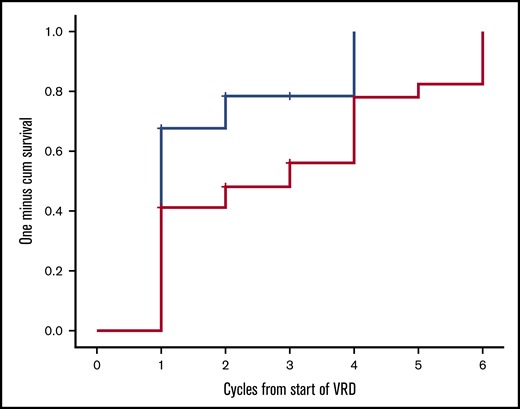
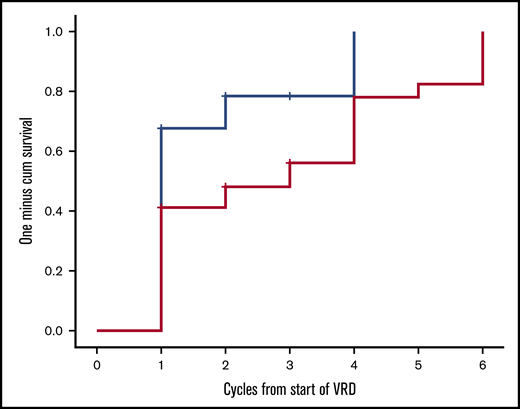
Twenty-two patients have completed the planned 8 cycles, of whom 18 received all 23 drugs for 8 cycles and 4 discontinued lenalidomide before the eighth cycle; 9 patients died prior to completion of planned therapy, 1 patient discontinued therapy for personal reasons (while in very good partial response [VGPR]), 1 patient discontinued therapy after the physician’s decision (while in VGPR), and 1 patient discontinued VRD due to severe bortezomib-related toxicity (while in CR). After the first cycle of VRD, 24 out of 34 evaluable patients (70.5%) had achieved a hematologic response; 42% of the patients had achieved a VGPR or CR and 27% a PR, and the ITT response rate at that point was also 70.5%. After 3 months of VRD, 82% of the evaluable patients (n = 27) had achieved at least a VGPR, including a CR in 6 out of 27 patients (22%) and a partial response (PR) in 5 out of 27 patients (18%), and the ITT response rate was 79% (27/34). After 6 cycles, CR, VGPR, and PR rates among evaluable (n = 24) patients were 11 (46%), 11 (46%), and 2 (7%), respectively (Table 2), and the ITT response rate was 88%. Overall, on ITT, a hematologic response was achieved in 88% of patients, and the best hematologic response was CR in 11 out of 34 patients (32%), and among CR patients, 5 out of 11 were tested as MRD negative at 10−5. VGPR was achieved in 17 out of 34 patients (50%), and in 9 of them, dFLC level was <10 mg/L but with either positive serum or urine immunofixation or an abnormal FLC ratio; 2 patients (2/34 [7%]) achieved a PR. Median time to first response (at least a PR) is depicted in Figure 1 and was 28 days (1 treatment cycle), while median time to at least a VGPR was 84 days (3 cycles of therapy). Cytogenetic data were available in 28 patients. The numbers are too small to make group comparisons; however, among patients with t(11;14) (n = 7), all achieved at least a VGPR (3 a CR and 4 a VGPR).
Time to first hematologic response (at least PR, blue line) and at least a VGPR (red line) for patients treated with VRD.
Time to first hematologic response (at least PR, blue line) and at least a VGPR (red line) for patients treated with VRD.
In Table 3, the organ response assessment is depicted at landmark time points at 6 and 12 months and for the overall ITT population. Overall organ responses were documented in 12 patients (35%); however, due to the known effect of lenalidomide on NTproBNP, cardiac responses may be underestimated (see supplemental Figure 1). During the follow-up period, 2 patients progressed to end-stage renal disease and required dialysis, both had a baseline eGFR <30 mL/min per 1.73 m2, with heavy proteinuria (in both >10 g/d); 1 patient had achieved a VGPR.
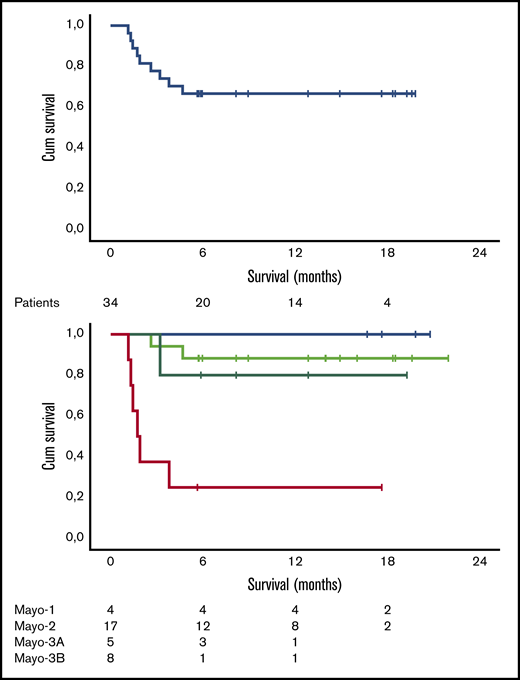
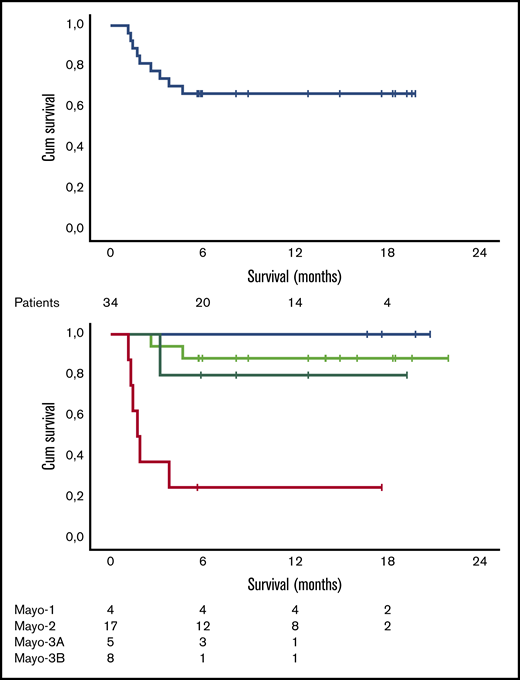
Median follow-up for all patients is 12.5 months, and 6- and 12-month survival is 73.5% (100%, 85%, and 71% for stage 1, 2, and 3A patients, respectively, but only 20% for stage 3B patients). As shown in Figure 2, the early death rate in patients with stage 3B disease was high. Patients who died within the first 3 months had quite advanced cardiac dysfunction (median NTproBNP was 11 916 pg/mL, median high-sensitivity troponin T was 99 pg/mL, all but 1 patient had stage 3B disease, median interventricular septum thickness was 16.75 mm, median left ventricular ejection fraction was 43%, and median global longitudinal strain was −7.5%; 2 patients had preexisting chronic atrial fibrillation, 1 patient had a recent history of paroxysmal atrial fibrillation prior to initiation of therapy, and 2 patients had a pacemaker; median dFLC was 507.2 mg/L). At the date of data cutoff, 1 patient has relapsed and has started second-line therapy. The patient had achieved a VGPR but stopped therapy after 4 cycles due to personal reasons.
Survival data. Overall survival (A) and survival per Mayo stage (B) of the 34 patients treated with VRD. Cum, cumulative.
Survival data. Overall survival (A) and survival per Mayo stage (B) of the 34 patients treated with VRD. Cum, cumulative.
Toxicity
The starting dose of lenalidomide was 5 mg in 30 patients (86%), 10 mg in 2 patients (7%), and 15 mg in 2 patients (7%) patients. No patient that started at 5 mg escalated to a higher dose. Hematologic toxicity of VRD was mild (grade ≥3 neutropenia, 3%; anemia, 6%; thrombocytopenia, 6%). Among the nonhematologic toxicities, rash was quite common (grade 2, 27%; grade 3, 12%; grade 4, 3%). Median time to the development of rash was 40 days (range, 5-155 days). In 3 patients, lenalidomide was discontinued due to rash, and the rest of the patients continued lenalidomide therapy with the addition of antihistamines or low-dose steroids, with or without dose reductions of lenalidomide. Other common nonhematologic adverse events (AEs) included infections (grade ≥3, 9%), constipation (grade ≥3, 9%), and peripheral neuropathy (grade 2, 20%). Thromboprophylaxis with aspirin was given in 47% of patients, low-molecular-weight heparin in 23.5%, novel oral anticoagulants in 15%, and coumadin in 12%. A thromboembolic event (pulmonary embolism) occurred in 1 patient with heavy nephrotic syndrome (proteinuria >20 g/d and serum albumin <2 g/dL) who was receiving low-molecular-weight heparin prophylaxis, but the patient continued therapy with additional prophylaxis. One patient developed a left ventricle thrombus while receiving NOACs but with no other evidence of embolism; however, the patient discontinued lenalidomide. An increase in serum creatinine was observed in 50% of patients and in many was temporary; most of the patients had severe proteinuria and were receiving diuretics. The toxicities are summarized in Table 4. In total, 37.5% of patients required lenalidomide dose reduction (in those receiving 5 mg daily, the dose was reduced to 5 mg every other day), 9 patients (27%) discontinued lenalidomide before planned therapy completion, 13 patients (38%) required bortezomib dose reduction, and 4 patients (12%) discontinued bortezomib before cycle 8. Reasons for early discontinuation of lenalidomide included rash in 3 patients, increase in serum creatinine in 5 patients, and development of left ventricle thrombus in 1 patient receiving novel oral anticoagulants. Hospitalization was required for 19 patients (56%), mostly for amyloidosis-related complications; 4 patients required hospitalization due to treatment-related complications (1 patient due to grade 4 rash related to lenalidomide, 1 patient due to development of grade 3 rash with grade 4 liver function test elevation and fever attributed to lenalidomide, 1 patient due to ileus related to bortezomib, and 1 patient due to thrombocytopenia with lower gastrointestinal hemorrhage, although lenalidomide treatment had been discontinued 45 days before admission).
Comparison with matched patients treated with CyBorD
Because CyBorD/VCD combinations are the most commonly used for the primary therapy of patients with AL amyloidosis, we also attempted to compare the efficacy of VRD to VCD/CyBorD in patients matched for Mayo stage and baseline dFLC levels. Table 5 presents the comparison of the 2 groups, which were similar in their characteristics. As shown, there was no significant difference in terms of depth of response at 1 month, however, there was a trend for deeper responses at 3 months in patients treated with VRD and a more clear difference at the rate of VGPR or better at 6 months (P = .049) and at best response in the overall ITT patient population (P = .088). On ITT, renal responses have been observed in 31% of patients with renal involvement and cardiac responses in 38% of patients with cardiac involvement treated with CyBorD/VCD, although at longer follow-up than VRD-treated patients. Regarding toxicity, although a direct comparison cannot be made, the main toxicities (grade ≥3) that were recorded during therapy with VCD/CyBorD in these patients were mostly neuropathy (grade 2, 15%; grade 3, 3%), constipation (grade ≥3, 9%), diarrhea (grade ≥3, 9%), and infections (grade ≥3, 7%). The bortezomib dose was reduced in 42% and was discontinued early due to toxicity in 8%; the cyclophosphamide dose was reduced in 2%. Regarding the need for hospitalization, 51% of patients treated with VCD/CyBorD required ≥1 admission for complications related to therapy or their disease. The 3-month and 6-month mortality rates were 18% and 23% for VRD and 11% and 17% for CyBorD/VCD (both were statistically nonsignificant).
Discussion
In this study, we evaluated a light combination of bortezomib with low-dose lenalidomide and dexamethasone (VRD), and we observed rapid and high activity, deep hematologic responses (including MRD negativity), and organ responses within a few weeks from initiation of therapy. To our knowledge, this is the first report of a VRD regimen in newly diagnosed patients with AL amyloidosis, despite the extensive use of such regimens in patients with myeloma. However, it is also important to emphasize that we also observed the challenges associated with the use of this combination, especially in frail patients with AL amyloidosis.
We started lenalidomide at low doses of 5 mg/d in 86% of the patients, based on our previous experience as well as the extensive experience from other centers, which has shown that lenalidomide therapy may be challenging in newly diagnosed AL patients, especially in those with renal and cardiac dysfunction.26,39,40 A recent study combining bortezomib with another IMiD (pomalidomide) in newly diagnosed, previously untreated patients with AL amyloidosis also showed the challenges that are associated with such regimens in terms of toxicity in newly diagnosed AL patients.41 Despite the use of low doses of lenalidomide, the nonhematologic toxicities were common; however, we believe that we need to be cautious before we attribute all AEs to the use of lenalidomide. With the exception of rash, which is quite common in AL patients treated with lenalidomide, it is important to note that in frail AL patients with multiorgan dysfunction, it may be difficult to define whether all these AEs are treatment or disease related. The observed toxicity with VRD is also another example of a regimen that is standard in MM but not as well tolerated in AL amyloidosis. The differences in the toxicity profile of lenalidomide in patients with AL amyloidosis (such as the quite high rate of skin rash) may also indicate underlying mechanism associated with the amyloid deposits or a more intact immune system (compared with more immunocompromised myeloma patients) that may “overreact.”
Currently, VCD/CyBorD remains the most commonly used regimen for the initial therapy of patients with AL amyloidosis (see supplemental Table 1) and has been the standard therapy/control regimen in the most recent prospective randomized trials in AL amyloidosis that recruited newly diagnosed patients. In several reports, the hematologic overall response rates with VCD range from 62% to 94%, with hematologic CRs in 17% to 71% of patients4,5,33,42,43 and at least hematologic VGPRs in 35% to 42% of patients (when hematologic VGPR was rated). However, VCD has not been compared prospectively to other regimens in patients AL amyloidosis. Retrospective comparisons indicated increased activity of VCD/CyBorD in terms of response rates and depth of response over cyclophosphamide, thalidomide and dexamethasone.42 BMDex, on the contrary, has been prospectively compared with melphalan and dexamethasone in a phase 3 randomized study and showed increased activity with higher response rates and deeper responses at 3 months and at best response and improved progression-free survival and probably overall survival.44 Although cross-trial comparisons cannot be made, especially considering the small numbers and the different characteristics of the patients, our data on VRD compare favorably to the bortezomib-based regimens above.
One of the characteristics of the VRD regimen is the rapid induction of deep responses (at least VGPR), which is important in order to reduce the toxic load of the free light chain to vital organs such as the heart; however, we do not have such detailed data for the other regimens reported above. Despite the rapid induction of response, we cannot recommend to discontinue lenalidomide early, because deeper responses require more time to achieve and early discontinuation may also compromise duration of response and relapse. We attempted to compare VRD to a group of patients treated with VCD/CyBorD in our department matched for Mayo stage and baseline dFLC levels. This comparison is not optimal, but in a rare disease, with very few randomized studies performed, it is unrealistic to expect a prospective comparison of these 2 regimens. Our findings indicated that VRD might induce deeper responses in the previously untreated patients with AL amyloidosis. This is not surprising given the data that have been published or reported in newly diagnosed myeloma patients. However, these comparisons should always be viewed in the context of the known limitations.
One may argue that the bortezomib combinations that are used for patients with AL amyloidosis seem to have efficacy that is rather similar whether bortezomib is combined with cyclophosphamide, melphalan, or lenalidomide. This observation is in alignment with a previous analysis from our center showing that the addition of cyclophosphamide to bortezomib/dexamethasone may offer some additional efficacy, but this benefit is not substantial.8 Taken together, these data indicate that while bortezomib is probably the most effective single drug in patients with AL amyloidosis, the optimal partner has not been defined. In this regard, the addition of daratumumab (or another anti-CD38 antibody) or another targeted therapy (such as venetoclax in patients with t(11;14)) may offer a new opportunity to improve significantly over current bortezomib combinations and is under investigation in a large randomized study (NCT03201965).
Given the efficacy and toxicity of VRD in our study, the use of this regimen must be weighed carefully against other available options. VCD/CyBorD is a standard regimen for most patients with AL amyloidosis, has better results in low- or intermediate-risk (Mayo stage 1, 2, and probably 3A) patients, and may have less efficacy in patients with t(11;14).45 BMDex has been compared in prospective randomized study to melphalan and dexamethasone44 and has proven its superiority, has good results in patients with Mayo stage 1-3A disease, and is probably more active in t(11;14) than CyBorD,45 but melphalan use is associated with myelotoxicity and perhaps a risk of secondary MDS.46 Based on our results, although in a relatively small but unselected group of patients, VRD is a rapidly acting regimen able to induce deep responses, including MRD negativity; it is more expensive than CyBorD and probably has more nonhematologic toxicity, but it may also be more effective in patients with t(11;14) (all patients in our series harboring t(11;14) achieved VGPR or CR), although more data in larger numbers of patients are needed. Given, however, the current standards, we believe that having an additional option for the treatment of patients with AL amyloidosis is very important. Unfortunately, despite the rapid activity of VRD, neither regimen seems able to salvage patients with very advanced cardiac dysfunction, such as patients with stage 3B disease, which, however, by all available regimens have a very poor outcome. While patients with stage 1 to 3A disease had very good outcome with VRD, those with stage 3B had very poor survival. Thus, there is an urgent need for more effective and safe therapies, probably acting beyond the plasma cell clone, for these patients. We have not included patients requiring dialysis in this report due to the dosing modifications required for lenalidomide, which would make dosing more complex.
We conclude that VRD with weekly bortezomib and low-dose lenalidomide is a very effective and rapidly acting regimen that can induce deep hematologic responses within 3 months of therapy. However, the toxicity of this combination in patients with AL amyloidosis is significant, despite the use of low doses of lenalidomide, and patients need close follow-up with appropriate interventions and thromboprophylaxis.
Authorship
Contribution: E.K. and M.A.D. designed the study, collected and analyzed data, and drafted the manuscript; and I.D., M.G., M.R., N.K., D.F., I.N.-S., E. Papadopoulou, D.C.Z., K.S., E.M., A.N., E.E.-P., A.P., M.M., A.-M.P., H.G., E. Psimenou, M.I.T., O.T., I.K., and E.T. collected and analyzed data and critically reviewed and modified the manuscript.
Conflict-of-interest disclosure: E.K. has received honoraria/personal fees from Amgen, Genesis Pharma, Janssen, Takeda, and Prothena and research grants from Amgen and Janssen. E.T. has received honoraria/personal fees from Amgen, Celgene, Janssen, and Takeda and research grants from Janssen. M.A.D. has received honoraria/personal fees from Amgen, BMS, Celgene, GSK, Janssen, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Efstathios Kastritis, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, “Alexandra” Hospital, 80 Vas. Sofias Ave, 115 28 Athens, Greece; e-mail: ekastritis@gmail.com and ekastritis@med.uoa.gr.
References
Author notes
The full-text version of this article contains a data supplement.