Key Points
ATG decreased OS and GRFS in CB, but improved OS and GRFS in 1-MMUD.
Abstract
Antithymocyte globulin (ATG) is widely used to reduce acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD). To clarify the different impacts of ATG for conditioning across different donor types, we retrospectively analyzed patients with acute leukemia (n = 6617) who underwent hematopoietic stem cell transplantation between 2008 and 2015 with ATG (n = 279) or without ATG (n = 6338). Because thymoglobulin is the only ATG drug approved for GVHD prophylaxis in Japan since September 2008, we included thymoglobulin alone in the present analysis. The survivors’ median follow-up time was 1081 days. Patients were categorized into 5 groups: cord blood (CB; n = 1915), matched related donor (n = 1772), 1-antigen mismatched related donor (1-MMRD; n = 225), matched unrelated donor (MUD; n = 1742), and 1-allele mismatched unrelated donor (1-MMUD; n = 963). In multivariate analysis, ATG decreased overall survival (hazard ratio [HR], 1.403; P = .054) and GVHD-free/relapse-free survival (GRFS) (HR, 1.458; P = .053) in association with increased nonrelapse mortality (NRM) (HR, 1.608; P = .03) with CB, whereas it improved GRFS (HR, 0.515; P < .01) and decreased grades II to IV aGVHD (HR, 0.576; P < .01), extensive cGVHD (HR, 0.460; P = .02), and NRM (HR, 0.545; P = .03) with 1-MMUD. ATG did not impact survival with 1-MMRD and MUD. The use of ATG in conditioning is beneficial due to the reduction in acute/chronic GVHD without increasing NRM or disease relapse only in 1-MMUD transplantation. On the other hand, ATG is not recommended for CB transplantation.
Introduction
Acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD) remain major causes of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT).1,2 Antithymocyte globulin (ATG) is often used in alternative donor transplantation, because several prospective studies have shown that ATG reduces acute/chronic GVHD (a/cGVHD) without increasing relapse.3-7 Nevertheless, a concern remains about an increased incidence of disease relapse with the use of ATG, because GVHD and the graft-versus-leukemia effect have been observed simultaneously.7,8
Furthermore, the impact of ATG on transplant outcomes may differ according to the HSCT setting. In cord blood transplantation (CBT), several studies have indicated that ATG increases infection-related mortality and decreases overall survival (OS) despite reducing a/cGVHD. This may be due to excessive immunosuppression characterized by impaired reconstitution of CD4+ T cells.9,10 In bone marrow transplantation (BMT), ATG decreases a/cGVHD without impacting OS.3,11 In peripheral blood stem cell transplantation (PBSCT), ATG reduces the incidence of a/cGVHD without increasing relapse and infection-related mortality.12 Thus, the outcomes after ATG use for conditioning are different among graft sources. Most of these previous studies have investigated the impact of ATG in a specific type of donor; however, a comprehensive analysis to assess the impact of ATG across different donor types is lacking. In addition, the optimal dose of ATG to be used in patients with acute leukemia has not been determined, because the impact of ATG may be affected by the HSCT setting.
In the present study, our specific aim was to comprehensively analyze the impact of ATG for conditioning in patients with acute leukemia undergoing HSCT while in complete remission (CR) across different donor types in an integrated way.
Patients and methods
Inclusion criteria
We included patients aged 4 months to 85 years old with acute myeloid leukemia (AML) or acute lymphocytic leukemia (ALL) who underwent a first HSCT in CR with single cord blood (CB), human leukocyte antigen (HLA)–matched related donor (MRD), HLA 1-antigen mismatched related donor (1-MMRD), HLA-matched unrelated donor (MUD), or HLA 1-allele mismatched unrelated donor (1-MMUD) through the Japan Marrow Donor Program or the Japan Cord Blood Bank Network between September 2008 and December 2015. All clinical data for patients were obtained from the Transplant Registry Unified Management Program.13 Of the 6650 patients who fulfilled the selection criteria, the following patients were excluded: 18 patients who received stem cells manipulated by CD34 selection and 15 patients who received a brand of ATG other than thymoglobulin (Sanofi, Paris, France), which is the only ATG drug approved for GVHD prophylaxis in Japan since September 2008. A total of 6617 patients was analyzed in this study. Graft sources included 4 to 6 of 6 HLA antigen-matched CB donors, 5 or 6 of 6 HLA antigen-matched related donors (1-MMRD or MRD), with matching considered at HLA-A, -B, and -DRB1 in the graft-versus-host direction, and 7 or 8 of 8 allele HLA-matched unrelated donor (1-MMUD or MUD) in the graft-versus-host direction.
This study was planned by the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation and approved by the data management committees of the Transplant Registry Unified Management Program and the Institutional Review Board of the Japanese Red Cross Nagoya First Hospital. Written informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
End points and definitions
The primary end point was defined as 3-year OS after HSCT. Secondary end points were neutrophil and platelet engraftment, cumulative incidence of aGVHD and cGVHD, cumulative 3-year relapse incidence (RI), cumulative incidence of 3-year nonrelapse mortality (NRM), 1-year GVHD and relapse-free survival (GRFS),14 and 3-year disease-free survival (DFS). Neutrophil recovery was defined as an absolute neutrophil count ≥500/μL for 3 consecutive days after transplantation. Platelet recovery was defined as an absolute platelet count ≥5 × 104/μL without platelet transfusion. GVHD was graded according to previously published criteria.15,16 The incidence of cGVHD was evaluated in patients who survived for ≥100 days after HSCT. NRM was defined as death without relapse, and DFS was defined as survival without relapse or second malignancy. GRFS was defined as survival without grade III or IV aGVHD or extensive cGVHD, and without relapse or death from any cause. We classified the conditioning regimens as myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC) according to the operational definitions of the National Marrow Donor Program/Center for International Blood and Marrow Transplant Research.17
Statistical analysis
Categorical variables were compared between groups using the Fisher exact test, and continuous variables were compared using the Mann-Whitney U test. The probabilities of OS, GRFS, and DFS were estimated according to the Kaplan-Meier method.18 The probabilities of neutrophil and platelet engraftment, a/cGVHD, RI, and NRM were estimated based on the cumulative incidence methods and compared among groups using the Gray test.19,20 For neutrophil and platelet engraftment, death, relapse, or second transplantation without engraftment was the competing event; for a/cGVHD, relapse or second transplantation without a/cGVHD was the competing event; for NRM, relapse was the competing event; and for RI, death without relapse was the competing event.
Multivariate analyses for OS, GRFS, and DFS were performed using the Cox proportional hazards model,21 whereas multivariate analyses for a/cGVHD, RI, NRM, and neutrophil and platelet engraftment were performed using the Fine and Gray regression model.20 The following variables were considered: the patient’s age at HSCT (≤20 years, 21-40 years, 41-60 years, or ≥61 years), patient sex (male or female), disease type (AML or ALL), disease status at HSCT (first CR [CR1] or at least second CR [CR2]), Eastern Cooperative Oncology Group Performance Status (ECOG-PS; 0-2 or 3-4), intensity of the conditioning regimen (MAC or RIC), GVHD prophylaxis (cyclosporine based or tacrolimus based), year of HSCT (2008-2011 or 2012-2015), stem cell source (bone marrow [BM] or peripheral blood [PB]), use of ATG (with ATG [ATG(+)] or without ATG [ATG(−)]), and HLA disparity (HLA antigen match or mismatch in CB). All P values were 2-sided, and values ≤.05 were considered statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University),22 which is a graphical user interface for R (version 3.0.2; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The median follow-up time for survivors was 1081 days (range, 13-2936 days). Patients were divided into 5 groups according to donor source: CB (n = 1915), MRD (n = 1772), 1-MMRD (n = 225), MUD (n = 1742), and 1-MMUD (n = 963) (Table 1). Few patients received ATG for CB, MRD, and MUD (2.8%, 1.0%, and 1.9%, respectively), but those who received ATG for 1-MMRD and 1-MMUD accounted for a large population (32.0% and 10.7%, respectively). We found no differences in the number of patients who underwent BMT and PBSCT with MRD or 1-MMRD, whereas almost all patients underwent BMT with MUD or 1-MMUD, because the Japan Marrow Donor Program began an unrelated PBSCT program in October 2010. As the conditioning regimen, MAC was used more frequently than RIC in all donor types.
Clinical impact of ATG for transplant outcome in each donor type
Engraftment.
We found no statistically significant differences in the incidence of neutrophil engraftment at day 60 or platelet engraftment at day 100 between ATG(+) patients and ATG(−) patients for each donor type. In multivariate analysis, ATG did not have a significant impact on neutrophil or platelet engraftment in any donor type (Table 2).
aGVHD.
Multivariate analysis showed that the use of ATG significantly decreased grades II to IV aGVHD with 1-MMRD and 1-MMUD, whereas it did not affect grades II to IV aGVHD with CB, MRD, or MUD (Table 2). Other risk factors for grades II to IV aGVHD included AML with 1-MMUD (supplemental Table 1). We did not find any difference in the incidence of grades II to IV aGVHD at day 100 between MRD-ATG(−) and 1-MMRD–ATG(+) or between MUD-ATG(−) and 1-MMUD–ATG(+) (Figure 1B-C). The incidence of grades III to IV aGVHD with 1-MMUD–ATG(+) was significantly lower than that with 1-MMUD–ATG(−), whereas we did not find any difference in grades III to IV aGVHD for other donor types, with or without ATG (supplemental Figure 1).
Cumulative incidences of grades II to IV aGVHD with various graft sources. (A) CB. (B) Related donor. (C) Unrelated donor.
Cumulative incidences of grades II to IV aGVHD with various graft sources. (A) CB. (B) Related donor. (C) Unrelated donor.
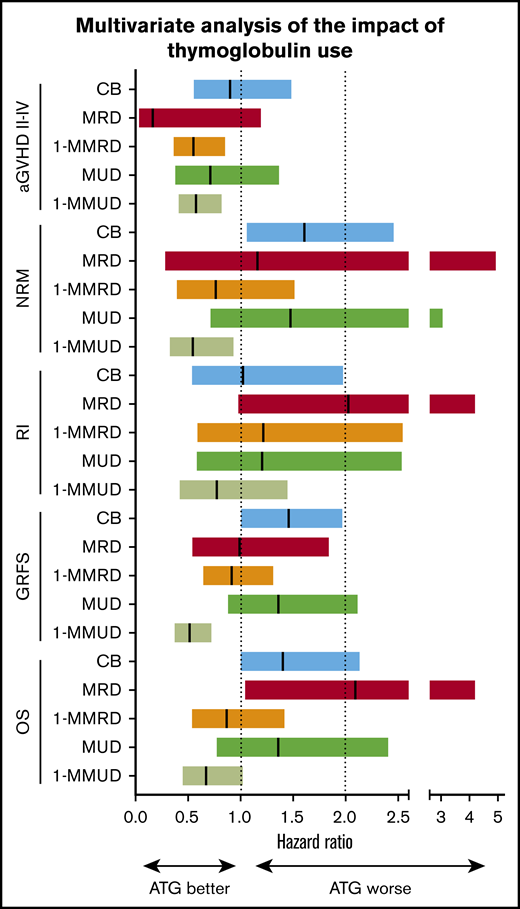
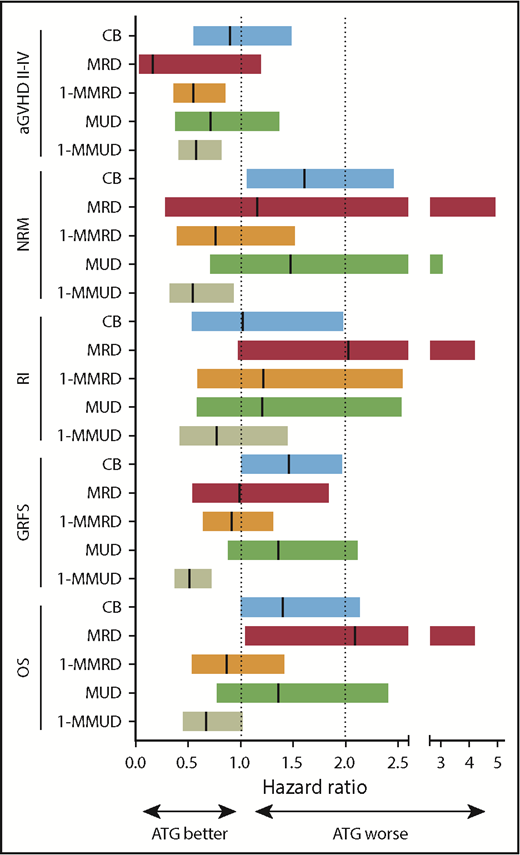
Forest plots demonstrated that the use of ATG had a similar positive impact on grades II to IV aGVHD with 1-MMRD and 1-MMUD. Although the use of ATG had no statistically significant impact on grades II to IV aGVHD with CB, MRD, or MUD, a tendency toward a decrease in grades II to IV aGVHD after ATG use was generally observed across 3 donor types (Figure 2).
Forest plot showing the different impact of ATG on transplant outcome across different donor types. Hazard ratios are shown by the middle line in the box, and 95% confidence interval ranges are indicated by the line at both ends.
Forest plot showing the different impact of ATG on transplant outcome across different donor types. Hazard ratios are shown by the middle line in the box, and 95% confidence interval ranges are indicated by the line at both ends.
cGVHD.
Multivariate analysis showed that the use of ATG significantly decreased extensive cGVHD with 1-MMUD, and it also decreased extensive cGVHD with CB, MRD, and 1-MMRD without a significant difference (Table 2). Nonuse of ATG was identified as an independent risk factor for extensive cGVHD with 1-MMUD (supplemental Table 1). The incidence of extensive cGVHD with 1-MMUD–ATG(+) was approximately half as low as that with 1-MMUD–ATG(−) (supplemental Figure 2C). Furthermore, the incidence with 1-MMUD–ATG(+) tended to be lower than that with MUD-ATG(−). Likewise, the incidence of extensive cGVHD with 1-MMRD–ATG(+) was lower than that with MRD-ATG(−) and 1-MMRD–ATG(−) (supplemental Figure 2B).
NRM.
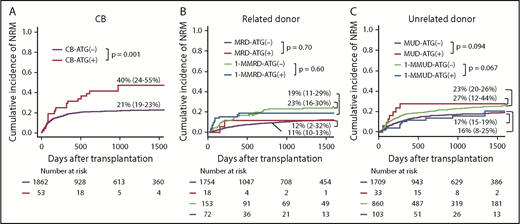
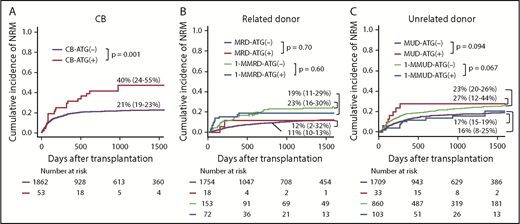
Multivariate analysis showed that the use of ATG significantly increased NRM with CB-ATG(+), whereas it significantly decreased NRM with 1-MMUD–ATG(+) (Table 2). In multivariate analysis, other risk factors for NRM were as follows: with CB, age at HSCT > 20 years and male sex and with 1-MMUD, age at HSCT > 40 years (supplemental Table 1). The incidence of 3-year NRM with CB-ATG(+) was significantly higher than that with CB-ATG(−) (40% vs 21%, respectively; P = .001) (Figure 3A). The incidence of 3-year NRM with 1-MMUD–ATG(+) was decreased to the same extent as that with MUD-ATG(−) (Figure 3C). The cumulative incidence curve of NRM with 1-MMRD demonstrated that early NRM within 100 days was higher with 1-MMRD–ATG(+) than with 1-MMRD–ATG(−), although late NRM after 3 years was lower with 1-MMRD–ATG(+) than with 1-MMRD–ATG(−) (Figure 3B).
Cumulative incidence of NRM with various graft sources. (A) CB. (B) Related donor. (C) Unrelated donor.
Cumulative incidence of NRM with various graft sources. (A) CB. (B) Related donor. (C) Unrelated donor.
The forest plots demonstrated that the use of ATG had a negative impact on NRM with CB, MRD, and MUD, although the impact was not significantly different between MRD and MUD. On the other hand, ATG had a positive impact on NRM with 1-MMRD and 1-MMUD; in particular, the hazard ratio (HR) reached statistical significance with 1-MMUD (Figure 2).
RI.
Multivariate analysis showed that the use of ATG did not have any significant impact on RI in any donor type. Although we observed a tendency for an increase in RI with MRD-ATG(+) compared with MRD-ATG(−), the difference was marginal but not significant (Table 2; supplemental Figure 3). The 3-year RI with 1-MMRD–ATG(+) was comparable to that with MRD-ATG(−); likewise, the same phenomenon was observed between 1-MMUD-ATG(+) and MUD-ATG(−) (supplemental Figure 3B-C).
The forest plots demonstrated that the use of ATG did not have a significant negative impact on RI with CB, MRD, 1-MMRD, MUD, or 1-MMUD (Figure 2).
GRFS, OS, and DFS.
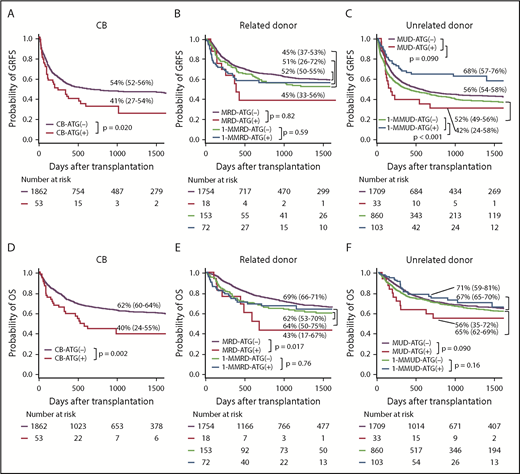
Multivariate analysis revealed that the use of ATG led to a marginally higher risk for GRFS and OS with CB; however, ATG use significantly improved GRFS and marginally improved OS with 1-MMUD (Tables 2 and 3). On the other hand, the use of ATG had a negative impact on OS with MRD. The use of ATG did not have a significant impact on GRFS or OS with 1-MMRD or MUD (Tables 2 and 3).
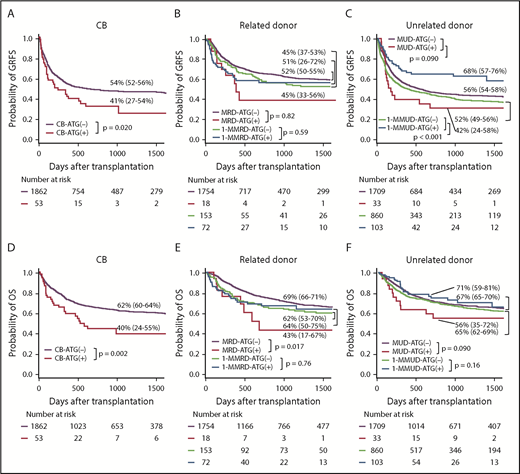
The 1-year GRFS was significantly lower with CB-ATG(+) than with CB-ATG(−), whereas it was significantly higher with 1-MMUD–ATG(+) than with 1-MMUD–ATG(−) (Figure 4A,C). The difference in 3-year OS was depicted with Kaplan-Meier curves, which demonstrated ∼20% lower OS with CB-ATG(+) than with CB-ATG(−). The 3-year OS was higher with 1-MMUD–ATG(+) than with 1-MMUD-ATG(−) (Figure 4D,F). The 3-year DFS was significantly lower with CB-ATG(+) than with CB-ATG(−) and with MRD-ATG(+) than with MRD-ATG(−) (supplemental Figure 4A-B).
Probability of GRFS and OS with various graft sources. (A,D) CB. (B,E) Related donor. (C,F) Unrelated donor.
Probability of GRFS and OS with various graft sources. (A,D) CB. (B,E) Related donor. (C,F) Unrelated donor.
The forest plots demonstrated that the use of ATG had a negative impact on GRFS with CB and a significant positive impact on GRFS with 1-MMUD. In terms of OS, the use of ATG had a marginal negative impact with CB and a significant negative impact with MRD. However, the use of ATG had a positive impact on 1-MMRD and 1-MMUD. In particular, the HR with 1-MMUD was almost statistically significant (Figure 2).
Cause of death with each donor type
Disease relapse was the most common cause of death in all groups with the exception of CB-ATG(+), in which infection-related death was the most common cause of death (supplemental Table 2). All donor types with ATG tended to show a lower incidence of a/cGVHD-related death than the corresponding donor types without ATG.
Discussion
In the present study, ATG decreased OS after CBT with significantly higher NRM. However, with 1-MMUD, ATG improved GRFS and OS in association with decreased rates of grades II to IV aGVHD, extensive cGVHD, and NRM. With MRD, ATG decreased the incidence of aGVHD but increased the incidence of relapse, thus the use of ATG eventually decreased the OS significantly. With MUD, ATG did not have an impact on transplant outcomes. With 1-MMRD, ATG did not have an impact on GRFS or OS, although it decreased grades II to IV aGVHD and extensive cGVHD.
The HSCT settings for the 5 donor types in this study were highly variable. Despite these different settings, ATG showed a clear tendency to reduce a/cGVHD in all donor types. However, particularly with CB, ATG had negative impacts on OS and GRFS. This may be related to delayed T cell recovery after CBT compared with BMT or PBSCT, resulting in an increased risk for NRM when adding ATG. Our results are consistent with several previous reports that demonstrated the negative impact of ATG use in CBT.9,23,24
Conversely, 1-MMUD–ATG(+) showed a higher probability of OS and GRFS than 1-MMUD–ATG(−), in association with lower incidences of a/cGVHD and NRM. Our results showed that ATG prominently decreased a/cGVHD and NRM and significantly improved GRFS with 1-MMUD BMT, as shown in a previous study.25 Based on these results, ATG may improve the long-term quality-of-life (QOL) of transplant survivors; therefore, it is recommended for use in HSCT with 1-MMUD.
Although ATG tended to reduce a/cGVHD, it did not impact OS or GRFS with 1-MMRD transplantation; however, the reduction in 3-year extensive cGVHD with 1-MMRD–ATG(+) may lead to a reduction in long-term immunosuppression and improve QOL. Indeed, ATG prevents cGVHD and chronic lung dysfunction, resulting in improvement in QOL.11 Although ATG had no significant impact on OS or GRFS, the use of ATG may be considered with 1-MMRD. In addition, we performed subgroup analysis of 1-MMRD, because peripheral blood stem cell grafts contained 1-log higher numbers of T cells than BM grafts,26,27 suggesting a different effect of ATG on PBSCT compared with BMT. We divided 1-MMRD into 2 groups (1-MMRD–BM or 1-MMRD–PB) according to graft sources and analyzed the potential difference in the effect of ATG; however, we did not find any difference in OS or GRFS in the presence or absence of ATG, although ATG decreased grades II to IV aGVHD more remarkably with 1-MMRD–PB (HR, 0.384; 95% confidence interval, 0.200-0.737; P < .01). Because our analyses included a small number of ATG(+) patients, further studies are warranted to elucidate the differential effect of ATG between BMT and PBSCT.
Finke et al previously demonstrated that the incidence of a/cGVHD is lower in patients receiving ATG-Fresenius in MUD transplantation without improving OS4 ; however, in our study, the use of ATG tended to be associated with worse OS and GRFS in the setting of MUD and MRD transplantation. This difference may be related to the lower incidence of a/cGVHD in Japanese patients compared with white patients, thus limiting the beneficial effects of ATG in Japanese patients.28 In addition, the proportion of patients who underwent MUD PBSCT was lower than that in Finke’s study. Also, the data about MRD and MUD showed relatively large confidence intervals compared with the other groups; thus, we need to be careful about the interpretation of results.
In the present study, a dose of ATG > 2.5 mg/kg was a risk factor for RI without reducing a/cGVHD or NRM with 1-MMUD transplantation (data not shown). Several studies suggested that the use of low-dose ATG results in more favorable transplant outcomes by reducing a/cGVHD and NRM without increasing RI.3-5,11,29-32 In addition to these factors, the timing of ATG administration is important, because the degree of immune suppression depends on biologically active ATG at the time of graft infusion.33 Although the appropriate ATG dose with 1-MMUD remains unclear, we suggest that a dose > 2.5 mg/kg may be unnecessary for this group. Because the analysis included only a small number of patients with ATG, we need careful consideration for interpretation of the results.
The present study has several limitations. First, we divided disease risk into CR1 or others, because data on cytogenetic or genetic abnormalities were insufficient. This risk classification may not be appropriate for accurate assessment of the impact of ATG on RI.34 Although a previous study reported that the type of conditioning regimen is associated with RI, the conditioning regimen was not identified as a risk factor for RI in the entire group.35 Second, data for the timing of ATG infusion were not available. Chawla et al demonstrated that higher ATG levels at the time of graft infusion are associated with a lower incidence of a/cGVHD.36 In addition to the ATG dose, the timing of ATG administration was probably related to the incidence of a/cGVHD. Third, the patient population was not balanced with regard to the use or nonuse of ATG in the CB, MUD, and MRD groups. Although the HR did not reach statistical significance in the analysis of OS for CB, it was considered to be primarily due to the small number of CB-ATG(+) patients. Conversely, in the analysis of MRD, there is a possibility that a significant difference was seen because the number of patients in the ATG(+) group is small.
In conclusion, ATG showed a different impact on transplant outcome across different donor types, and its use is recommended because it may be beneficial by reducing a/cGVHD without increasing NRM or RI. ATG decreased OS and GRFS with CB after a higher NRM. With MRD, ATG decreased OS due to the increase of RI. With 1-MMUD, ATG improved GRFS with lower a/cGVHD and NRM. Therefore, the only potential advantage for thymoglobulin use in the Japanese patients was observed in those receiving mismatched MUD transplants, and that should be confirmed in a prospective randomized trial.
Presented at the 2018 BMT Tandem meetings, Salt Lake City, UT, 21-25 February 2018.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all of the physicians and staff members of the collaborating institutes of the Japan Society for Hematopoietic Stem Cell Transplantation.
This work was supported by a grant from the Japan Society for the Promotion of Science KAKENHI (18k08351) (S.T.).
Authorship
Contribution: M.W. designed the research, analyzed the data, and wrote the manuscript; S.T. analyzed the data and helped to write the manuscript; K.O., T.F., Y.O., H.K., M.S., N.U., S.O., A.M., Y.K., and H.N. contributed to data collection; T.I. and Y.A. supervised data management; K.K., M.M., and T.T. designed and supervised the research; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation appears in the supplemental appendix.
Correspondence: Seitaro Terakura, Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Aichi 466-8560, Japan; e-mail: tseit@med.nagoya-u.ac.jp.