Key Points
CXCR4S338X clonality ≥25% is associated with lower very good partial response and shorter progression-free survival to ibrutinib.
CXCR4S338X clonality assessment represents a novel biomarker to predict outcomes to ibrutinib in Waldenström macroglobulinemia patients.
Introduction
Waldenström macroglobulinemia (WM) is an immunoglobulin M (IgM)–secreting lymphoplasmacytic lymphoma.1 Activating somatic mutations in MYD88 and CXCR4 are present in 95% to 97% and 30% to 40% of WM patients, respectively.2,,-5 Mutated MYD88 triggers prosurvival NF-κB signaling via Bruton tyrosine kinase (BTK) and hematopoietic cell kinase, both direct targets of ibrutinib.6,7 MYD88WT patients harbor NF-κB pathway mutations downstream of BTK, and derive minimal benefit from ibrutinib.8,9 CXCR4 mutations occur nearly exclusively with mutated MYD88 and promote enhanced AKT and extracellular signal-regulated kinase 1/2 activation.5,10,11 CXCR4 mutations also confer both in vitro and clinical resistance to ibrutinib, particularly nonsense variants such as CXCR4S338X.10,,,,,-16 In WM patients, CXCR4S338X constitutes the most common CXCR4 mutation identified.3,5 CXCR4S338X is primarily subclonal to mutated MYD88, but shows a highly variable clonal distribution.5 These findings prompted us to examine the impact of CXCR4S338X clonality on outcomes to ibrutinib in WM patients.
Methods
We identified consecutive patients seen at our institution between May 2012 and January 2018 who met the consensus criteria for WM and received ibrutinib monotherapy.1 The presence of MYD88 and CXCR4 mutations was assessed by allele-specific polymerase chain reaction (AS-PCR) and Sanger sequencing in sorted CD19 cells derived from bone marrow (BM) aspirates.4,5,17 Cancer cell fraction (CCF) analysis was performed for patients with CXCR4S338X using synchronous, parallel quantitative AS-PCR analyses for MYD88L265P and CXCR4S338X, as previously described.5 The CCF was determined as the ratio of cells expressing CXCR4S338X/MYD88L265P. Patients with non-S338X CXCR4 mutations unamenable to AS-PCR were excluded, and their outcomes to ibrutinib are provided in supplemental Table 1. Time-dependent receiver operator curve estimation with area under the curve (AUC) analysis was used to determine the optimal cutoff for CXCR4S338X clonality. Time to events was estimated using the Kaplan-Meier method, and comparisons were made using the log-rank test. A multivariate model for progression-free survival (PFS) was not pursued given the lack of statistically significant covariates in univariate analysis. P values were considered to be statistically significant if <.05. Calculations were performed with R (R Foundation for Statistical Computing, Vienna, Austria).
Results and discussion
A total of 147 patients with WM met inclusion criteria for this analysis. The MYD88L265P and CXCR4S338X mutations were identified in 147 patients (100%) and 37 patients (25%), respectively. Baseline clinical characteristics at the time of ibrutinib initiation are shown in supplemental Table 2. The median treatment duration of ibrutinib was 21.1 months (range, 0.3-69 months). Patients with CXCR4S338X had lower rates of major response (62% vs 85%; P = .001) and very good partial response (11% vs 35%; P = .006) vs CXCR4WT, as well as delayed attainment of both minor (1.8 vs 1.1 months; P < .001) and major responses (7.4 vs 1.8 months; P < .001). No difference in overall response rate was observed (92% vs 96%; P = .27). At the time of this report, 23 patients (16%) have progressed on ibrutinib therapy. CXCR4S338X was the only variable associated with worse progression-free survival (PFS) (hazard ratio [HR], 5.03; 95% confidence interval [CI], 1.91-13.2; P = .001) with a significantly shorter PFS compared with CXCR4WT (44.1 months vs not reached [NR]; supplemental Table 3).
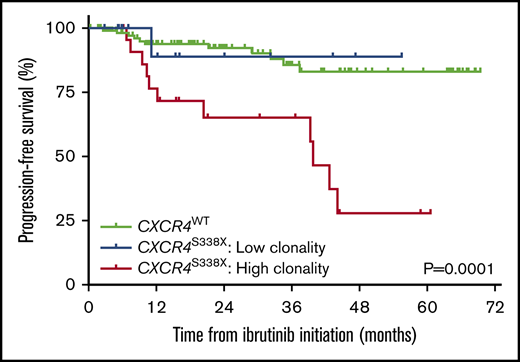
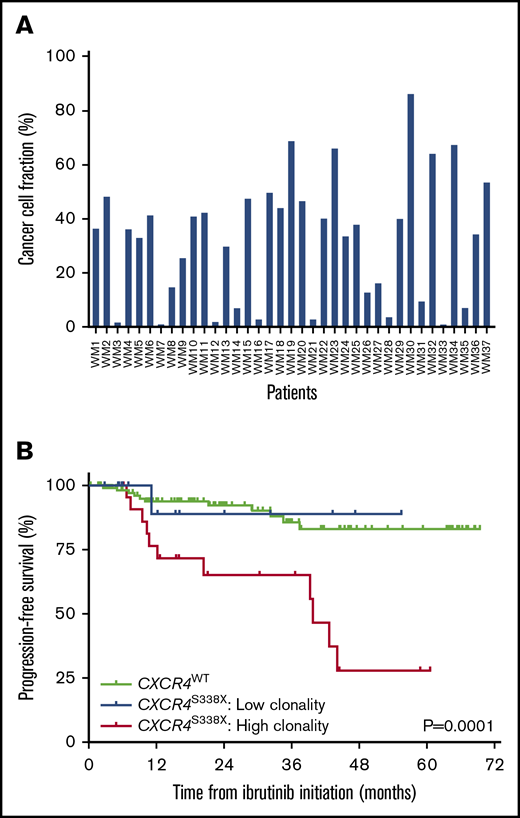
Among the 37 patients with CXCR4S338X, the median clonality was 35.3% (range, 0.94% to 86.2%; Figure 1A). By receiver operator curve analysis, a CXCR4S338X clonality of 25% was calculated as the optimal cutoff for disease progression within 12 months (sensitivity, 71%; specificity, 72%; AUC, 0.73) or 24 months (sensitivity, 72%; specificity, 69%; AUC, 0.69) of ibrutinib initiation. Patients were stratified into 2 groups based on CXCR4S338X clonality: high clonality (n = 23; 62%) defined as ≥25% and low clonality (n = 14; 38%) defined as <25%. Baseline clinical characteristics as well as response rates and kinetics to ibrutinib therapy according to CXCR4S338X clonality are shown in supplemental Table 4 and Table 1, respectively. Patients with high CXCR4S338X clonality were more likely to have a baseline BM involvement ≥50% (87%, 57%, and 50%; P = .005), serum IgM level >7000 mg/dL (17%, 7%, and 3%; P = .02), and platelet count ≤100 × 109/L (17%, 14%, and 4%; P = .03) vs patients with low CXCR4S338X clonality and CXCR4WT, respectively. In addition, patients with high CXCR4S338X clonality had lower rates of very good partial response (4%, 21%, and 35%; P = .01) and delayed major response attainment (9.7, 7.4, and 1.9 months; P < .001) to ibrutinib. Compared with patients with CXCR4WT, high CXCR4S338X clonality was associated with significantly worse PFS (HR, 10.44; 95% CI, 3.43-31.8; P < .0001), whereas low CXCR4S338X clonality did not impact PFS (HR, 0.90; 95% CI, 0.13-6.44; P = .92). Patients with high CXCR4S338X clonality also had a significantly shorter median PFS vs patients with low CXCR4S338X clonality and CXCR4WT (39.9 months, NR, NR, respectively; P = .0001; Figure 1B).
Clonality assessment of CXCR4S338X. (A) CCF analysis for CXCR4S338X clonality in CD19-selected BM cells from WM patients. (B) Kaplan-Meier curves for PFS on ibrutinib in WM patients stratified by CXCR4S338X mutant clonality.
Clonality assessment of CXCR4S338X. (A) CCF analysis for CXCR4S338X clonality in CD19-selected BM cells from WM patients. (B) Kaplan-Meier curves for PFS on ibrutinib in WM patients stratified by CXCR4S338X mutant clonality.
These findings demonstrate that CXCR4S338X mutations adversely impact clinical outcomes to ibrutinib monotherapy in WM patients. Our data are consistent with preclinical studies showing that WM cells transduced with CXCR4S338X have increased CXCL12-triggered AKT and extracellular signal-regulated kinase 1/2 activation, and decreased in vitro ibrutinib-related apoptosis.10,11 Prospective studies have also reported that CXCR4 mutations are associated with lower response rates, delayed response attainment, and shorter PFS on ibrutinib.12,,-15 However, these studies included heterogenous groups of CXCR4-mutated patients, and were not large enough to discriminate the effect of individual somatic variants.
An important finding was the identification of CXCR4S338X clonality as a predictor of long-term outcomes to ibrutinib therapy. Our study shows that WM patients with both low and high CXCR4S338X clonality have evidence of intrinsic resistance to ibrutinib (ie, lower response rates, delayed response attainment), but only high clonality was associated with inferior PFS, suggesting that ibrutinib was unable to overcome the pejorative impact of CXCR4S338X in these patients. Although the mechanism for this finding remains to be delineated, tumors with higher CXCR4S338X clonality may be less amenable to eradication by ibrutinib due to enhanced prosurvival signaling. Likewise, it is possible that tumors with high CXCR4S338X clonality could have an increased tropism for the BM stroma, which provides a permissive niche for drug resistance as well as malignant cell survival and IgM release.10,11,18,-20 This may account for the higher baseline BM involvement and serum IgM levels observed herein for patients with high CXCR4S338X clonality, as well as the lower incidence of ibrutinib-triggered peripheral lymphocytosis in CXCR4-mutated WM patients previously reported.12 Prospective longitudinal studies as well as in vitro signaling studies are needed to further elucidate these hypotheses. A phase 2 trial evaluating ibrutinib in previously untreated WM patients with serial whole-genome sequencing is now fully enrolled (NCT02604511).
Our study highlights the importance of developing novel strategies to overcome ibrutinib resistance in CXCR4S338X-mutated WM patients. The use of CXCR4 inhibitors has been shown to restore the sensitivity of CXCR4S338X-mutated cells to ibrutinib,10 and a phase 1/2 study investigating the CXCR4-blocking antibody ulocuplumab and ibrutinib is ongoing in CXCR4-mutated WM patients (NCT03225716). Moreover, the iNNOVATE study recently evaluated the combination of ibrutinib and rituximab, and updated results demonstrate shorter 36-month PFS among CXCR4MUT vs CXCR4WT WM patients (64% vs 84%).15,21 Preliminary data suggest that the second-generation BTK inhibitor zanubrutinib has some activity in MYD88WT WM patients, but the impact of CXCR4 mutations is currently unknown.22
The present study is not without limitations. Despite the largest cohort of WM patients on ibrutinib with CXCR4S338X clonality determined, the results are based on a limited number of patients and a clonality cutoff with modest sensitivity and specificity. In addition, we were unable to evaluate the impact of CXCR4 clonality on ibrutinib for non-S338X mutations unamenable to AS-PCR. Larger studies are needed to provide external validation for our preliminary findings.
In summary, high CXCR4S338X clonality adversely impacts clinical outcomes to ibrutinib therapy in WM patients. Clonality assessment represents a novel biomarker for predicting outcomes on ibrutinib in WM patients carrying CXCR4S338X nonsense mutations.
The full-text version of this article contains a data supplement.
Acknowledgments
J.J.C. was supported by the WMR Fund.
J.N.G. was awarded Young Investigator Awards for this research at the 10th International Workshop for Waldenström’s Macroglobulinemia, New York, NY (October 2018), and at the 17th International Myeloma Workshop, Boston, MA (September 2019).
Authorship
Contribution: J.N.G., L.X., Z.R.H., S.P.T., and J.J.C. conceived and designed the experiments, performed the data analysis, and wrote the manuscript; N.T., M.G.D., A. Kofides, and L.X. performed the sequencing studies; J.G.C., X.L., M.M., M.L.G., G.G.C., C.J.P., and G.Y. prepared samples; and K.M., A. Keezer, T.D., J.N.G., J.J.C., and S.P.T. provided patient care, obtained consent, and were responsible for sample collection.
Conflict-of-interest disclosure: J.J.C. has received honoraria and/or research funds from AbbVie, BeiGene, Janssen, Millennium, Pharmacyclics, and TG Therapeutics. S.P.T. has received research funding and consulting fees from Pharmacyclics and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Jorge J. Castillo, Bing Center for Waldenström’s Macroglobulinemia, Dana-Farber Cancer Institute, M221, 450 Brookline Ave, Boston, MA 02215; e-mail: jorgej_castillo@dfci.harvard.edu.