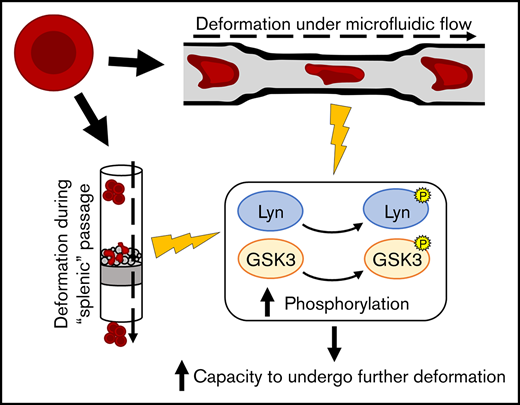
Key Points
Specific phosphorylation events are associated with RBC deformation.
Inhibition of GSK3 and Lyn impairs RBC capacity to undergo successive deformation and resist shear stress.
Abstract
The capacity to undergo substantial deformation is a defining characteristic of the red blood cell (RBC), facilitating transit through the splenic interendothelial slits and microvasculature. Establishment of this remarkable property occurs during a process of reticulocyte maturation that begins with egress through micron-wide pores in the bone marrow and is completed within the circulation. The requirement to undertake repeated cycles of deformation necessitates that both reticulocytes and erythrocytes regulate membrane-cytoskeletal protein interactions in order to maintain cellular stability. In the absence of transcriptional activity, modulation of these interactions in RBCs is likely to be achieved primarily through specific protein posttranslational modifications, which at present remain undefined. In this study, we use high-throughput methods to define the processes that underlie the response to deformation and shear stress in both reticulocytes and erythrocytes. Through combination of a bead-based microsphiltration assay with phosphoproteomics we describe posttranslational modification of RBC proteins associated with deformation. Using microsphiltration and microfluidic biochip-based assays, we explore the effect of inhibiting kinases identified using this dataset. We demonstrate roles for GSK3 and Lyn in capillary transit and maintenance of membrane stability following deformation and show that combined inhibition of these kinases significantly decreases reticulocyte capacity to undergo repeated deformation. Finally, we derive a comprehensive and integrative phosphoproteomic dataset that provides a valuable resource for further mechanistic dissection of the molecular pathways that underlie the RBC’s response to mechanical stimuli and for the study of reticulocyte maturation.
Introduction
The capacity to undergo substantial deformation is a defining characteristic of the red blood cell (RBC). Establishment of this remarkable property occurs during a process of reticulocyte maturation that begins in the bone marrow and is completed within the circulation in which the nascent reticulocyte, having expelled its nucleus, remodels its membrane to acquire the recognizable biconcave morphology of the mature erythrocyte. While the deformability of early reticulocytes is known to be lower than that of erythrocytes1-3 but increase progressively throughout the maturation process, reticulocytes themselves must undergo extreme deformations without damage or lysis. These include bone marrow egress (before entering peripheral circulation) as well as multiple traversals of the splenic interendothelial slits (during circulatory maturation) and the microvasculature. As a result, reticulocytes are necessarily exposed to high pressures and shear stress, which in turn may partially drive the processes underlying maturation.
Modulation of reticulocyte and erythrocyte properties through response to shear stress is a well described phenomenon. Shear stress has been reported to be implicated in the regulation of adenosine triphosphate (ATP) release by erythrocytes,4,5 removal of autophagic vesicles from circulating reticulocytes,6 and general induction of autophagy in other mammalian cells.7 In erythrocytes, shear stress is known to induce calcium ion entry through activation of the mechanically activated cation channel Piezo1,8 regulating the volume changes necessary for the maintenance of homeostasis during deformation.9 Increases in intracellular calcium are known to promote phosphorylation of multiple proteins through interaction with sensors such as calmodulins and C2 domain-containing proteins.10-13 Phosphorylation, in turn, regulates cytoskeletal alterations and interactions14,15 as well as the stability of the erythrocyte membrane.10,16
Understanding the biochemical mechanisms that enable or are activated upon RBC deformation is an extremely difficult proposition given the challenges associated with isolating cells undertaking this process. Various types of shear stress/deformation-inducing systems exist specifically for the study of RBC properties,17-19 but most of the focus of the use of those systems has been on the assessment of the mechanical and physical properties of the cells or the high-throughput screening of chemical compound effects on these characteristics20 rather than elucidation of the molecular basis of RBC deformation.
In this study, we aim to better define the phosphorylation events that underlie the response to deformation and shear stress in both reticulocytes and erythrocytes. Using in vitro systems that mimic deformation through the spleen and microvasculature, respectively, we have captured cells in states immediately pre-, mid-, and postdeformation and used phosphoproteomics to obtain a unique and comprehensive shear-response dataset, providing a valuable resource for further studies of the processes involved in RBC deformation and reticulocyte maturation. We then validate the importance of 2 of these modifications in the cellular response to shear stress through chemical inhibition of the respective proteins.
Methods
Erythrocyte isolation and in vitro erythroid culture
Erythrocytes were isolated by leukofiltration from the RBC fraction obtained by Histopaque separation of healthy donor platelet apheresis waste blood. In vitro–cultured reticulocytes were differentiated from CD34+ cells isolated from the mononuclear cell fraction according to previously published protocols.21 All source material was provided with written informed consent for research use given in accordance with the Declaration of Helsinki (National Health Service Blood and Transplant, Filton, Bristol, United Kingdom). The research into the mechanisms of erythropoiesis was reviewed and approved by the Bristol Research Ethics committee (REC Number 12/SW/0199).
Proteomics experimental design, data acquisition and analysis
Two experiments were performed: (1) A comparison of erythrocytes and in vitro–derived reticulocytes lysed before, during, and after shear stress exposure through microsphere filtration (microsphiltration) was performed, with at least 3 biological repeats per cell type and shear stress exposure condition, generating a total of 21 individual samples that were processed through qualitative titanium dioxide–enriched phosphoproteomics; and (2) a comparison of reticulocytes lysed before and after shear stress exposure through passage in successive microfluidic constrictions with 3 biological repeats and generating a total of 6 individual samples.
Each sample corresponds to 10 × 106 cells. The protein content between samples was equalized through hemoglobin estimation via the use of Drabkin’s reagent, using different standard curves for reticulocytes and erythrocytes due to their differences in average hemoglobin concentration. Data are available via ProteomeXchange with identifiers PXD013652 and PXD013960, with corresponding DOIs 10.6019/PXD013652 and 10.6019/PXD013960. Further information and reasoning for the chosen proteomics design may be found in the supplemental Rationale material. Extended methods for sample preparation, data acquisition and analysis are provided in the supplemental Methods.
ARCA sample preparation and analysis
A total of 2 × 106 cells were diluted in 200 µL of a polyvinylpyrrolidone solution (viscosity, 28.1 mPa·s; Mechatronics Instruments). Samples were assessed in an Automated Rheoscope and Cell Analyzer (ARCA)22 according to previously published protocols.23 At least 1500 valid cells per sample were analyzed using bespoke ARCA analysis software.
Microsphiltration
A microsphiltration device was built as originally described by Deplaine et al.24 A 1:1 mixture of 15 to 25 µm and 5 to 15 µm diameter metal microspheres (1 g; 1 g consisting of 96.50% tin, 3.00% silver, and 0.50% copper; Industrie des Poudres Sphériques) was resuspended in 5 mL PBSAG (phosphate-buffered saline + 1 mg/mL bovine serum albumin + 2 mg/mL glucose). A total of 600 µL of the microsphere suspension were loaded directly on top of the antiaerosol filter of the inverted pipette tip. The remaining volume was filled with PBSAG. A 50-mL syringe was loaded with PBSAG and connected to a 3-way stopcock, which was connected to the pipette tip using silicone tubing (Cole-Parmer). A 1-mL syringe was used for loading the cell suspension into the microsphiltration system through the stopcock. Pressure was created manually through slow and constant depression of the 50-mL syringe plunger.
For the proteomics experiment, cells were lysed at 3 points: (1) before use of the microsphiltration system, (2) during the point of maximum cell density in the bead layer, and (3) postrecovery of the cells from the microsphiltration system. For recovery during the point of maximum cell density in the bead layer, pressure was stopped immediately, and the top of the pipette filter tip was cut open. Then, the PBSAG present in the system was aspirated. Forty microliters of lysis buffer were carefully added to the bead layer and incubated for 10 minutes at 4°C. The bead layer and lysate were collected into a microcentrifuge tube and centrifuged for 10 minutes at 16 100g, 4°C. The supernatant was then collected. For recovery after the microsphiltration system, the resulting suspension was centrifuged for 5 minutes at 400g, 4°C, and the cells were counted and lysed. Lysates were then equalized in regard to cell number through quantitation of hemoglobin using Drabkin’s reagent.
For the percentage of cell recovery experiment, cells were treated overnight with 1:1000 dimethyl sulfoxide (DMSO) as a control, or a pharmacological inhibitor (3 µM bafetinib [specific Lyn inhibitor, 50% inhibition concentration (IC50): 19 nM; Insight Biotechnology], 10 nM CHIR-98014 [specific GSK3 inhibitor, GSK3α IC50: 0.65 nM, GSK3β IC50: 0.58 nM, closest homolog Cdc2 IC50: 3.7 µM; Stratech Scientific], or a combination of both). Following treatment, the cells were stained with CellTracker Green CMFDA Dye (Invitrogen) for 45 minutes at 37°C. After staining, the cells were mixed with untreated, unstained cells in a 5:95 mixture and resuspended in PBSAG. The resulting cell suspension was subjected to microsphiltration and analyzed through flow cytometry.
For fixation of cells in the bead layer, the filter tip was cut and the PBSAG aspirated as described earlier. A total of 100 µL of fixing solution (1% paraformaldehyde and 0.0075% glutaraldehyde [v/v] in PBSAG) were carefully added to the bead layer and incubated for 10 minutes at room temperature. One milliliter of PBSAG was added for resuspension of the bead layer and the suspension was collected into microcentrifuge tubes. The beads were let to sediment by gravity and, 5 minutes later, the supernatant containing the fixed cells was collected into new microcentrifuge tubes. The cell suspension was centrifuged, and the cells were washed twice in PBSAG to remove any remaining microbeads. The fixed cells were then stored at 4°C.
Microfluidic multiconstriction assay
The microfluidic biochip was designed and fabricated as described by Lizarralde Iragorri et al.19 An MFCS-EZ microfluidic flow control system (Fluigent) was used to regulate the flow pressure in the microfluidic biochip. The biochip was connected to the pump by 1.6-mm silicone tubing and male Luer connectors and mounted on the stage of an inverted AxioObserver Z1 microscope (Zeiss) coupled with a Phantom Miro M 320S high-speed camera, with imaging at ×100 magnification.
For each assay, 60 µL of reticulocyte or erythrocyte suspensions at 2.5% hematocrit were loaded in the input well of the biochip and perfused at constant pressure (150 mBar). The 2.5% hematocrit, lower than the literature value of 30%, was used to allow for the identification of individual cells in the acquired images. For inhibitor treatment, the cells were treated with 1:1000 DMSO as a control, or an adequate inhibitor (3 µM bafetinib, 10 nM CHIR-98014, or a combination of both) for an hour and resuspended in PBS with the respective inhibitor for use in the microfluidics chip. High-speed videos were obtained at 900 to 1500 frames per second and converted into .tiff frame sequences with the use of Phantom Camera Control (PCC) v2.2 or Cine Viewer (CV) v3.1 software.
High-speed video analysis
All images resulting from the high-speed videos were processed with the use of Fiji v. 2.0.0-rc-54/1.51h.25 A detailed method for high-speed video analysis is provided in the supplemental Methods.
Raw proteomics data and data preprocessed with Proteome Discoverer are available via ProteomeXchange with identifiers PXD013652 and PXD013960, with corresponding DOIs 10.6019/PXD013652 and 10.6019/PXD013960. Proteomics data tables from preprocessing with Proteome Discoverer may also be found in the supplemental Data.
Filtered proteomics data from the microsphiltration and microfluidics proteomics analyses may be respectively found in supplemental Tables 1 and 2.
Results
The RBC phosphoproteome is modulated in response to deformation
Due to the relatively short timeframe required for the deformation and recovery of RBCs and the general lack of transcriptional machinery, we hypothesized that any underlying mechanism for responding to deformation would necessarily be driven by posttranslational modification of proteins via phosphorylation. Altered phosphorylation of cytoskeletal and membrane proteins is known to influence multiple properties in the erythrocyte (eg, membrane stability and tension, deformability, effective viscosity16,26-28 ), principally through changes in cytoskeletal stability and in the association between cytoskeletal proteins and the multiprotein membrane complexes. Given the reported increased kinase activity of reticulocytes29 and the fact that many proteins are lost during reticulocyte maturation to the erythrocyte,30 investigating reticulocyte response to deformation may allow for increased sensitivity in the detection of deformation-induced signaling cascades. Thus, to investigate any possible changes underlying deformation and recovery, we produced 2 qualitative phosphoproteomics datasets comparing erythrocytes from 3 donors and in vitro CD34+-derived cultured reticulocytes from 4 donors lysed before, during, and after passage through the spleen-like microsphiltration system originally developed by Deplaine et al.24 In vitro–generated reticulocytes were used in preference to reticulocytes isolated from donors because of the high number of cells required per complete sample set in the microsphiltration experiment, although we have previously shown that in vitro and in vivo reticulocytes are proteomically similar both in quantitative protein abundance and presence of specific phosphopeptides.23
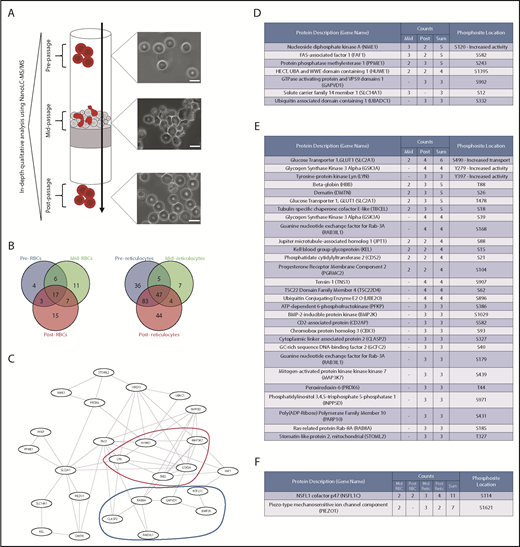
Figure 1A summarizes the design of the experiment and includes micrographs of erythrocytes in the 3 conditions. Cells analyzed in the beginning of the experiment possess the characteristic biconcave morphology, whereas a majority of the cells fixed during passage through the microsphiltration system are in the process of being deformed (although the degree to which they are deformed varies for each cell). Cells that have undergone passage through the system regain their biconcave morphology without any visible alteration. Resulting processed proteomics data from the experiment may be found in supplemental Table 1.
Red blood cell deformation induces measurable changes in the phosphoproteome. (A) Experimental design for proteomic comparison: RBCs and cultured reticulocytes were subjected to flow in a microsphiltration system and lysed before, during, and after passage. The lysates were equalized through hemoglobin quantification using Drabkin’s reagent and analyzed through NanoLC-MS/MS. Cells were fixed and imaged under brightfield at all 3 stages, showing the extent of their deformation within the system and their recovery after passage. Scale bars, 10 μm. (Microsphiltration schematic adapted from Duez et al.48 ) (B) Venn diagram of differentially phosphorylated proteins with ≥50% detection across all samples in RBCs (left, n = 63 with 33 modifications detected exclusively in cells mid- and postdeformation) and reticulocytes (right, n = 226 with 55 modifications detected exclusively in cells mid- and postdeformation). (C) Top-scoring protein-protein interaction network from the joint dataset of mid- and postdeformation exclusive phosphoproteins in reticulocytes, as predicted by STRING.49 Two main subsets of proteins were present in the network, kinases (marked in red) and vesicle transport-related proteins (marked in dark blue). (D) List of phosphoproteins exclusive to the RBC mid-deformation and mid-/postintersection datasets. Count indicates the number of observed instances of the phosphopeptide (n = 3); the phosphopeptides were sorted by the sum of their counts in both datasets and filtered for their presence in at least 2 samples per condition. PhosphoSite location indicates the predicted location of the phosphorylation site in the protein sequence (as predicted by SEQUEST and/or PhosphoSitePlus data50 ), as well as the effect of its phosphorylation if described in low-throughput studies according to PhosphoSitePlus.50 (E) List of phosphoproteins exclusive to the reticulocyte mid-deformation and mid-/postintersection datasets (n = 4). Dataset analysis, filtering, and annotation were performed as previously described. (F) List of phosphoproteins present among both reticulocyte and RBC mid-/postdeformation datasets, with no significant detection in the predeformation dataset; dataset analysis, filtering, and annotation were performed as previously described.
Red blood cell deformation induces measurable changes in the phosphoproteome. (A) Experimental design for proteomic comparison: RBCs and cultured reticulocytes were subjected to flow in a microsphiltration system and lysed before, during, and after passage. The lysates were equalized through hemoglobin quantification using Drabkin’s reagent and analyzed through NanoLC-MS/MS. Cells were fixed and imaged under brightfield at all 3 stages, showing the extent of their deformation within the system and their recovery after passage. Scale bars, 10 μm. (Microsphiltration schematic adapted from Duez et al.48 ) (B) Venn diagram of differentially phosphorylated proteins with ≥50% detection across all samples in RBCs (left, n = 63 with 33 modifications detected exclusively in cells mid- and postdeformation) and reticulocytes (right, n = 226 with 55 modifications detected exclusively in cells mid- and postdeformation). (C) Top-scoring protein-protein interaction network from the joint dataset of mid- and postdeformation exclusive phosphoproteins in reticulocytes, as predicted by STRING.49 Two main subsets of proteins were present in the network, kinases (marked in red) and vesicle transport-related proteins (marked in dark blue). (D) List of phosphoproteins exclusive to the RBC mid-deformation and mid-/postintersection datasets. Count indicates the number of observed instances of the phosphopeptide (n = 3); the phosphopeptides were sorted by the sum of their counts in both datasets and filtered for their presence in at least 2 samples per condition. PhosphoSite location indicates the predicted location of the phosphorylation site in the protein sequence (as predicted by SEQUEST and/or PhosphoSitePlus data50 ), as well as the effect of its phosphorylation if described in low-throughput studies according to PhosphoSitePlus.50 (E) List of phosphoproteins exclusive to the reticulocyte mid-deformation and mid-/postintersection datasets (n = 4). Dataset analysis, filtering, and annotation were performed as previously described. (F) List of phosphoproteins present among both reticulocyte and RBC mid-/postdeformation datasets, with no significant detection in the predeformation dataset; dataset analysis, filtering, and annotation were performed as previously described.
A significant proportion of the detected phosphopeptides were unique to the deformed or recovery states in erythrocytes (52.4% of detected phosphopeptides) and reticulocytes (24.3% of detected phosphopeptides), as summarized in Figure 1B. Further analysis of these phosphopeptide groups through use of the STRING database revealed a predicted common underlying protein-protein interaction network, which is shown in Figure 1C. Two main groups of proteins were observed to be connected through this network, namely kinases and intracellular transport proteins (as identified by red and blue outlines, respectively). The only KEGG pathway detected in the dataset through enrichment analysis was related to AMPK signaling (KEGG enrichment performed with the use of DAVID 6.8,31,32 P = .029), although no AMPK-related phosphopeptides were detected (suggesting we are capturing downstream phosphorylation events). Phosphopeptides detected only in erythrocytes are listed in the table in Figure 1D, whereas phosphopeptides detected only in reticulocytes are listed in Figure 1E. As expected, a much broader variety of novel peptides phosphorylated during or after deformation was detected in reticulocytes compared with erythrocytes. Although this finding may be related to the previously mentioned higher kinase activity in reticulocytes,29 it may also potentially highlight the existence of only a temporary role for the detected proteins and respective phosphorylations. Interestingly, only a small overlap was detected between erythrocytes and reticulocytes (Figure 1F), which could also be related to differences in the activation kinetics for signaling and recovery of the cell from deformation (or, again, due to known differences in kinase abundances between the cell types). It is noteworthy that only the phosphopeptide corresponding to NSFL1C was consistently detected among the 2 cell types in both the mid- and postdeformation states, despite no information existing on its effect in erythroid cells.
Inhibition of Lyn and GSK3 decreases RBC capacity to undergo repeated deformation
Notably, phosphorylations with well-defined effects on kinase activity were also detected by our experimental approach in reticulocytes. Because of the ubiquitous presence of their phosphopeptides in the reticulocyte postdeformation samples, we focused our attention on the kinases Lyn and GSK3α. The tyrosine kinase Lyn is a known regulator of the erythrocyte protein band 3, a cytoskeleton-interacting protein known to be the most abundant membrane protein in RBCs.33 Its kinase activity is partially regulated through autophosphorylation of the tyrosine residue in site Y397, which was detected in this dataset and has been well-described to induce further activation of the protein.34 Conversely, serine/threonine kinases of the GSK3 family have been shown to participate in the phosphorylation of β-adducin,35 which is also known to interact with the spectrin-actin cytoskeleton in erythrocytes.36 Tyrosine phosphorylation of GSK3α at the Y279 site, which we have detected in this dataset, is known to increase enzymatic activity.37
Given that modulating the activity of both kinases could have a quantifiable impact on the erythroid cytoskeleton, we decided to attempt chemical inhibition and subsequent cell microsphiltration to investigate a putative effect on the ability of reticulocytes to undergo successive deformations. In addition to the 2 kinase inhibitors, treatment with an anti-glycophorin A (GPA) antibody was used as a positive control for detrimental effects on cell deformability, as GPA ligation has been demonstrated to increase cell rigidity.38 After treatment, the cells were mixed at approximately 5:95 ratio with untreated erythrocytes, as per a previously published method by Cluitmans et al,39 and allowed to settle on the surface of the microbead layer. Even, manual pressure was applied and the percentage of reticulocytes that traversed the system was evaluated using flow cytometry through the detection of CellTracker Green CMFDA-labeled cells. The results for this experiment are shown in Figure 2A.
GSK3 and Lyn inhibition impacts capillary traversal of reticulocytes and red blood cells. (A) Percentage recovery of reticulocytes treated with the respective reagent after microsphiltration. After treatment, the cells were added to an untreated RBC suspension in a 5:95 ratio and the mixture then subjected to microsphiltration. Anti-GPA antibody was used as a positive control for negative effects on cell deformability. Bafetinib (3 µM) was used as a Lyn inhibitor and CHIR-98014 (10 nM) was used as a GSK3 inhibitor. Error bars correspond to the standard deviation (n = 3). All comparisons were performed with a paired 2-tailed Student t test. The P values for each comparison are shown below the bar graph. *P < .05, **P < 0.01. (B) Image of the microfluidic PDMS biochip used for the capillary traversal experiment. The magnified image shows a section of the microcapillary channels, through which reticulocytes and red blood cells were subjected to successive deformations. Frame sequences were obtained through the use of a high-speed camera and then subjected to image analysis for automated cell tracking with the use of TrackMate.51 Scale bars, 50 μm. (C) Percentage microcapillary traversal velocity of reticulocytes and red blood cells treated with the respective reagents. The cells were treated for a minimum period of 1 hour and subjected to passage through the microfluidic biochip. The graph shows the average cell microcapillary traversal velocity of each treated sample as a percentage of the respective DMSO control sample traversal velocity. All comparisons were performed with a 1-sample Student t test. The error bars correspond to the standard deviation (n = 3). The P values for each comparison are shown below the dot plot. *P < .05, **P < .01. A minimum of 1000 cell tracks were analyzed per sample. n.s.s., not statistically significant.
GSK3 and Lyn inhibition impacts capillary traversal of reticulocytes and red blood cells. (A) Percentage recovery of reticulocytes treated with the respective reagent after microsphiltration. After treatment, the cells were added to an untreated RBC suspension in a 5:95 ratio and the mixture then subjected to microsphiltration. Anti-GPA antibody was used as a positive control for negative effects on cell deformability. Bafetinib (3 µM) was used as a Lyn inhibitor and CHIR-98014 (10 nM) was used as a GSK3 inhibitor. Error bars correspond to the standard deviation (n = 3). All comparisons were performed with a paired 2-tailed Student t test. The P values for each comparison are shown below the bar graph. *P < .05, **P < 0.01. (B) Image of the microfluidic PDMS biochip used for the capillary traversal experiment. The magnified image shows a section of the microcapillary channels, through which reticulocytes and red blood cells were subjected to successive deformations. Frame sequences were obtained through the use of a high-speed camera and then subjected to image analysis for automated cell tracking with the use of TrackMate.51 Scale bars, 50 μm. (C) Percentage microcapillary traversal velocity of reticulocytes and red blood cells treated with the respective reagents. The cells were treated for a minimum period of 1 hour and subjected to passage through the microfluidic biochip. The graph shows the average cell microcapillary traversal velocity of each treated sample as a percentage of the respective DMSO control sample traversal velocity. All comparisons were performed with a 1-sample Student t test. The error bars correspond to the standard deviation (n = 3). The P values for each comparison are shown below the dot plot. *P < .05, **P < .01. A minimum of 1000 cell tracks were analyzed per sample. n.s.s., not statistically significant.
Predictably, pretreatment with the anti-GPA antibody significantly affected cell traversal through microsphiltration. Inhibition of Lyn kinase showed a nonsignificant reduction in the percentage of cells obtained after passage through the system, whereas inhibition of GSK3 showed a significant reduction that was comparable to treatment with the anti-GPA antibody. Importantly, and in contrast to cells treated with the anti-GPA antibody, treatment with either inhibitor did not affect intrinsic capacity to undergo initial deformation as assayed through the Deformability Index distributions obtained by an Automated Rheoscope and Cell Analyzer (supplemental Figure 1). To further investigate the effect of inhibiting these 2 kinases, we used a microfluidics-based system (previously published by Lizarralde-Iragorri et al19 ) to subject the cells to shear stress and successive deformations in an observable manner. The chip is shown in Figure 2B, with an accompanying example of cells passing through the system (which may also be observed in supplemental Video 1) and being subjected to automatic tracking (supplemental Video 2). Through use of this system, we identified a general decrease in microcapillary traversal velocity in both reticulocytes and erythrocytes after treatment to inhibit Lyn or GSK3, as summarized in Figure 2C. In cases in which the comparison P value was higher than the significance cutoff, a decrease in cell velocity is still observed across all samples, with a large variation between samples causing the low significance.
Concomitant treatment with both inhibitors had a compounding effect, resulting in near-complete inability of cells to traverse the microfluidics chip and leading to highly variable results (eg, complete blockage of the system led to extremely slow traversal as shown in supplemental Video 3). We thus identify for the first time a requirement for both Lyn and GSK3 for regulating the capacity of cells to undergo successive deformations and resist shear stress.
Cells passing through the microfluidics system can be lysed near-immediately after deformation (we estimate there to be a maximum interval of 3 seconds between the last constriction point and the end of the capillary traversal), an interval that we considered significantly different to the approximately 5-minute period necessary to obtain samples following microsphiltration. Therefore, we combined the phosphoproteomics data from both experiments to create a comprehensive integrative dataset (shown in Table 1, with processed phosphoproteomics data from the microfluidics experiment provided in supplemental Table 2), which lists the phosphorylation sites most likely to be modulated as a result of shear stress recognition in the reticulocyte, generating a unique and unprecedented dataset that characterizes a highly transient state of this dynamic cell.
Discussion
Studying the dynamic processes involved in reticulocyte maturation or changes in the erythrocyte in response to mechanical stimulus presents inherent challenges due to the short-lived nature of said processes and, consequently, the technical difficulties of obtaining cells in a physiologically relevant state of deformation/posttranslational modification. Previous proteomic studies of the RBC and reticulocyte have mostly focused mainly on deriving definitive descriptive proteomes or studying quantitative differences in protein content between these cell types,23,40-43 although we have also recently published a dataset that characterizes important differential phosphorylation between erythrocytes and endogenous/cultured reticulocytes.23 However, none of these studies have investigated the possibility of posttranslational modifications actively changing within the cell over time as a direct consequence of the physiological stress involved in circulation. Previous studies have indicated that signaling pathways are at least involved in the changes induced in RBC aging,13 but the use of pharmacological compounds to induce a response will most likely generate outcomes that range beyond what the cell normally experiences.
Therefore, in this study, we report the first qualitative phosphoproteomic dataset that dissects the response to deformation and shear stress in the human reticulocyte and erythrocyte, identifying previously unreported protein modifications and signaling pathways and investigating the impact of inhibiting part of those pathways.
To achieve this, we have combined the use of 2 established systems that mimic physiological deformation of RBCs19,24 with high-throughput proteomics based on titanium dioxide enrichment, allowing us to investigate the difference in phosphorylation events present before, during and after deformation. By combining datasets derived from systems that mimic splenic passage and capillary deformation, we identify posttranslational modifications in several reticulocyte membrane and cytoskeletal proteins, including ankyrin, dematin, α/β-spectrin, and Piezo1, which we show are associated with mechanical stimulus and hypothesize may be modulated as part of the shear-induced reticulocyte maturation process due to their detection in reticulocytes occurring only after exposure to deformation.
Focusing on the signaling pathways that seemingly activate upon reticulocyte deformation, we used pharmacological kinase inhibitors to inhibit Lyn and GSK3, 2 kinases with a predicted impact on the erythroid cytoskeleton, and thus identified an effect of those kinases on the cell’s capacity to undergo successive deformations.
Together with Syk, the tyrosine kinase Lyn has been reported to phosphorylate the erythrocyte membrane protein band 3,33 leading to changes in red cell properties, including membrane stability and intracellular pH.44 Altered Lyn kinase activity resulting in band 3 hyperphosphorylation and disruption in erythrocyte membrane-cytoskeletal connectivity and compromised RBC stability is among the defining features of the disease chorea-acanthocytosis.45 The regulation of Lyn activity occurs partially through autophosphorylation of the tyrosine residue in site Y397, which we have detected in this dataset and is well-described to induce further activation of the protein.34 Interestingly, no phosphorylated tyrosine residues of band 3 were found to be exclusive to mid- or postdeformation samples, although the residue targeted by Lyn kinase (tyrosine 359) was observed to be phosphorylated across all samples. It is important to note that our experimental analysis is focused on the identification of phosphopeptides which are uniquely associated with deformation. Therefore, basally phosphorylated proteins for which deformation-induced changes constitute an alteration in abundance of the phosphorylated population would not be included in our list. This approach will undoubtedly result in a smaller list of candidate deformation-associated modifications relative to that which could be achieved using comparative quantitative alternatives (such as TMT labeling). Nevertheless, it both enables a greater input of material to be used (increasing the likelihood of detecting phosphopeptides that are only present in low abundance) and, crucially, generates targets that are easier to modulate experimentally because no quantity-based effects need to be accounted for when interfering with protein activity (given that these modifications are not present in the nondeformed cell and thus are not essential for their survival, at least in vitro).
In contrast to Lyn, the impact of GSK3α in RBCs is markedly less well-characterized. GSK3 is a serine/threonine kinase which has been shown to phosphorylate multiple sites in β-adducin and consequently regulate its activity in cortical neurons.35 In erythrocytes, β-adducin interacts with band 3 and with the spectrin-actin cytoskeleton, stabilizing the junctional complex and regulating membrane deformability.36 No phosphorylated residue of β-adducin was detected in our datasets to be exclusive to cells undergoing or recovering from deformation; however, given the presence of the GSK3-phosphorylated β-adducin peptide in the predeformation samples and the previously described limitations, it is tantalizing to speculate that modification of this site could be involved in the regulation of membrane-cytoskeletal connectivity of RBCs.
Interestingly, a compounding effect was repeatedly observed when inhibiting the 2 kinases, suggesting the existence of independent mechanisms that are activated by the same initial stimulus. However, neither inhibitor affected the inherent capacity of the cells to undergo deformation (as assessed by rheoscopy) in contrast to treatment with an anti-GPA antibody. Although we cannot exclude that varying shear stress levels or exposure times induced by the different modalities may contribute to this result, we hypothesize that inhibiting these mechanisms results in an inability to recover from successive deformations rather than an inability to deform. The initial deformability of the cell could, therefore, be primarily related to its inherent biophysical properties46 and not necessarily to a signaling pathway response (especially given the very short timeframe required for deformation). Nonetheless, as showcased by our functional assay studies, kinase activity and thus phosphorylation is clearly an important event in the regulation of physiological deformation.
In conclusion, our results not only present previously undescribed mechanisms for shear stress and deformation response in human erythrocytes and reticulocytes, but also serve as a valuable resource for further mechanistic dissection of the molecular pathways that underlie the RBC’s response to mechanical stimuli and for the study of reticulocyte maturation.
For original data, please contact t.satchwell@bristol.ac.uk or ash.m.toye@bristol.ac.uk.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE47 partner repository with the dataset identifiers PXD013652 and PXD013960, with corresponding DOIs 10.6019/PXD013652 and 10.6019/PXD013960.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Stephanie Pellegrin (University of Bristol, Bristol, United Kingdom) for her contributions to CD34+ cell isolation and culture; Kate Heesom and Marieangela Wilson, as well as the proteomics facility of the University of Bristol, for proteomics sample processing and data acquisition; Stephen Cross, as well as the Wolfson Bioimaging Facility of the University of Bristol, for helpful discussions and macro creation for imaging data preprocessing; and Judith Cluitmans (Radboud University Medical Center, Nijmegen, The Netherlands) for support in initial establishment of microsphiltration assays.
P.L.M. was funded by the European Union (H2020-MSCA-ITN-2015, “RELEVANCE,” grant agreement number 675117). M.A.L.I. and W.E.N. were funded by the INSERM, the Institut National de la Transfusion Sanguine, and the Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051; GR-Ex is funded by the program “Investissements d’avenir” of the French National Research Agency, reference ANR-11-IDEX-0005-02. M.A.L.I. was additionally funded by the Ministère de l’Enseignement Supérieur et de la Recherche (Ecole Doctorale BioSPC) and received financial support from the Club du Globule Rouge et du Fer and the Société Française d’Hématologie. O.F. and B.L.P. acknowledge the Labex LaSIPS (ANR-10-LABX-0040-Lasips), the Institut d’Alembert and the CNRS for project funding (microfluidics). A.M.T. and T.J.S. were funded by a National Health Service Blood and Transplant research and development grant (WP15-04 and WP15-05) and the National Institute for Health Research Blood and Transfusion Research Unit (NIHR BTRU) in Red Cell Products (NIHR-BTRU-2015-10032).
The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or the Department of Health and Social Care.
Authorship
Contribution: P.L.M. performed the majority of experiments, analyzed data, and prepared figures; M.A.L.I. and P.L.M. performed microfluidics experiments under supervision of W.E.N.; O.F. and B.L.P. provided essential reagents for microfluidics experiments; J.G.G.D. and G.J.S. provided essential Automated Rheoscope and Cell Analyzer equipment and analysis software; P.L.M, M.A.L.I., W.E.N., A.M.T., and T.J.S. conceived and designed experiments; P.L.M., A.M.T., and T.J.S. wrote the manuscript; A.M.T. and T.J.S. contributed equally to conception and supervision of the work; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy J. Satchwell, School of Biochemistry, University of Bristol, Medical Science Building, University Walk, Bristol BS8 1TD, United Kingdom; e-mail: t.satchwell@bristol.ac.uk; or Ashley M. Toye, School of Biochemistry, University of Bristol, Medical Science Building, University Walk, Bristol BS8 1TD, United Kingdom; e-mail: ash.m.toye@bristol.ac.uk.
References
Author notes
A.M.T. and T.J.S. contributed equally to this work.