Key Points
MAC leads to significantly increased infections per 100 days at risk (infection density) compared with RIC/NMA in the first 100 days.
These higher infection rates were caused by a significantly higher bacterial infection density in the MAC group.
Abstract
Presumably, reduced-intensity/nonmyeloablative conditioning (RIC/NMA) for allogeneic hematopoietic cell transplantation (alloHCT) results in reduced infections compared with myeloablative conditioning (MAC) regimens; however, published evidence is limited. In this Center for International Blood and Marrow Transplant Research study, 1755 patients (aged ≥40 years) with acute myeloid leukemia in first complete remission were evaluated for infections occurring within 100 days after T-cell replete alloHCT. Patients receiving RIC/NMA (n = 777) compared with those receiving MAC (n = 978) were older and underwent transplantation more recently; however, the groups were similar regarding Karnofsky performance score, HCT–comorbidity index, and cytogenetic risk. One or more infections occurred in 1045 (59.5%) patients (MAC, 595 [61%]; RIC/NMA, 450 [58%]; P = .21) by day 100. The median time to initial infection after MAC conditioning occurred earlier (MAC, 15 days [range, <1-99 days]; RIC/NMA, 21 days [range, <1-100 days]; P < .001). Patients receiving MAC were more likely to experience at least 1 bacterial infection by day 100 (MAC, 46% [95% confidence interval (CI), 43-49]; RIC/NMA, 37% [95% CI, 34-41]; P = .0004), whereas at least a single viral infection was more prevalent in the RIC/NMA cohort (MAC, 34% [95% CI, 31-37]; RIC/NMA, 39% [95% CI, 36-42]; P = .046). MAC remained a risk factor for bacterial infections in multivariable analysis (relative risk, 1.44; 95% CI, 1.23-1.67; P < .0001). Moreover, the rate of any infection per patient-days at risk in the first 100 days (infection density) after alloHCT was greater for the MAC cohort (1.21; 95% CI, 1.11-1.32; P < .0001). RIC/NMA was associated with reduced infections, especially bacterial infections, in the first 100 days after alloHCT.
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) is increasingly used for the treatment of hematologic malignancies. Due to the development of nonmyeloablative (NMA) or reduced-intensity conditioning regimens (RICs), older patients and patients with comorbidities are more frequently offered alloHCT.1-4 Overall survival seems similar in patients with hematologic malignancies after either a myeloablative (MAC) or an RIC/NMA regimen. However, an increased relapse is offset by a reduced nonrelapse mortality (NRM) and affects relapse-free survival.5-14 Bacterial infections early after alloHCT are associated with increased mortality.15 Factors associated with decreased infections after alloHCT include less mucositis,16-19 shorter duration and decreased severity of neutropenia/lymphopenia,16 and faster immune recovery,20 all of which are observed more frequently with RIC/NMA than with MAC.21,22 The incidence of infections, a common and often severe complication of alloHCT, is expected to be lower after RIC/NMA compared with MAC and thus contribute to the decreased NRM.23-25
In this large Center for International Blood and Marrow Transplant Research (CIBMTR) retrospective study, we compared the incidence and outcomes of bacterial, viral, and fungal infections occurring in the first 100 days after alloHCT in adult patients with acute myeloid leukemia (AML) in first complete remission receiving RIC/NMA or MAC.
Methods
The CIBMTR is a working group of >400 transplantation centers worldwide that contribute detailed data on HCT patients to the statistical center at the Medical College of Wisconsin. Participating centers are required to report all consecutive transplantations and to follow up patients longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality.
The CIBMTR performs observational studies in compliance with all applicable federal regulations pertaining to the protection of human research participants. The CIBMTR collects data at 2 levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, sex, pretransplantation disease stage and chemotherapy responsiveness, date of diagnosis, graft type (bone marrow–derived and/or blood-derived stem cells), conditioning regimen, posttransplantation disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR centers contribute TED data. A subset of registered patients selected by weighted randomization has CRF data that include more detailed disease and pretransplantation and posttransplantation clinical information, including infection data. TED- and CRF-level data are collected pretransplantation, 100 days and 6 months post-HCT, and annually thereafter or until death. The current analysis includes only CIBMTR CRF data. All patients provided written informed consent.
The Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin approved this study.
Patients
The study population consisted of: (1) patients aged ≥40 years (patients aged <40 years were excluded, given that younger patients with AML were expected to receive a MAC but actually received an RIC most likely indicates an existent comorbid condition and would have led to inherent biases); (2) patients who received alloHCT for AML in first complete remission (to decrease heterogeneity in previous chemotherapy and thus in previous infectious exposures); and (3) patients whose data were reported to the CIBMTR between 2006 and December 2013. All T-replete donor sources (ie, sibling, unrelated donors, umbilical cord blood [UCB]) except haploidentical donors and all graft sources (ie, peripheral blood, bone marrow, UCB) were included. HLA matching criteria were defined as previously reported.26 Previous alloHCT, ex vivo T-cell depletion, HIV seropositivity, ex vivo manipulated UCB, and UCB accompanied by another source of stem cells were excluded. There was no exclusion regarding conditioning regimen (eg, antithymocyte globulin [ATG], total body irradiation). Centers without patients in both the MAC and RIC/NMA categories were excluded to minimize center bias.
Definitions
Infection data reported to the CIBMTR are captured as a microorganism, site(s) of infection, and date of onset of infection. No information on symptoms, diagnostic criteria, or therapy of infection is collected. Infections are determined by the center as significant for reporting based on educational information and center-specific diagnostic test results. Because patients can have multiple infections, recurrent infections require a period of negative cultures, which differ by microrganism.27 Furthermore, multiple infections require assessment of infection density determined by the number of infections per potential days at risk during the first 100 days after transplantation.28 Conditioning intensity was determined by using standard criteria.29 Neutropenia is defined as the time from transplantation until the absolute neutrophil count was >0.5 × 109/L for the first of 3 days after the nadir.
End points
The primary end point was the comparison of the incidence of any infection within the first 100 days posttransplantation based on conditioning intensity (MAC vs RIC/NMA). Secondary end points specifically compared the incidence of bacterial, viral, and fungal infections between conditioning intensities as well as infection density for total infections and according to microbial category to account for multiple infections.
Statistical analysis
Patient-, disease-, and treatment-related factors were compared by using the χ2 test if variables were categorical or the Mann-Whitney U test if variables were continuous. Cumulative incidence of infection examined the first event of infection (overall) or according to the type of infection (viral, fungal or bacterial) using death as a competing risk. Poisson regression examined infection density as a ratio of MAC to RIC/NMA to account for multiple infections for both overall infections and according to microbial category.
A cause-specific hazards model was conducted for multivariable analysis for infections to handle competing risks and was examined for the primary and secondary outcomes of infection.30 Variables examined in each model included the main effect variable (MAC vs RIC/NMA). Presumed risk factors for infection, including age, Karnofsky performance score, cytogenetic risk group, time from diagnosis to transplantation, HCT–comorbidity index, donor/recipient HLA match, use of total body irradiation, use of ATG/alemtuzumab, history of pre-HCT fungal infection, occurrence of neutrophil recovery before infection, and occurrence of acute graft-versus-host disease (GVHD) before infection, were examined in each model. For each bacterial, viral, and fungal infection, the other 2 infections are added as time-dependent covariates examined as occurring before the infection of interest. The stepwise selection method was used to identify significant risk factors associated with the outcomes. Factors significantly associated with the outcome variable at a 5% level were retained in the final model. Neutrophil recovery and acute GVHD were then tested as time-dependent covariates. If at least 1 of either neutrophil recovery or acute GVHD was significant and at least 1 of the other 2 infections was significant, the interaction between them was examined. The proportional hazards assumption was examined. If violated, the variable was reported as a time-dependent covariate. The center effect was examined by using the score test of Commenges and Andersen.31 Interactions between the main effect and significant covariates were examined. In particular, an interaction between the main effect and recipient age was checked for all outcomes. Models using propensity scoring were examined, with outcomes similar to results of cause-specific modeling; therefore, only cause-specific models are reported.
Results
Patient characteristics and general outcomes
Table 1 shows the characteristics of the patient cohort. All patients had AML in first remission, with 978 patients receiving MAC and 777 patients receiving RIC/NMA. Patients receiving RIC/NMA were older and underwent transplantation more recently. The groups were similar regarding Karnofsky performance score, HCT–comorbidity index, and cytogenetic risk; however, patients receiving RIC/NMA had more frequent previous myelodysplastic syndrome (MDS). As pertains to infection risks, the groups had a similar frequency of fungal infection before alloHCT and planned use of growth factors but those receiving RIC/NMA had more in vivo T-cell depletion with either ATG or alemtuzumab.
Although the median time from diagnosis to transplantation for both MAC and RIC/NMA transplant recipients was 5 months, the ranges differed (2-12 months for MAC recipients and <1-13 months for RIC/NMA recipients), with 73% of the MAC group receiving their transplant <6 months from diagnosis vs 65% in the RIC/NMA group (P < .001). The median time to engraftment and GVHD, as well as the cumulative incidence of acute (grade II-IV) and chronic GVHD, is presented in Table 2. The median follow-up of survivors was longer after MAC (MAC, 59 months [range, 3-102 months]; RIC/NMA, 48 months [range, 3-105 months]; P = .002). At 1 year posttransplant, relapse predominated as the primary cause of death (MAC, 47%; RIC/NMA, 54%) (Table 3 ). Infection as a primary or contributing cause of death was reported in 33% of MAC patients and 25% of RIC/NMA patients.
Effect of conditioning regimen on posttransplantation infections by day 100
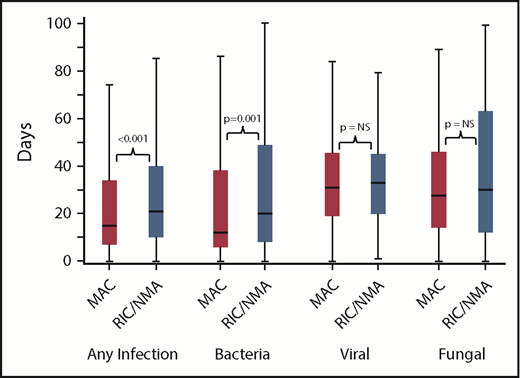
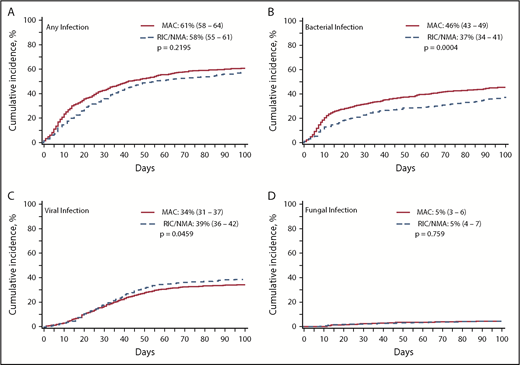
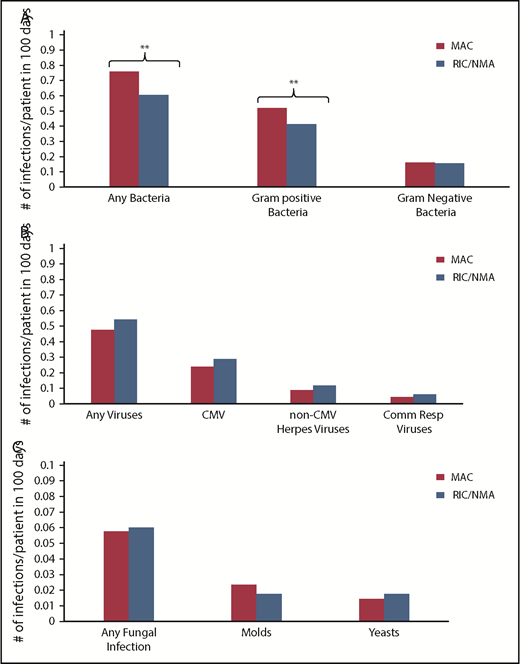
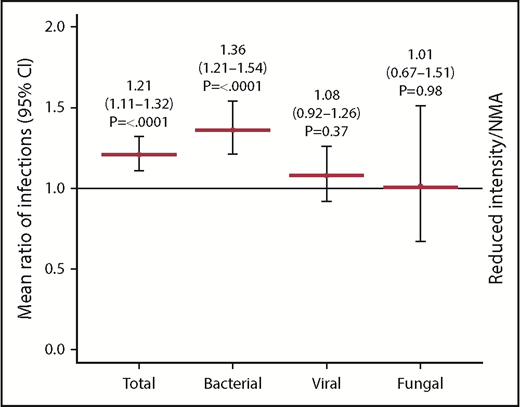
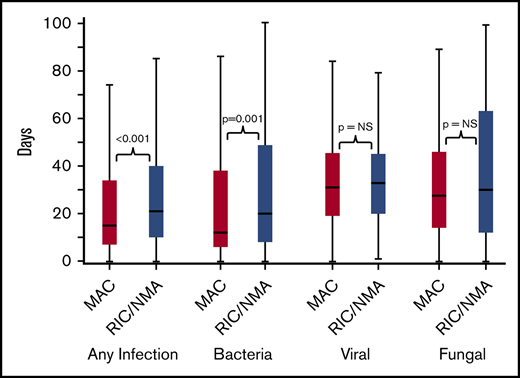
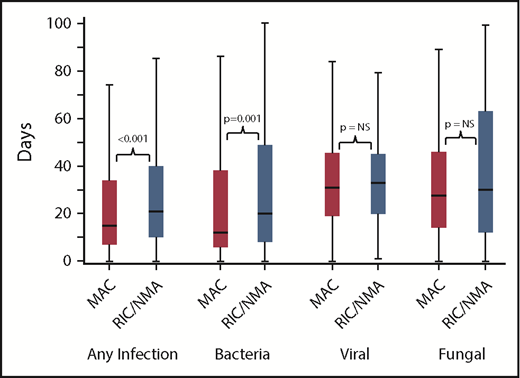
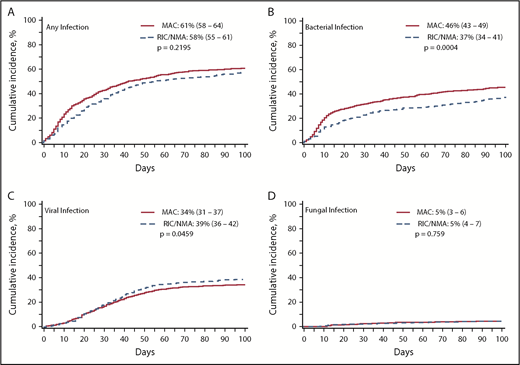
Of 1755 patients, 1045 (59.5%) patients (MAC, 595 [61%]; RIC/NMA, 450 [58%]; P = .21) experienced at least 1 infection. The median time to infection for patients after MAC conditioning occurred at 15 days (range, <1-99 days) and was 21 days (range, <1-100 days) for the RIC/NMA cohort (P < .001) (Figure 1). The cumulative incidence of infections is shown in Figure 2. Patients receiving MAC had a greater probability of developing at least 1 bacterial infection by day 100 (cumulative incidence: MAC, 46% [95% confidence interval (CI), 43-49]; RIC/NMA, 37% [95% CI, 34-41]; P = .0004), whereas patients in the RIC/NMA cohort were more likely to develop at least 1 viral infection (MAC, 34% [95% CI, 31-37]; RIC/NMA, 39% [95% CI, 36-42]; P = .046). In addition, the rate of any infection, accounting for multiple infections, per patient days at risk in the first 100 days (infection density) after HCT was greater for the MAC cohort. Infection densities for broad subsets of bacterial, viral, and fungal infections are shown in Figure 3. The increased bacterial infections after MAC were due to gram-positive bacteria, and the increased viral infections in RIC/NMA were due to cytomegalovirus (CMV). Setting the mean of RIC/NMA infection rate at 1, the mean ratio of any infection in the MAC group was significantly higher at 1.21 (95% CI, 1.11-1.32) infections per patient in the first 100 days (P < .0001) (Figure 4). These higher infection rates were caused by a significantly higher bacterial infection density in the MAC group at 1.36 (95% CI, 1.21-1.54) bacterial infections per patient in the first 100 days (P < .0001). Fungal and viral infection rates were similar between the MAC and the RIC/NMA groups.
Time to infections. The median onset and interquartile range for development of infections (any, bacterial, viral, or fungal) comparing patients receiving MAC vs RIC/NMA conditioning. NS, not significant.
Time to infections. The median onset and interquartile range for development of infections (any, bacterial, viral, or fungal) comparing patients receiving MAC vs RIC/NMA conditioning. NS, not significant.
Incidence of infections. The cumulative incidence of any infection (A), bacterial infection (B), viral infection (C), and fungal infection (D) occurring by day 100 in patients receiving MAC or RIC/NMA conditioning.
Incidence of infections. The cumulative incidence of any infection (A), bacterial infection (B), viral infection (C), and fungal infection (D) occurring by day 100 in patients receiving MAC or RIC/NMA conditioning.
Density of infections. Infection density for specific bacterial (A), viral (B), and fungal (C) infections according to conditioning intensity regimen. **P ≤ .001. The remaining comparisons were not significant.
Density of infections. Infection density for specific bacterial (A), viral (B), and fungal (C) infections according to conditioning intensity regimen. **P ≤ .001. The remaining comparisons were not significant.
Relative ratio of density of infections. Infection density, defined as the number of infections according to type (overall, bacterial, viral, and fungal) based on the patient days at risk in the first 100 days. The Poisson regression normalizes the rate as a ratio with RIC/NMA at 1 and shows a higher risk of overall infections and bacterial infections in recipients of MAC in the first 100 days after alloHCT.
Relative ratio of density of infections. Infection density, defined as the number of infections according to type (overall, bacterial, viral, and fungal) based on the patient days at risk in the first 100 days. The Poisson regression normalizes the rate as a ratio with RIC/NMA at 1 and shows a higher risk of overall infections and bacterial infections in recipients of MAC in the first 100 days after alloHCT.
Risk factors for infection
In multivariable analysis (Table 4), a center effect was observed and adjusted for when assessing the risk of developing any infection and viral infections by day 100 but was not observed for either bacterial or fungal infection. Patients receiving MAC conditioning, compared with those receiving RIC/NMA conditioning, had a significantly increased risk of any infection, bacterial, fungal, or viral (relative risk [RR], 1.28; 95% CI, 1.11-1.48; P < .001) and bacterial infections (RR, 1.44; 95% CI, 1.23-1.67; P < .0001) but not viral (RR, 1.00; 95% CI, 0.82-1.21; P = .9930) or fungal (RR, 0.99; 95% CI, 0.64-1.54; P = .969) infections.
Additional factors associated with an increased risk of any infection include UCB as the stem cell source (RR, 1.81; 95% CI, 1.40-2.34; P < .0001), use of ATG/alemtuzumab (RR, 1.33; 95% CI, 1.13-1.55; P = .0004), HCT–comorbidity index ≥3 (RR, 1.19; 95% CI, 1.02-1.38; P = .0008), and development of acute GVHD grades II to IV (RR, 1.53; 95% CI, 1.33-1.77; P < .0001). In addition to MAC, there was an increased risk of bacterial infections for patients receiving UCB (RR, 1.57; 95% CI, 1.27-1.95; P ≤ .0001), having a previous viral infection (RR, 1.82; 95% CI 1.46-2.26; P < .0001), or experiencing a previous fungal infection (RR, 2.13; 95% CI, 1.38-3.30; P = .0007). As expected, neutrophil recovery (ie, resolution of neutropenia due to engraftment) decreased the risk of bacterial infection (RR, 0.60; 95% CI, 0.44-0.81; P = .0009). An increased risk of viral infections was observed in patients with an HCT–comorbidity index ≥3 (RR, 1.26; 95% CI, 1.03-1.55; P = .0270), UCB (RR, 2.63; 95% CI, 2.06-3.35; P < .0001), partially matched/mismatched unrelated donors (RR, 1.46; 95% CI, 1.10-1.93; P = .0078), previous acute GVHD (RR, 1.73; 95% CI, 1.46-2.06; P < .0001), and previous bacterial infection (RR, 1.40; 95% CI, 1.18-1.67; P = .0002). In addition, patients receiving ATG/alemtuzumab had an increased risk of viral infections during the first 39 days posttransplantation (RR, 2.48; 95% CI, 1.94-3.15; P < .0001); however, by day 40, this negative impact was lost. Moreover, there was no interaction between conditioning intensity and ATG/alemtuzumab. For fungal infections, the risk increased for recipients of UCB (RR, 2.65; 95% CI, 1.41-5.00; P = .0026), those with previous bacterial infections (RR, 1.81; 95% CI, 1.41-2.89; P = .0119), or those with previous viral infections (RR, 2.17; 95% CI, 1.28-3.67; P = .0038) but was decreased with neutrophil recovery (RR, 0.37; 95% CI, 0.18-0.74; P = .0053). Total body irradiation was not associated with infections in multivariable analysis.
Discussion
Our data show that, for patients receiving alloHCT for AML in first remission, MAC was associated with greater infection events of any type (bacterial, fungal, or viral) in the first 100 days after transplantation. This effect was driven primarily by bacterial infections. Viral infections were slightly higher in patients receiving RIC/NMA conditioning, and fungal infections were similar regardless of conditioning intensity. Beyond conditioning intensity, additional risk factors for infection varied according to the infection examined, although receipt of a UCB graft and a previous infection of another type were consistent across the multivariable analyses.
A higher incidence of bacterial infections after MAC has been previously reported. Kim et al32 reported that bacterial infections were higher during the entire follow-up period of ∼700 days (14% vs 5%; P = .012) after MAC, whereas the incidence of fungal and viral infections was similar after MAC compared with RIC. That study reported that both bacterial infections preengraftment and from engraftment to day 100 were greater in the MAC group compared with the RIC group (8% vs 4% [P = .269] and 4% vs 0% [P = .095], respectively). Of note, cumulative incidence of infections was less common in the study of Kim et al than that of our study, perhaps due to a much younger population (median age, 35 years). Neutropenia is a known risk for bacterial infections. Although several studies have reported shortened time to engraftment after RIC/NMA, our cohorts had a similar median time to neutrophil recovery.6,33-36 However, the current data show that neutrophil recovery decreased the risk of bacterial infection by 40%. It is possible that bacterial infections are influenced not only by duration of neutropenia but also by the severity of neutropenia.24,34 Unfortunately, the CIBMTR does not capture severity, only the time to neutrophil engraftment; severity was therefore not examined in this analysis. Interestingly, UCB, known to result in prolonged time to engraftment, was an independent risk factor for bacterial infections and corroborates a previously published CIBMTR analysis.20,37 Another hypothesis for increased bacterial infections after MAC is that most early bacterial infections originate from gastrointestinal (GI) flora and that MAC causes increased mucosal damage in the GI tract.36,38,39 Acute GVHD, reportedly more common with MAC compared with RIC,5,7,8,40,41 might be another reason for more GI mucosal damage. Although our data showed an increased incidence of acute GVHD grades II to IV after MAC, the development of acute GVHD before an infection was associated with an increased risk of viral infections specifically, but it also increased the risk of any infection in the first 100 days. Our data indicate that these bacterial infections are driven by gram-positive organisms often associated with oropharyngeal flora rather than the gram-negative bacteria associated with lower GI tract damage. Consequently, the impact of mucosal damage from conditioning may be the driver for bacterial infection, whereas the increased immunosuppression associated with acute GVHD treatment may trigger the higher risk for viral infections.
We identified a slightly higher probability of developing a single viral infection by day 100 in the RIC/NMA group compared with the MAC group; however, the number of viral infections when accounting for multiple viral infections per patient per days at risk (infection density) was similar between the 2 groups. This result suggests that those patients receiving MAC and developing at least a single viral infection were either more likely to have multiple viral infections more frequently than the RIC/NMA group, that the days at risk in the RIC/NMA group were less because of early mortality, or a combination of both factors. The published data are conflicting regarding the effect of conditioning intensity on viral infections. Similar to our data, Satwani et al42 reported that viral infections were not significantly affected by conditioning intensity in a pediatric cohort. However, when examining only CMV, a small, randomized prospective study reported that CMV infection was more common in patients receiving MAC (14 of 19) compared with RIC (6 of 18; P = .02).19 Notably, a larger study found that CMV-seropositive recipients had less high-grade CMV viremia (CMV pp65 antigenemia >10/200 000 peripheral blood leukocytes or polymerase chain reaction [PCR] >1000 copies/mL) (hazard ratio, 0.7; 95% CI, 0.5-0.9; P = .02) after NMA than after MAC; however, there was no difference in overall CMV infection rates.23 Although our study did not examine CMV alone, a comparison of the median infection density found similar rates of CMV infection after MAC and RIC/NMA conditioning. As expected, factors associated with increased immunosuppression were associated with viral infections by day 100. Interestingly, use of ATG or alemtuzumab as a component of conditioning or GVHD prophylaxis was nonproportional in the multivariable analysis and associated with early viral infections before 40 days; however, by 40 days after transplantation, the impact was lost. ATG use was reportedly associated with increased NRM in a previous CIBMTR study.43 Furthermore, the development of acute GVHD preceding infection was associated with increased risk of viral infections, most likely due to the initiation or addition of immunosuppression to treat the episode of acute GVHD, as well as the innate immune dysfunction associated with GVHD precluding the patient from clearing a viral infection and/or preventing viral reactivation.
Fungal infections, fortunately, remain a rare event in the first 3 months after transplantation, with a similar occurrence regardless of conditioning intensity. In accordance with our data, other studies have not identified a difference in fungal infections or an increased risk of atypical mold infections based on conditioning intensity.42,44 Notable factors that contributed include receipt of UCB and lack of neutrophil recovery by the time of infection. Furthermore, a previous bacterial infection and a previous viral infection both resulted in an increased risk of subsequent fungal infection. Congruent with our data, Yong et al45 previously reported that previous CMV reactivation is associated with risk of invasive fungal infection (IFI). This association may simply sow profound immune compromise, although it is notable that neither ATG or alemtuzumab use nor preceding acute GVHD was associated with an increased risk of fungal infection. However, this scenario may simply be a function of too few events. Preexisting IFIs are a known risk factor for posttransplantation IFI and increased mortality.46 In the current study, the numbers of pre-HCT fungal infections were small and similar in each conditioning cohort, potentially accounting for the lack of significance in the multivariable analysis. However, in a CIBMTR study examining the impact of pretransplantation IFI, Maziarz et al46 reported a greater likelihood of experiencing a posttransplantation IFI in patients with preexisting IFI compared with those with no history of IFI (RR, 1.35; 95% CI, 1.16-1.58; P = .001). Notably, conditioning intensity was not an independent risk factor for posttransplantation IFI in that the analysis enriched for patients with pretransplantation IFI.
Our study has limitations. One significant limitation is examination of infections occurring only in the first 100 days. However, the differences in infections mediated by conditioning intensity are expected to occur early, in the peri-transplantation period. Other studies have shown that very early (first 30 days) infections were less common after RIC/NMA compared with MAC but that this differences does not persist.34,35,47 Junghanss et al34 reported that NMA was associated with fewer episodes of bacteremia during the first 30 days (9% vs 27%; P = .01) and a trend to fewer episodes of bacteremia during the first 100 days posttransplantation (27% vs 41%; P = .7). Later infections from ongoing immunocompromise are likely similar, regardless of initial conditioning intensity. As patients leave the transplantation center, common infections such as bacterial pneumonia and viral upper respiratory tract infections are less likely to be reported back to the transplantation center and, consequently, to the CIBMTR, which therefore necessitated the truncation of infections by day 100 for this analysis. The current study did not examine the impact of these infections on survival specifically. This approach was chosen to focus on the frequency and likelihood of infections, in total and by broad category, after these 2 conditioning intensities. However, infection as the reported primary cause of death by 1 year after HCT was similar (MAC, 17%; RIC/NMA, 13%) in our cohorts. Finally, we do not have detailed information regarding antimicrobial prophylaxis, severity of infection, or treatment of infection. In addition, diagnostic criteria for infection or reactivation, such as PCR viral loads, are not available. The prophylaxis data, in particular, may alter frequency of infection. To account for these, our data set was limited to centers with patients in both the MAC and RIC/NMA cohorts because centers would be expected to have fairly uniform antimicrobial prophylaxis regimens, indications for treatment based on viral load PCRs, and antimicrobial management. Furthermore, we also assessed for a center effect to account for potential biases of these unavailable, but pertinent, data. Although patients in the RIC/NMA group had more previous MDS and we did not examine directly its effect, time to transplantation was not significant for any infection in the multivariable analysis.
In conclusion, RIC/NMA alloHCT is associated with a decreased risk of any infection and particularly early bacterial infections. The risk of viral and fungal infections per days at risk is similar. Furthermore, a preceding infection of one type (bacterial/fungal/viral) increases the likelihood of subsequent infection. Recipients of UCB have increased risk of all types of infection. In the future, efforts to modify conditioning regimens, perhaps with more targeted therapies with less toxicity, may decrease infections, especially bacterial infections. Moreover, antibiotic prophylaxis might be modified by conditioning intensity.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health; Grant/Cooperative Agreement 4U10HL069294 from the National Institues of Health, National Heart, Lung, and Blood Institute and National Cancer Institute; contract HHSH250201200016C with the Health Resources and Services Administration (Department of Health and Human Services); and 2 grants (N00014-17-1-2388 and N0014-17-1-2850) from the Office of Naval Research. The CIBMTR is also supported by grants from Actinium Pharmaceuticals, Inc., Amgen, Inc., Amneal Biosciences, Angiocrine Bioscience, Inc., an anonymous donation to the Medical College of Wisconsin, Astellas Pharma US, Atara Biotherapeutics, Inc., Be the Match Foundation, bluebird bio, Inc., Bristol-Myers Squibb Oncology, Celgene Corporation, Cerus Corporation, Chimerix, Inc., Fred Hutchinson Cancer Research Center, Gamida Cell Ltd., Gilead Sciences, Inc., HistoGenetics, Inc., Immucor, Incyte Corporation, Janssen Scientific Affairs, LLC, Jazz Pharmaceuticals, Inc., Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Inc., medac GmbH, MedImmune, The Medical College of Wisconsin, Mediware, Merck & Co., Inc., Mesoblast, MesoScale Diagnostics, Inc., Millennium, the Takeda Oncology Co., Miltenyi Biotec, National Marrow Donor Program, Neovii Biotech NA, Inc., Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Co, Ltd.–Japan, PCORI, Pfizer Inc., Pharmacyclics, LLC, PIRCHE AG, Sanofi Genzyme, Seattle Genetics, Shire, Spectrum Pharmaceuticals, Inc., St. Baldrick’s Foundation, Sunesis Pharmaceuticals, Inc., Swedish Orphan Biovitrum, Inc., Takeda Oncology, Telomere Diagnostics, Inc., and the University of Minnesota.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: C.U., M.L.R., C.A.L., K.V.K., J.J.A., S.K., and M.C. conceived the study and its design; S.K. and M.C. performed the statistical analysis; C.U., M.L.R., C.A.L., K.V.K., J.J.A., S.K., and M.C. analyzed and interpreted the data; C.U., M.L.R., and C.A.L. drafted the manuscript; and all authors contributed patients to the CIBMTR for data acquisition, and all authors revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Celalettin Ustun, Rush University, 1725 W Harrison St, Suite 834, Chicago, IL 60612; e-mail: celalettin_ustun@rush.edu.