Key Points
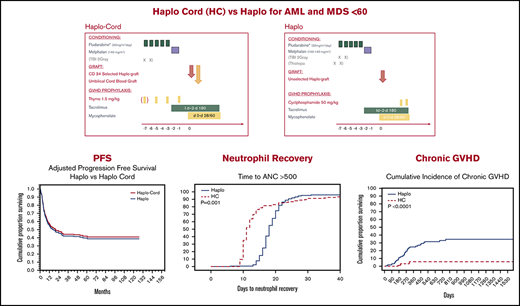
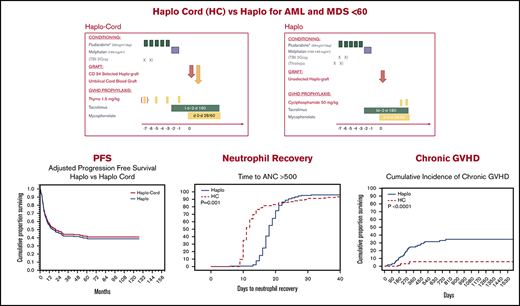
Haploidentical transplant and haplo-cord transplant result in similar and promising survival and progression-free survival.
Neutrophil recovery was considerably faster after haplo-cord transplant; acute and chronic GVHD were decreased after haplo-cord transplant.
Abstract
Haplo-identical transplant with posttransplant cyclophosphamide (haplo) and umbilical cord blood transplant supported by third-party CD34 cells (haplo-cord) are competing approaches to alternative donor transplant. We compared, in adults younger than age 60 years, the outcomes of 170 haplo at 1 institution with that of 137 haplo-cord at 2 other institutions. All received reduced intensity conditioning with fludarabine and melphalan ± total body irradiation. GVHD prophylaxis for haplo consisted of cyclophosphamide, tacrolimus, and mycophenolate, whereas haplo-cord received antithymocyte globulin, tacrolimus, and mycophenolate. Haplo transplant used mostly bone marrow, and peripheral blood stem cells were used in haplo-cord transplants. Haplo-cord were older and had more advanced disease. Haplo-cord hastened median time to neutrophil (11 vs 18 days, P = .001) and platelet recovery (22 vs 25 days, P = .03). At 4 years, overall survival (OS) was 50% for haplo-cord vs 49% for haplo. Progression-free survival (PFS) was 40% for haplo-cord vs 45% for haplo. In multivariate analysis, the disease risk index was significant for OS (hazard ratio, 1.8; 95% confidence interval, 1.48-2.17; P = .00) and PFS. Total body irradiation was associated with decreased recurrence and improved PFS, age >40 with increased nonrelapse mortality. The type of transplant had no effect on OS, PFS, relapse, or nonrelapse mortality. Cumulative incidence of grade 2-4 acute graft-versus-host disease (GVHD) by day 100 was 16% after haplo-cord vs 33% after haplo (P < .0001), but grade 3-4 GVHD was similar. Chronic GVHD at 1 year was 4% after haplo-cord vs 16% after haplo (P < .0001). Haplo or haplo-cord results in similar and encouraging outcomes. Haplo-cord is associated with more rapid neutrophil and platelet recovery and lower acute and chronic GVHD. Institutional review board authorization for this retrospective study was obtained at each institution. Some patients participated in trials registered at www.clinicaltrials.gov as #NCT01810588 and NCT 01050946.
Introduction
Older patients, smaller families, ethnic diversity, and multiethnicity all contribute to lower likelihoods of identifying matching donors. Haploidentical transplantation (haplo) with posttransplant cyclophosphamide (PTCy) has quickly emerged as an appealing option for those lacking matching donors.1 Haplo relies on partially matched first- or second-degree relatives, readily available in ∼80% of patients.2 Reduced-intensity conditioning partnered with PTCy achieves low rates of nonrelapse mortality (NRM), and encouraging survival, with acceptable rates of graft-versus-host-disease (GVHD). The Haplo procedure is based on the powerful GVHD prevention potential of high-dose cyclophosphamide that seems, however, to spare aldehyde-low T cells, which may preserve immunity and graft-versus-leukemia (GVL) activity, and quiescent hematopoietic stem cells that reconstitute recipient’s hematopoiesis.3 But recent data raise concerns over HLA loss as a mechanism of relapse in haplo transplant.4
Other centers have used umbilical cord blood (UCB) transplants which may mediate superior GVL effects, cause less chronic GVHD and are nearly universally available.5,6 Many have observed decreases in relapse rates after UCB transplant that may be further enhanced by careful selection of the cord blood graft.5-7 To hasten hematopoietic recovery after UCB transplant, double UCB grafts or in vitro expansion of UCB cells have been used, with varying success.6,8 The delayed engraftment of cord blood cells can be readily avoided by third-party donor cells, usually derived from family members, but occasionally also from unrelated donors.9 This procedure, which we labeled haplo-cord transplant, requires addition of antithymocyte globulin (ATG) to prevent violent rejection of the haplo-graft and a second nadir before cord blood engraftment.10
Here, we compare in patients up to age 59 the outcomes of melphalan-based reduced-intensity conditioning and haploidentical transplantation with PTCy vs haplo-cord transplantation.
Methods and patients
This is a retrospective study of consecutive patients age 18 to 59 undergoing haplo-cord transplantation using conditioning that included fludarabine and melphalan at the University of Chicago and Weill Cornell Medical College in New York. Second transplants were excluded as were 2 patients with hematological malignancy and HIV, 1 with human T-cell lymphotropic-related leukemia, and 1 with simultaneous breast cancer and leukemia. Haplo-cord transplant in whom unrelated donors of CD34+ cells were used, was also excluded.11 Patients participated in trials registered at www.clinical trials.gov as #NCT01810588 and NCT 01050946. The outcomes were compared with those of consecutive patients ages 18 to 59, undergoing haplo transplantation using conditioning that included fludarabine and melphalan, and GVHD prophylaxis that included PTCy, tacrolimus, and mycophenolate at MD Anderson Cancer Center. Second transplants were excluded. All patients and donors signed institutional review board-approved consent forms that allowed analysis and dissemination of their outcome data. A Material Transfer Agreement was signed between the institutions to share and analyze data.
Donor selection criteria, stem cell processing, conditioning, and GVHD prophylaxis for haplo-cord recipients
Cord-blood units were selected based on HLA-typing and total nucleated cell (TNC) count. Grafts were matched for at least 4 of 6 HLA loci by the standard cord criteria (ie, low resolution for HLA-A and HLA-B and high resolution for HLA-DR) and contained a minimum cell count of 1.5 × 107 TNC per kgrec (kilogram recipient body weight) before freezing. In contrast with common practice, we prioritized matching over TNC. As of mid-2012, we used high-resolution HLA typing for HLA A, B, C, and DR for UCB graft selection.
The haploidentical donor providing CD34+ cells was a first- or second-degree relative. Donors received filgrastim for 4 consecutive days. Apheresis was started on day 5 and continued until 5 × 106 CD 34+ cells/kgrec were collected. Haploidentical grafts were T cell–depleted initially using the Isolex 300i CD34 selection device. As of early April 2010, the Miltenyi CliniMACS was used under an investigational new drug filing from the US Food and Drug Administration. In the initial protocol (NCT00943800), the cell dose of the haplo donor was based on CD3+ cell dose (<1 × 106 CD3/kgrec).12 Subsequently, the cell dose of the haplo graft was based on CD34+ dose with a target dose of 3 to 5 × 106 CD34/kgrec.
As of the tenth patient enrolled on the initial protocol, UCB and haploidentical donor selection were also based on avoidance of donor specific HLA antibodies (DSA). A donor targeted by preexisting DSA was avoided or, when unavoidable, various strategies were used to limit exposure of the graft to DSA.13
The conditioning for haplo-cord patients consisted of fludarabine 30 mg/m2 × 5, melphalan 140 mg/m2, thymoglobulin 1.5 mg/kg for 3 or 4 doses. Total body irradiation (TBI) 400 cGy was added in 25 cases. Posttransplant GVHD prophylaxis consisted of tacrolimus until day 180, mycophenolate mofetil initially until day 60, and, for more recent patients, until day 28.
Donor selection criteria, stem cell collection, conditioning, and GVHD prophylaxis for haplo transplant recipients
The haploidentical donors were first-degree relatives who underwent bone marrow harvest. Occasionally, donors underwent stem cell mobilization using filgrastim. Apheresis was started on day 5 and continued until at least 5 × 106 CD 34+ cells/kgrec were collected.
Conditioning included melphalan 140 mg/m2 (100 mg/m2 for patients older than 55 and/or significant comorbidities) fludarabine 40 mg/m2 × 4 days and 1 dose of 200 cGy TBI or thiotepa 5 mg/kg. GVHD prophylaxis included PTCy 50 mg/kg on days +3 and +4, tacrolimus until day 180, and mycophenolate until day 90 followed by a taper, unless otherwise indicated. Patients with DSA were avoided or received treatment as previously described.13
End point definitions
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of 0.5 × 109/L, and platelet engraftment as the first of 7 consecutive days with a platelet count of 20 × 109/L without platelet transfusion. Acute GVHD was diagnosed and graded according to consensus criteria. Chronic GVHD was assessed by traditional criteria (limited vs extensive).14 NRM was defined as death without evidence of relapse/progression of malignancy.
Statistical analysis
Comparison between groups used χ2 testing for categorical variables and t tests for continuous variables. Probabilities of NRM and relapse were generated using cumulative incidence estimates to accommodate competing risks. The Gray test was used to assess the statistical significance of a prognostic factor in cumulative incidence analysis. The incidences of acute GVHD and chronic GVHD were calculated using the cumulative incidence function, with death, relapse, or disease progression as competing risks. The cumulative incidence of fatal GVHD, fatal infection, or fatal posttransplant lymphoproliferative disorder was calculated with death from any other causes, or relapse as competing risks. Probabilities of overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier estimator. Patients alive without evidence of disease relapse or progression were censored at last follow-up, and the PFS event was summarized by a survival curve.
Cox proportional hazards regression was used to compare outcomes between the groups. The following variables were considered in univariate models: age (18-39 vs ≥40 years), patient sex, disease (lymphoma/chronic lymphocytic leukemia vs acute leukemia/myelodysplastic syndrome [MDS] vs other leukemia), Center for International Blood and Marrow Transplant Research (CIBMTR) disease risk indices (DRI) (low vs medium vs high vs very high),15 and whether or not TBI was used. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. A stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for graft source. Potential interaction between main graft source effect and all significant risk factors were tested. Adjusted probabilities of OS and PFS were generated from the final regression models stratified on both groups.
Results
Patients
Patient characteristics are summarized in Table 1. A total of 137 patients underwent haplo-cord transplantation: 77 at the University of Chicago between January 2007 and August 2015 and 60 at Weill Cornell Medical College/New York Presbyterian Hospital in New York between August 2012 and December 2016.
A total of 170 patients underwent haplo transplant between January 2009 and October 2016 at MD Anderson Cancer Center. Conditioning consisted of fludarabine-melphalan 140 mg/m2 for 42 patients, fludarabine melphalan with the addition of thiotepa for 129 patients, and fludarabine melphalan with addition of TBI 200 cGy for 26 (Table 1). The majority of patients in both cohorts had AML or MDS. Patients undergoing haplo-cord transplantation were significantly older, and had, on average, a higher DRI score. Haplo-cord recipients more frequently had an ABO-mismatched graft, and somewhat less frequently were cytomegalovirus (CMV) positive with CMV-positive donors.
Median follow-up for survivors was 54 months for haplo-cord recipients and 41 months for haplo transplant recipients.
Engraftment and chimerism
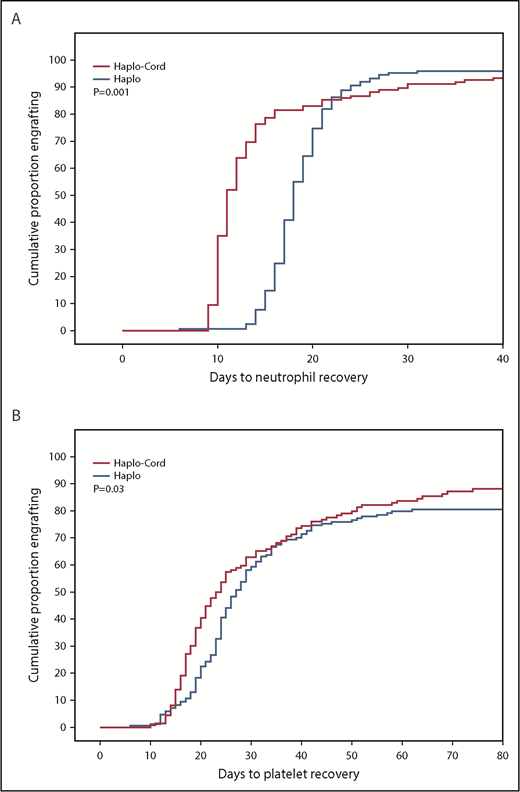
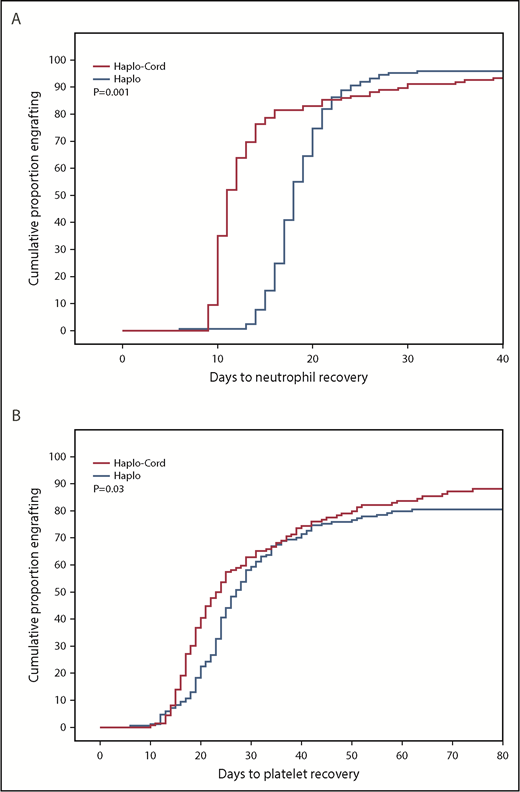
Median time to neutrophil recovery after haplo-cord transplant was 11 days vs 18 days after haplo-identical transplant (Figure 1A; Table 2). By day 15, 78% % of the haplo-cord recipients had recovered neutrophil counts vs 15% of haplo recipients (P = .001). Median time to platelet recovery after haplo-cord transplant was 22 days vs 25 days after haplo-identical transplant (Figure 1B). By day 20, 40% of the haplo-cord recipients had recovered platelets vs 22% of haplo recipients (P = .025).
Hematopoietic recovery after transplant. (A) Neutrophil recovery. (B) Platelet recovery.
Hematopoietic recovery after transplant. (A) Neutrophil recovery. (B) Platelet recovery.
Primary graft failure was documented in 3 haplo transplant recipients and irreversible secondary graft failure in an additional 9 patients. The rate of graft failure is therefore 7%. Ten haplo-cord recipients (7%) also developed graft failure. It was more difficult to differentiate primary vs secondary graft failure.
Haplo recipients who engrafted nearly always had full donor chimerism. There were only 4 cases of mixed chimerism. Engraftment of haplo-cord recipients is characterized by gradual transition from initial haplo chimerism to full or majority cord blood unit (CBU) chimerism. By day 100, 56% of recipients had >80% CBU chimerism, 35% had mixed haplo and cord chimerism, and 9% had less than 5% CBU chimerism.
Relapse, NRM, PFS, and OS
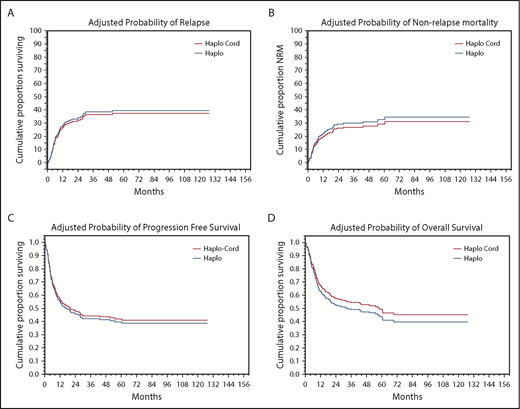
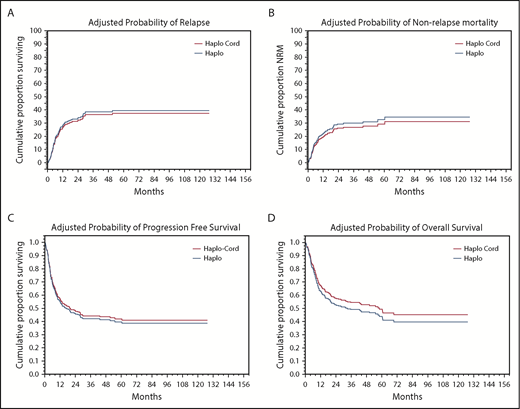
In univariate analysis, NRM was nearly identical at 18% (95% confidence interval [CI], 12-24) at 1 year for haplo-cord recipients vs 19% (95% CI, 13-25) for haplo recipients (Figure 2A; Table 3). Significant factors for NRM in multivariate analysis included DRI (hazard ratio [HR], 1.7; 95% CI, 1.25-2.295; P = .0006) and age 40 years or older (HR, 2.0; 95% CI, 1.18-3.27; P = .01).
Adjusted probabilities of outcomes. (A) Relapse. (B) NRM. (C) PFS. (D) OS.
Causes of death are listed in Table 4. Thirty-seven haplo-cord recipients died of nonrelapse causes, as did 39 haplo recipients. Distinct patterns emerge among causes of death. Fatal GVHD occurred in 9 haplo transplants (7 acute GVHD and 2 chronic GVHD). The cumulative incidence of fatal GVHD at 1 year was 3.5% in haplo recipients and reached 5% at 2 years. By contrast, only 1 haplo-cord patient died of GVHD, a cumulative incidence of less than 0.5% (P = .02)
Epstein-Barr virus (EBV) reactivation was addressed in haplo-cord transplant by systematic surveillance, twice weekly in hospital and at every clinic visit thereafter. Reactivation led to diagnostic workup for EBV and to preemptive treatment with rituximab. Surveillance was not conducted in the haplo recipients and diagnosis testing for EBV reactivation and posttransplant lymphoproliferative disorder (PTLD) was based on signs, symptoms, and clinical suspicion.
Among the haplo-cord patients, rituximab was administered for EBV reactivation or PTLD in 30 patients. Eleven had PTLD, of which 4 died. By contrast, only 5 patients in the haplo cohort received rituximab, and there was only 1 case of PTLD. There were no cases of fatal PTLD among haplo transplant recipients. The cumulative incidence of fatal PTLD of 3% at 1 year in haplo-cord was significantly higher (P = .01) than in the haplo patients. There were no significant differences in other causes of death.
Cumulative incidence of relapse at 1 year was identical at 27% (95% CI, 20-34) for haplo-cord recipients vs 27% (95% CI, 40-53) for haplo recipients (Figure 2B). In multivariate analysis, DRI (HR, 2.2; 95% CI, 1.72-2.89; P = .0000) and use of TBI as part of the conditioning (HR, 0.4; 95% CI, 0.19-0.747; P = .005) were significant predictors for relapse.
PFS at 1 year was 55% for haplo-cord vs 56% for haplo recipients. At 4 years, PFS was 40% for haplo-cord vs 45% for haplo recipients. In multivariate analysis, CIBMTR DRI (HR, 1.9; 95% CI, 1.59-2.35; P = .0000) and use of TBI in conditioning (HR, 0.6; 95% CI, 0.41-0.97; P = .03) were significant predictors for PFS (Figure 2C). Age >40 was also associated with worse PFS, but the increase did not reach statistical significance (HR, 1.3; 95% CI, 0.98-1.8; P = .07)
OS at 1 year was 63% for haplo-cord vs 65% for haplo recipients, whereas at 4 years, it was 50% for haplo-cord vs 49% for haplo recipients. In multivariate analysis, CIBMTR DRI was the only significant predictor for overall survival (HR, 1.8; 95% CI, 1.48-2.17; P = .0000) (Figure 2 D). Age > 40 was also associated with worse OS, but this was not statistically significant (HR, 1.4; 95% CI, 0.84-1.85; P = .08).
GVHD and NRM
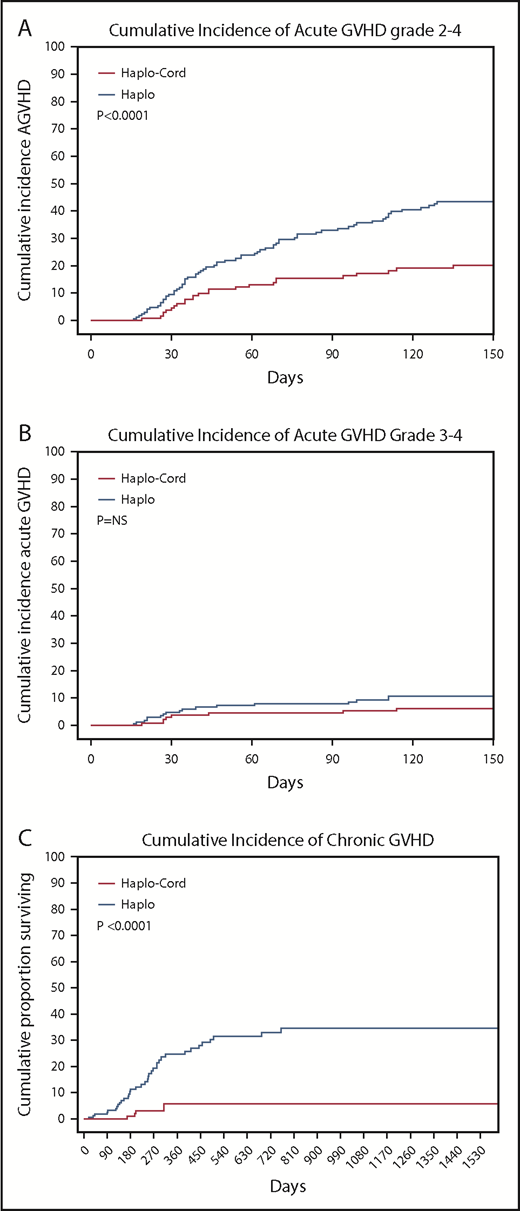
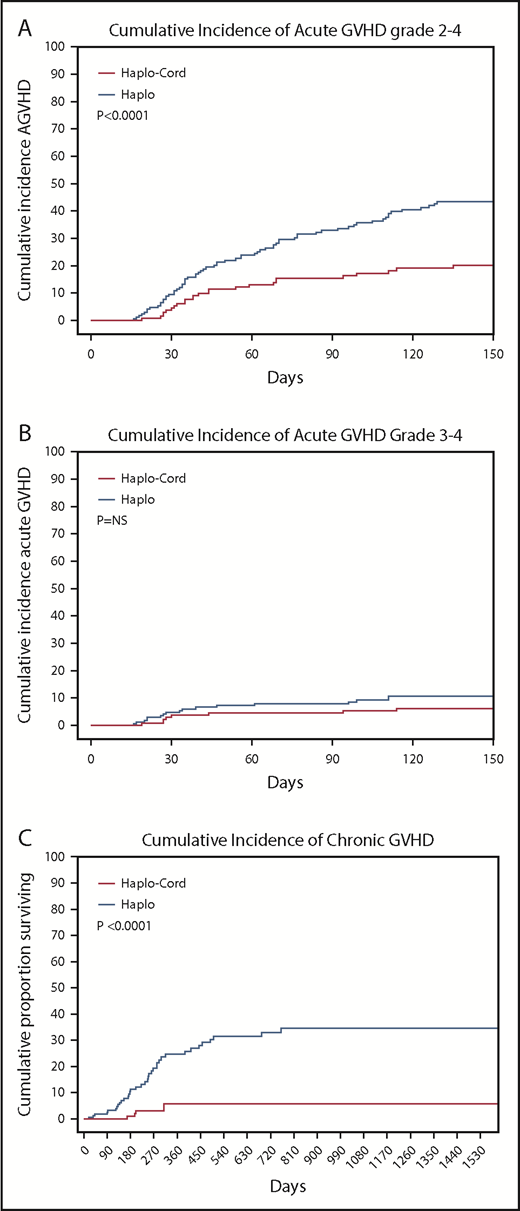
The cumulative incidence of acute GVHD grade 2-4 by day 100 was 16% (95% CI, 9-23) in the haplo-cord patients vs 33% (95% CI, 27-39) in the haplo group (P < .0001). Incidence of grade 3-4 acute GVHD by day 100 was 5% (95% CI, 1-9) in the haplo-cord patients compared with 9% (95% CI, 5-3) in the haplo group (P = .27) (Table 2; Figure 3A-B).
Cumulative incidences. (A) Acute GVHD grade 2-4. (B) Acute GVHD grade 3-4. (C) Chronic GVHD.
Cumulative incidences. (A) Acute GVHD grade 2-4. (B) Acute GVHD grade 3-4. (C) Chronic GVHD.
Finally, chronic GVHD was significantly reduced in haplo-cord with a cumulative incidence at 1 and 2 years of 4% (95% CI, 0-10) vs 16% (95% CI, 12-20) at 1 year, and 21% (95% CI, 15-27) at 2 years for haplo transplant (P < .0000) (Figure 3C). Of the 34 haplo recipients with chronic GVHD, 14 were extensive. Of the 5 cases of chronic GVHD after haplo-cord transplant, 2 were extensive and 3 were limited.
Discussion
Recent developments in allogeneic transplantation have led to major improvements in alternative donor transplantation. In the United States and Europe, 2 competing platforms have evolved over the past decades, those relying on haploidentical donors with GVHD prophylaxis containing PTCy and those using umbilical cord blood transplantation.
Here we compare transplantation outcomes among adults <60 years of age using haplo transplantation to umbilical cord transplantation supported by third-party donor grafts (ie, haplo-cord). There is one such prior study, but the patient numbers were much smaller, the cohorts not contemporaneous, different conditioning regimens were used,16 and the haplo recipients received peripheral blood stem cells rather than bone marrow, which may have increased their risk of GVHD. Our study results are strengthened by the relatively large sample size, use of the same melphalan-based conditioning and consistent institutional management, and complete data on DRI and hematopoietic cell transplantation-comorbidity index. The haplo transplants used mostly bone marrow grafts. The haplo-cord transplants used haplo CD34+ cells to hasten neutrophil recovery. Low-dose TBI was added in some patients because of its encouraging effects on relapse.17 Thiotepa was used before the routine introduction of TBI in all haplo grafts.
The overall results were gratifying, with both procedures achieving favorable and similar results for NRM, relapse, and survival. At 1 year, almost two-thirds of patients were alive and 4-year OS approximated 50%. After adjustment for prognostic factors, OS and PFS did not differ between haplo-cord and haplo grafts. Disease risk more than donor platform influenced outcomes. For subgroups with low and intermediate DRI, the outcome of transplantation was excellent, with 4-year survival of 65% for low DRI and 62% for intermediate DRI. For more advanced disease, both higher rates of relapse and NRM produced less favorable long-term survival, with 4-year survival of 36% for high DRI and 22% for very high DRI. Age >40 was associated with a significant increase in risk for NRM. Although its adverse effect on PFS and OS was considerable, it did not reach statistical significance. Last, we confirm that the use of low doses of TBI reduces relapse rates and improves PFS.17
The unique GVL effects of UCB have been repeatedly described.5,6,16,18 Our own group has shown that persistence of the UCB graft (as contrasted with dominance of the haplo graft) is associated with improved outcomes.7 Kwon et al, in their comparison of haplo vs haplo-cord, found that, despite higher DRI risk groups, haplo-cord did not have more relapse.16 Wang et al, from China, also showed that the additional infusion of a CBU with a haplo-graft resulted in reduction of relapse rates and improved OS.18 There are several plausible hypotheses for the GVL effects of UCB grafts. Maternal microchimerism may mediate reactivity against inherited paternal antigens.19 Alternatively, the infusion of a second graft might protect from relapse caused by HLA loss of AML clones.
We had therefore hypothesized that haplo-cord procedure might have lower rates of disease recurrence, but we did not find such a difference. The UCB-mediated GVL effects were partially abrogated by ATG. Additionally, there were major differences in patient characteristics, with more very high-risk patients and a higher median age in the haplo-cord group. It remains possible that, despite adjustment for covariates, imbalances in occult variables persist between the groups. Also, graft failure continues to adversely affect outcomes in small proportion of patients. In a small percentage of haplo-cord recipients, only the CBU graft fails and a durable haplo graft is established. This issue and its implications were discussed previously.7
The incidence of GVHD was limited with either procedure, but lower after haplo-cord than after haplo transplant. Grade 2-4 acute GVHD was lower after haplo-cord, but severe grade 3-4 acute GVHD was similar in both groups. The haplo-cord patients had extremely low rates of chronic GVHD with a cumulative incidence of 4% for the haplo-cord cohort vs 16% at 1 year for the haplo patients. Nine fatalities were attributed to GVHD (7 acute and 2 chronic) among haplo transplant recipients and only 1 in haplo-cord recipients. This suggests that, despite PTCy-based GVHD prophylaxis, GVHD remains a more serious problem with haplo transplants, even when bone marrow is used for the graft. Although PTCy has been associated with acceptable rates of chronic GVHD, haplo-cord may have almost entirely abolished this complication, as previously reported by us and others.16 We have attributed this to the dual effects of the high degree of matching in single UCB units and on the use of ATG.20 Chronic GHVD has repeatedly been shown to be a major risk factor for long-term morbidity and late mortality in survivors of allogeneic transplantation. It also affects long-term health care expenses.21,22 We consider its near absence one of the major advantages of haplo-cord.
Effective prevention of acute and chronic GVHD in the haplo-cord group came at the expense of a high incidence of EBV reactivation and development of PTLD in 11 cases, 4 of which were fatal. ATG likely contributed to the high incidence of PTLD. Beginning in 2017, we have administered 1 dose of pretransplant rituximab to haplo-cord. This has nearly eliminated EBV reactivation and PTLD, without obvious side effects.23 Many fewer cases of EBV reactivation were detected after haplo transplant and no fatal cases of PTLD encountered. Because surveillance was not conducted, it is possible that some cases remained undetected.
Other opportunistic infections are even more affected by surveillance and prophylaxis strategies (CMV) or by seasonal or local environmental factors and are therefore not compared. Studies of lymphocyte recovery have been previously reported.24,25 After haplo transplant, T-cell recovery occurs rapidly and both CD4 and CD8 numbers reach 200 by 6 months after transplantation. After haplo-cord transplant, CD4 and CD8 recovery was slower and reached 200 cells per microliter by 1 year after transplant. Natural killer cell recovery occurred rapidly after both procedures, but B-cell recovery occurred much faster after haplo-cord transplantation with normal numbers by day 30 after transplantation. The clinical implications of this rapid B-cell recovery are unclear. In a subsequent study, we evaluated T-cell diversity by next-generation sequencing and found that higher degree of CBU chimerism was associated with higher T-cell diversity.26 Higher T-cell diversity in turn was associated with decreased GVHD and lower rates of disease recurrence.
Although single or double UCB transplantation is known to be associated with slow hematopoietic recovery, such is not the case after the haplo-cord procedure. The infusion of third-party mobilized peripheral blood CD34+ cells from haplo donor was followed by rapid neutrophil and platelet recovery, as previously reported.16,27 In haplo transplants with PTCy, the myelosuppressive effects of cyclophosphamide and the use of bone marrow rather than peripheral blood stem cells results in a delay in primary engraftment, although this does not seem to affect early NRM in this group of younger patients.
Our results add to the growing literature on alternative donor transplants. Haplo transplant requires only 1 graft and no in vitro manipulation, which reduces initial costs and requires limited technical expertise compared with haplo-cord. Haplo-cord transplant results in more rapid count recovery which serves to reduce length of stay and costs of initial hospitalization.28 The near absence of chronic GVHD should, in addition to very substantial cost-savings,22 result in a reduced incidence of late end-organ damage, improved quality of life, and decreased NRM among long-term survivors.21 Last, we and others have found that approximately 20% of our patients lack suitable haplo donors.2,11 In haplo-cord transplant, this problem can be circumvented by the use of unrelated third-party donors instead of haplo identical donors.11
The retrospective nature of our study introduces typical limitations. Although the multivariate analysis adjusts for baseline differences including older age and higher disease risk in the haplo-cord cohort, additional imbalances may exist. Further, differences in method of assessment of chronic GVHD and potential differences in supportive care precluded an accurate comparison of chronic GVHD, and GVHD- and relapse-free survival between the 2 types of transplants. Important infectious complications such as CMV reactivation may also have been affected by institutional differences in screening and management. The haplo-cord approach has been performed exclusively on trial with open eligibility criteria driven by the need to have US Food and Drug Administration investigational new drug or investigational device exemption for CD34+ selection and previously for unlicensed cord units. A formal cost analysis was beyond the scope of this study, although this remains an area of interest for the future.
Despite these limitations, our results establish that both procedures provide excellent alternatives for patients lacking HLA-matched related or unrelated donors. The choice between the 2 procedures then should be based on (1) availability, (2) cost and strain on hospital resources, (3) expertise, and (4) long-term outcomes. These data offer reassurance that either approach can be considered to move quickly to transplant when no HLA-matched donor is immediately available.
Authorship
Contribution: K.v.B. and A.A. designed study, analyzed data, wrote the manuscript, and cared for patients; S.O.C. designed the study, wrote the manuscript, and cared for patients; R.E.C., M.R.B., U.G., T.S., H.L., S.A.M., S.A.S, and W.S. reviewed the manuscript and cared for patients; and D.G., J.C., and G.R. undertook data collection and review of the manuscript.
Conflict-of-interest disclosure: K.v.B. and A.A. receive an unrestricted research grant from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Koen van Besien, Department of Hematology and Oncology, Weill Cornell Medical College, 50 East 70th St, F561, New York, NY 10021; e-mail: kov9001@med.cornell.edu.