Abstract
Oral anticoagulants (OACs) are indicated for treatment and prevention of thromboembolic diseases. Supplemental patient education (education) has been proposed to improve outcomes, and this systematic review assesses the effect of education on mortality, thromboembolic events (TEEs) including venous thromboembolism (VTE), and bleeding in patients taking OACs. Randomized controlled trials were included, and 2 authors independently screened articles and assessed risk of bias. In 9 trials (controls, n = 720; intervention group patients, n = 646), 4 assessed critical outcomes of mortality, TEEs (VTE, stroke, and systemic embolism), and bleeding to estimate absolute risk ratios. When comparing education with usual care, in 1000 patients, there may be 12 fewer deaths (95% confidence interval [CI], 19 fewer to 154 more) and 16 fewer bleeding events (95% CI, 34 fewer to 135 more), but this evidence is uncertain; the evidence also suggests 6 fewer VTEs (95% CI, 10 fewer to 16 more) and 8 fewer TEEs (95% CI, 16 fewer to 18 more). The mean difference in time in therapeutic range may be 2.4% higher in the education group compared with usual care (95% CI, 2.79% lower to 7.58% higher). We also found very low certainty of evidence for a large increase in knowledge scores (standardized mean difference, 0.84 standard deviation units higher; 95% CI, 0.51-1.16). Overall, the certainty of evidence was low to very low because of serious risk of bias and serious imprecision. Additional sufficiently powered trials or different approaches to education are required to better assess supplemental education effects on outcomes in patients taking OACs.
Introduction
Oral anticoagulants (OACs) are indicated for treatment and prevention of thromboembolic diseases including venous thromboembolism (VTE)1 for prevention of stroke and systemic embolism in patients with atrial fibrillation (AF)2 and increasingly for cardiovascular indications. OACs are considered high-alert medications, because they are also among the top drug-related causes of hospitalization in seniors.3,4 OACs include traditional vitamin K antagonists (VKAs), requiring monitoring and dose adjustment to maintain blood coagulation parameters within narrow therapeutic ranges to optimize the risk/benefit ratio as well as direct OACs (DOACs). Although the latter do not require monitoring, they still require education about the importance of adherence, proper dosing, bleeding risk, and drug interaction potential.
Supplemental patient education (education) provides information beyond what is typically provided by a health care provider as part of usual care. Because of the complexity of patient management using OAC treatments, usual care for patients initiating OACs is likely to involve more extensive education than is typical with other cardiovascular medications. The content of these educational interventions would be expected to cover information such as indications for treatment, including chances of benefits and harms, drug intake information (eg, dose, frequency, and timing of doses relative to food intake), drug interaction management, recognition and management of bleeding and therapeutic failure, importance of medication adherence, and strategies if doses are missed.
The effect of educational intervention strategies in patients taking DOACs is of major importance given their relatively shorter half-lives, rapid onset and offset of action, and absence of international normalized ratio (INR) monitoring. Because of the pharmacokinetics of DOACs, missed doses may create critical transient gaps in OAC coverage, exposing patients to increased risk of thromboembolic events (TEEs).5 Adherence is therefore potentially a more essential educational issue with DOACs than VKAs.
Improving patient OAC knowledge may result in better adherence to prescribed treatments (influencing both TEE and bleeding risks) or promote early recognition of signs and symptoms of adverse events such as bleeding. Patient education may modify other behaviors or lifestyle factors that could affect well-known and established cardiovascular risk factors, such as hypertension, smoking, hypercholesterolemia, and diabetes.6-8
A previous systematic review of supplemental patient education for OACs found a lack of evidence of benefit for clinical outcomes9 ; however, that systematic review predated the launch of DOACs. The objective of our systematic review was to evaluate the effect of supplemental patient education for OACs on patient-important outcomes, including death, TEEs (VTE, stroke, myocardial infarction [MI], and systemic embolism), and bleeding. Secondarily, the impact of supplemental patient education on time in therapeutic INR range (TTR) and patient knowledge was assessed. Information from the earlier systematic review was critically appraised and synthesized together with new evidence, including information about DOACs (which became available after the review by Wong et al9 was published).
Methods
This systematic review was performed as part of the American Society of Hematology guideline on VTE, developed in partnership with the McMaster University Grading of Recommendations Assessment, Development and Evaluation (GRADE) Centre, and investigates 1 of the questions prioritized for the new guideline.10 Review and meta-analysis methodology followed the Cochrane Handbook,11 with reporting according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.12
Randomized controlled trials (RCTs) of patients treated with OACs (including patients at risk for or diagnosed with deep venous thromboembolism [DVT]/pulmonary embolism [PE], AF, or prosthetic heart valves), with any length of follow-up, were included if they had at least 1 supplemental educational component as the intervention and at least 1 control group comprising no supplemental education (usual care). Diverse approaches comprised usual care, with unstructured education or unrestricted VTE education serving as controls. Supplemental education was defined as information in addition to basic drug information provided as part of usual care and varied in modality, content, and intensity. Some educational programs were more intensive, such as visual material augmented with daily visits by nurses and physicians to repeat some items13 ; in another program, sessions up to 2 hours were held 3 times per week, providing information about the blood coagulation system and effects of some substances on treatments (eg, alcohol, diet, and medication, among others)14 ; 1 study provided targeted educational intervention based on knowledge gaps assessed in patients.15 Other programs were less intensive and more self-directed, such as sessions including a brief educational video16 or educational booklets.17 A brief summary of interventions and description of control groups is presented in Table 1.
Broad types of supplemental education interventions aimed at improving patient knowledge, TTR, or clinical outcomes were considered; however, the ability to evaluate the educational component alone was required. For example, educational interventions administered together only with patient INR self-monitoring, whereby the effect of supplemental education could not be separated, were not considered for pooling in the meta-analysis.
There were no restrictions for cointerventions administered, and in cases where different OACs were assessed for efficacy within the same study, treatments were pooled, and the educational component of the assessment across treatments was included in the meta-analysis. Cluster RCTs were eligible for inclusion, but observational and quasirandomized studies (eg, allocation of treatments by nonrandom methods, such as date of birth, or randomization after delivery of the educational component) were excluded.
MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials were searched, in addition to other sources (www.clinicaltrials.gov, article citations, American Society of Hematology guideline panel experts, and published guidelines18 ). Efforts were made to identify unpublished studies, such as those identified only in abstracts, by contacting authors. Search terms aimed to identify all anticoagulant agents, including synonyms, related terms and variants (including DOAC agents), parenteral agents, VKAs, educational interventions, patient compliance, and health behaviors as potential targets of intervention. The initial search was performed on 28 January 2017; an updated search was performed on 23 October 2018 using an identical search strategy (additional details found in supplemental Table 1A-B).
Independent screening and review of titles and abstracts for inclusion eligibility were conducted by 2 reviewers. Authors were contacted to obtain information on studies, including 2 with only abstract proceedings available at the time of search15,19 and another for which additional information on mean knowledge and TTR values was required.20 Because this review was an update of the review conducted by Wong et al,9 references before 2012 were excluded from the screening process. Studies identified for inclusion from the previous systematic review were reviewed and considered for inclusion in the data synthesis.
One reviewer independently extracted data for review by the second reviewer, who verified the information, with discrepancies resolved by discussion. Risk of bias (RoB) was assessed using the recommended categories in the Cochrane Handbook for Systematic Reviews of Interventions.11 RoB was assessed separately for 2 cluster RCTs using the Risk of Bias 2.0 tool assessing the effect of assignment to intervention. Evidence was assessed using the GRADE framework for the primary and secondary outcomes of interest.
Primary outcomes rated as critical using the GRADE approach included all-cause mortality, TEEs (including VTE [DVT and PE], stroke, MI, and peripheral embolism), and bleeding events of any severity. Secondary outcomes rated as important using the GRADE approach included TTR and knowledge-based measurements related to the disease condition and/or anticoagulation treatment. Outcomes were pooled and analyzed by meta-analytic techniques using RevMan software (version 5.3; released in June 2014).
For dichotomous outcomes (mortality, TEEs, and bleeding events), the Mantel-Haenszel method was used for analyzing and pooling the data for risk ratios of total patient events for each group and calculating 95% confidence intervals (CIs). For secondary outcomes with continuous variables (TTR and knowledge measures), results were analyzed as (standardized) mean differences, with higher TTR and knowledge scores indicating better outcomes. Individual VTE outcomes, PE and/or DVT, were not assessed, because studies did not include sufficient detail to present this information, and no MIs were reported. Bleeding events, irrespective of location or severity, were pooled together for calculation of risk. Only 1 study provided a definition for major bleeding,21 so major bleeding was not separately analyzed.
For studies in patients who were using VKAs, TTR means and standard deviations (SDs) were pooled to compare mean differences using a random-effects model. For 1 study that only presented medians and interquartile ranges for TTR,20 data analysis was completed with means imputed using the method described by Hozo et al.22
For the 2 cluster RCTs,21,23 adjustments were made to correct both the sample size (effective sample size) and number of events for the dichotomous outcomes by dividing the sample size and number of events by the design effect, as described in the Cochrane Handbook.11 For continuous variables, only the effective sample size was corrected using the design effect.23
Authors were contacted to obtain data identified from abstract screening; however, the data were not published at the time of the analysis.19 Two authors were contacted to provide means and SDs for knowledge scores, and data were obtained for 1 study15 but not the other.20
Heterogeneity of the eligible studies was assessed using the χ2 test, with significance at P < .10, and the I2 statistic,11 which was the primary measure used to assess degree of heterogeneity. I2 values between 50% and 90% were considered substantially heterogeneous, and values between 30% and 60% were considered moderately heterogeneous.24 For the purposes of the analysis in this investigation, random-effects models were used. The Mantel-Haenszel method was used for analyzing risk ratios for the pooled results of the dichotomous outcomes. Effect estimates for comparisons were calculated using median risks from pooled event rates from the control group of patients from the included RCTs. Absolute effects were similarly based on the control group event rates from the included RCTs.
Results
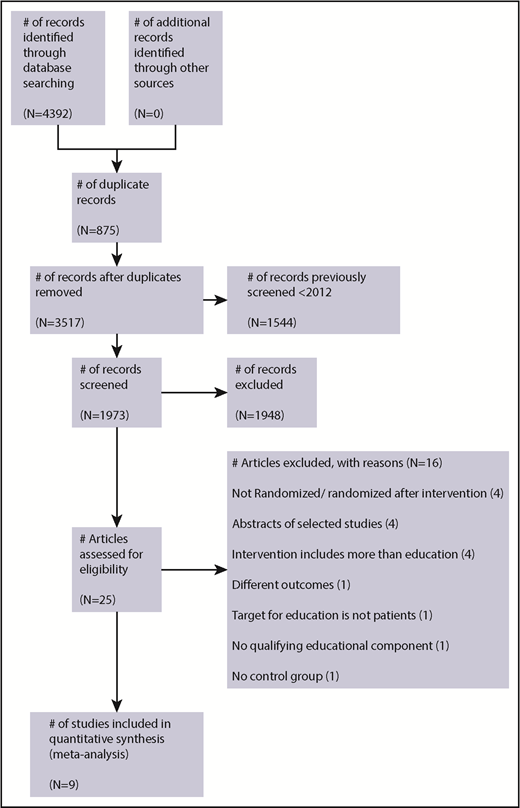
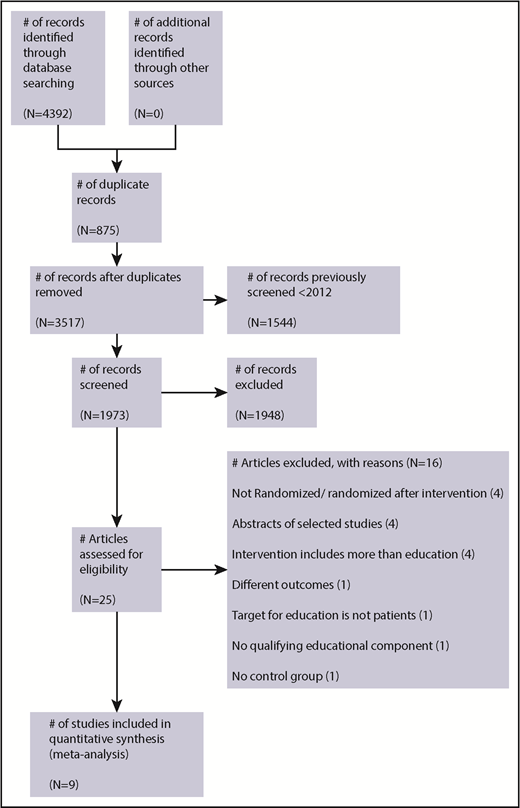
The search retrieved a total 4392 articles from all sources. Studies to February 2012 were reviewed by the Wong et al9 systematic review and therefore were not screened (n = 1544). Once the remaining titles and abstracts were screened and the full text reviewed, a total of 25 studies were identified for potential eligibility. Details of the studies reviewed and reasons for exclusion are summarized in Figure 1.
A total of 9 studies were included (5 studies from the previous systematic review,9 3 additional studies from the 28 January 2017 search, and 1 from the 23 October 2018 search), and these studies recruited 1366 patients (control group patients, n = 720; intervention group patients, n = 646). The characteristics of included studies in Table 1 provide further information for the studies that comprised data for the meta-analyses, which included patients ranging from age 18 to 91 years who were followed from 24 to 72 hours up to 12 months.
The studies included a mix of OAC indications, including 2 studies that exclusively studied VTE patients,21,25 1 study in patients with generally described TEEs,13 2 studies in mixed populations,14,23 2 studies in AF populations,15,20 and 2 studies with unspecified indications.16,17 All but 1 of the studies addressed VKAs, and 1 included DOACs.15 Two of the studies were cluster randomized.21,23
A brief summary of reasons for excluded studies is presented in Figure 1; detailed reasons are given in supplemental Table 2. Two studies previously included in the earlier systematic review9 were excluded. One study was excluded because patients were randomized after delivery of the educational intervention, the control group was historical, and adverse events were not monitored in the control group.26 A second study was excluded because there was no qualifying educational component (only a visual analog scale with a brief teach-back session of several minutes).27 One study presented only median knowledge scores at subsequent follow-up points and was excluded from the analysis of knowledge outcomes.20
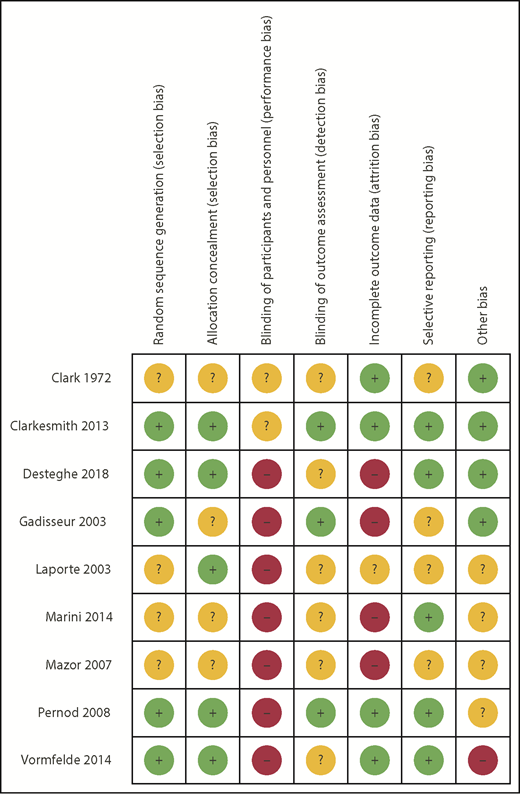
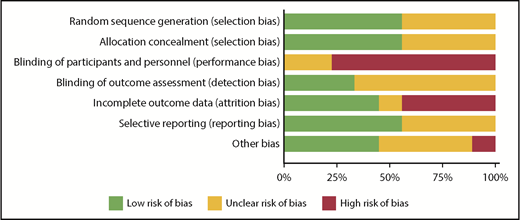
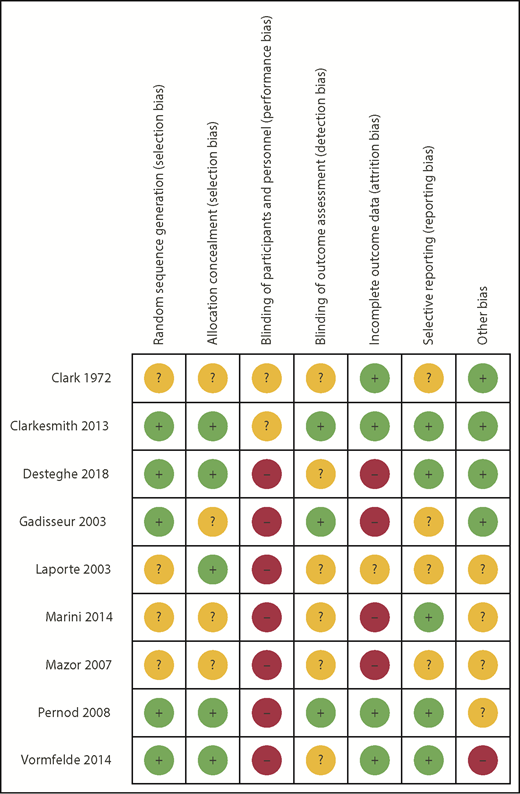
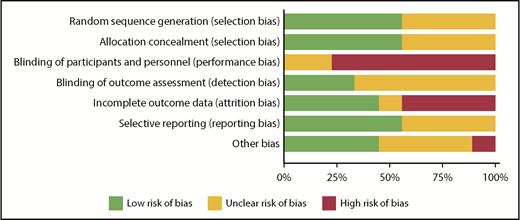
RoB using the Cochrane tool was assessed in each of the eligible studies and is further detailed in supplemental Table 3. Figure 2 shows the assessment of RoB for each study, and Figure 3 summarizes the RoB for each domain assessed. Most studies had high RoBs as a result of absence of blinding of participants or personnel,13-16,21,23,25 and 4 studies had identified high RoB with respect to incomplete outcome data.14-16,25 Supplemental Table 4 provides a summary of findings, including a summarization of certainty of evidence for all outcomes.
There was only 1 death resulting from an unknown cause reported in the control group of a study including 97 participants.20 The absolute risk reduction was 12 fewer deaths per 1000 patients in the intervention group compared with the control (95% CI, 19 fewer to 154 more). Overall, the evidence was uncertain about the effect of supplemental education on mortality (because of very serious imprecision). The 95% CIs included appreciable benefit and harm, with evidence of moderate heterogeneity (I2 = 52%). A forest plot is available in supplemental Figure 1.
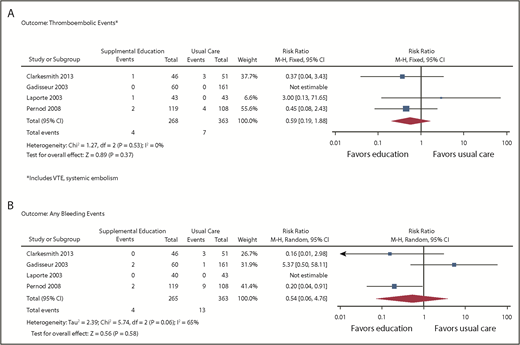
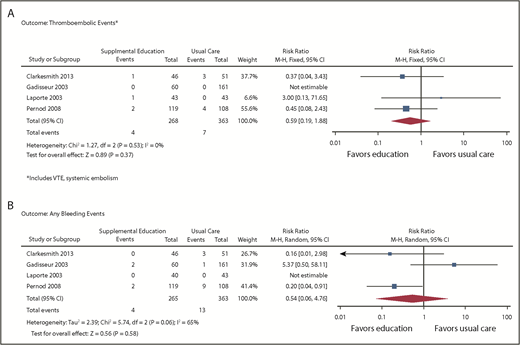
Outcomes of VTE (supplemental Figure 2) were assessed from 4 studies enrolling a total of 706 patients (631 after adjustment for cluster RCTs).13,14,20,21 From the pooled analysis, we calculated 6 fewer VTEs per 1000 patients in the intervention group (95% CI, 10 fewer to 16 more) and 8 fewer TEEs per 1000 patients (95% CI, 16 fewer to 18 more), with CIs showing appreciable benefit and harm (Figure 4A). Events were only reported in 1 study, so heterogeneity could not be assessed. The overall certainty of the evidence was low (primarily because of high RoB and imprecision).
Forest plots: supplemental education vs usual care. (A) Outcome thromboembolic events. (B) Outcome: any bleeding events.
Forest plots: supplemental education vs usual care. (A) Outcome thromboembolic events. (B) Outcome: any bleeding events.
Bleeding was assessed in 4 studies,13,14,20,21 enrolling a total of 706 patients (631 after adjustment for cluster RCTs). From the pooled analysis, we calculated 16 fewer bleeding events per 1000 when supplemental education was provided (95% CI, 34 fewer to 135 more; Figure 4B). There was substantial heterogeneity in the studies for this outcome (I2 = 65%), and CIs included both substantial benefit and harm. The overall certainty of the evidence was very low because of high RoB, inconsistency, and imprecision of estimates.
Four studies randomizing 749 patients (505 after adjustment for cluster randomization) measured the effect of supplemental education on TTR; the mean difference in TTR was 2.40% higher with education (95% CI, 2.79% lower to 7.58% higher; supplemental Figure 3). The mean TTR in the usual care group was 64.4%. Heterogeneity in the studies was moderate (I2 = 31%). The overall certainty of the evidence was low because of high RoB and imprecision.
For knowledge scores, 6 studies (936 patients in total; 643 after adjustment for cluster RCTs) reported results that were pooled.15-17,20,21,23,25 The standardized mean difference in knowledge score was 0.84 SD units higher with supplemental education (95% CI, 0.51-1.16 SDs higher; absolute increase of 15.1% [8.4%]; supplemental Figure 4) compared with usual care (higher scores indicating better knowledge). However, heterogeneity between studies was substantial (I2 = 70%). The certainty of evidence was very low, primarily because of high RoB, imprecision, and inconsistency.
Discussion
Despite additionally searching the 6 most recent research-intensive years and including additional studies, there was low to very low certainty in the evidence for improving patient-important outcomes with supplemental education. Although absolute risk of harm with education tended to be lower with supplemental education, we were uncertain about this effect on critically important outcomes. The small magnitude of improvement (<3%) on TTR alone is unlikely to be sufficient to improve critical patient outcomes.28 Because of the small number of events and limited follow-up in some of the studies, there was serious to very serious concern with the overall precision of the estimated effects, precluding the possibility of drawing conclusions of benefit with a high level of certainty. Consequently, recent guidelines have issued a conditional recommendation based on very low certainty evidence that health care practitioners consider incorporating supplemental patient education in addition to basic education as part of the management strategy for patients receiving OACs for VTE treatment.10
The main challenges to arriving at definitive conclusions around the impact of education on outcomes were related to methodological concerns, with serious RoB and imprecision resulting from the small number of observed events, variability in duration of follow-up among studies (affecting the period of time over which to observe mortality, bleeding, and thromboembolic outcomes and possibly resulting in differential decay of knowledge-based measures), and variability in content, delivery, and intensity of educational interventions. The challenges and methodological biases should be addressed in future studies, especially with respect to patient and health care provider blinding and allocation concealments.
In another systematic review of OAC treatment in AF patients evaluating the effect of self-monitoring plus education on the primary outcome of TTR, the authors concluded that the effect of these interventions was uncertain compared with usual care, in 11 trials of >2000 AF patients with very low quality evidence, citing similar challenges around the lack of standardization of interventions and differing conditions of education reflective of usual care.29
Despite the paucity of evidence, belief in the inherent value of education and the low perceived risk of causing harm may lead some health systems to continue promoting the use of different forms of supplemental education in these patient groups. However, the opportunity cost of doing so may be underestimated. Studies included did not consider the cost effectiveness, resource implication, or potential patient burden of supplemental OAC education, which could be significant depending on the format, frequency, and intensity of patient education programs.
In patients taking DOACs,15 there was only 1 study evaluating supplemental education impact on knowledge and none evaluating educational impact on patient-important outcomes such as mortality or bleeding. Therefore, additional information is needed as the uptake of DOACs and their integration into clinical practice become more widespread. Patient education may be even more important in promoting treatment adherence and persistence because of the comparatively shorter half-life of these agents.
Attempts to minimize bias in the review process were made by using multiple databases, not limiting the search by language, and ensuring that the screening of studies to be included was done independently by 2 reviewers. We also contacted authors of unpublished studies and contacted other authors to obtain additional data. Data extraction and analysis were conducted by 1 researcher but checked by a second, reducing potential bias in the review process.
Although we did not detect publication bias, it is possible that bias exists but was not found. This is of particular importance with interventions such as supplemental education, which may be particularly prone to participant selection and attrition and reporting bias.
There was significant heterogeneity in some measures, namely bleeding and knowledge score-based outcomes, with variability that could not easily be controlled before comparing treatment groups. This underscores the importance of standard measures to improve interpretation and further highlights the importance of using standardized definitions for bleeding and validated measures for knowledge assessment outcomes. For TTR, moderate heterogeneity may also reflect systematic and important differences in the usual care delivered among institutions as well as the intensity and/or effectiveness of the supplemental education interventions.
Furthermore, this review did not aim to assess patient values or preferences or address the feasibility and acceptability of such programs by patients, health care providers, payers, institutions, or granting agencies. Finally, the resources to implement and sustain such programs were not addressed as part of this review, and these are important to consider for any future recommendations.
In conclusion, although absolute risks for outcomes with supplemental education were generally lower than with usual care, there was low to very low certainty in these effects on critical patient outcomes such as mortality, bleeding, and TEEs in patients taking OACs. Longer follow-up, additional studies, and different approaches to education are needed, and future studies should also examine potential harms and costs to make definitive conclusions around the benefit of supplemental patient education in OAC use, particularly in patients using DOACs, where information is lacking and follow-up may be less frequent in the absence of INR monitoring.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Itziar Etxeandia Ikobaltzeta for setting up the search strategy and executing the searches, Yuan Yuan Gu for assistance with screening for systematic reviews, Lien Desteghe for providing unpublished data for use in the meta-analysis of knowledge scores, and Nancy Santesso for her critical review of the manuscript.
Supported by the American Society of Hematology.
The review question focused on all available OACs, and 8 of the 9 studies included in the review were conducted exclusively in patients receiving a vitamin K antagonist, mainly warfarin. In our opinion, Boehringer Ingelheim will not be affected by this review regardless of its conclusions.
Authorship
Contribution: M.P. contributed to study design, search strategy, study selection, data extraction, statistical analysis, interpretation of findings, and writing of the report; D.M.W., A.H., J.S., J.A., H.J.S., and W.W. contributed to study design, interpretation of findings, and writing of the report; and R.N. contributed to study design, search strategy, study selection, data extraction, statistical analysis, interpretation of findings, and writing of the report.
Conflict-of-interest disclosure: M.P. is an employee of Boehringer Ingelheim, Ltd, which markets dabigatran etexilate, a DOAC. (M.P.’s work on each step of this review was supervised by R.N., who reports no conflicts of interest). A.H. receives honoraria as a drug policy expert advisor from federal and provincial governments as well as publicly funded peer-reviewed grants to improve the quality of OAC management. The remaining authors declare no competing financial interests.
Correspondence: Miney Paquette, Department of Health Research Methods, Evidence and Impact, McMaster University, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: miney.paquette@boehringer-ingelheim.com.