Key Points
Gilteritinib induces 2 distinct marrow responses in FLT3-mutated AML: responses with and without differentiation.
Ongoing clonal hematopoiesis is ubiquitous during gilteritinib therapy and may promote genetic evolution and drug resistance.
Introduction
The potent, selective FLT3 inhibitors gilteritinib, quizartinib, and crenolanib have significant clinical activity in relapsed and/or refractory (R/R) FLT3-mutated (FLT3mut+) acute myeloid leukemia (AML).1-6 However, standard response metrics based on the Revised International Working Group criteria,7 which were established for cytotoxic chemotherapy, suboptimally characterize responses and expected clinical outcomes in patients treated with the selective FLT3 inhibitors or other leukemic differentiating agents.6,8,9 We previously described that quizartinib induces the terminal differentiation of leukemic myeloblasts in approximately half of responding patients and clearance of marrow blasts with a substantial reduction in overall marrow cellularity in the remaining responders.10 Whether these findings are specific to quizartinib or represent a class effect of the selective FLT3 inhibitors is unknown, and both the small sample size and short response duration limited the interpretation of our quizartinib data.
We therefore sought to perform a detailed analysis of clinical responses to the selective FLT3 inhibitor gilteritinib. Gilteritinib inhibits both FLT3-internal tandem duplication (ITD) and FLT3-D835 tyrosine kinase domain (TKD) mutations11 and was recently approved by the US Food and Drug Administration for the treatment of R/R FLT3mut+ AML. We performed a clinicopathologic study of adults with R/R FLT3mut+ AML who received gilteritinib at our institution on a multicenter phase 1/2 study (Chrysalis study; NCT02014558).
Methods
Patient cohort
All FLT3mut+ subjects enrolled on the Chrysalis study at the University of Pennsylvania were considered for this study. Patients who received gilteritinib at FLT3-inhibitory doses (≥80 mg/day)1 and had response data including bone marrow evaluations available for review were included. Research protocols were approved by the University of Pennsylvania’s institutional review board. Written informed consent for trial participation and tissue banking was provided by all subjects according to the Declaration of Helsinki. Clinical, demographic, and laboratory data were abstracted from the clinical charts.
Morphologic response assessment
Bone marrow aspirate and core biopsy samples were collected per the Chrysalis protocol1 or as clinically indicated and processed by our institution’s clinical laboratory. All available histomorphologic material was analyzed by 2 blinded pathologists using a Leica DM 2500 clinical light microscope.
FLT3 allelic ratio measurement
FLT3-ITD or FLT3-TKD mutant:wild-type allelic ratios were obtained from polymerase chain reaction and capillary electrophoresis as previously described.10 To facilitate the visual representation of data and statistical analysis, FLT3 mutation frequencies were estimated by transforming the ratio data for each mutation; ie, ITD ratio value/(ITD ratio value + wild-type ratio value).
Statistical analyses
Statistical analyses were performed using Stata/IC 15.0 (College Station, TX). P < .05 was considered statistically significant.
Results and discussion
Gilteritinib induces responses with and without differentiation
Twenty-one of 25 subjects had evaluable marrow specimens at baseline and at least 1 time point during therapy (supplemental Figure 1); 19 of 21 subjects (90.5%) had FLT3-ITD mutations, including 3 with both FLT3-ITD and TKD mutations, and 2 subjects (9.5%) had FLT3-D835 mutations only. Patient characteristics are summarized in supplemental Table 1. A marked reduction or elimination of circulating blasts occurred in all patients. Examination of marrow specimens revealed that 17 of 21 subjects (81.0%) had a ≥50% reduction in marrow blast percentage from baseline. Among these 17 subjects, we observed 2 distinct patterns of marrow responses: those with and without clear evidence of terminal differentiation of leukemic blasts.
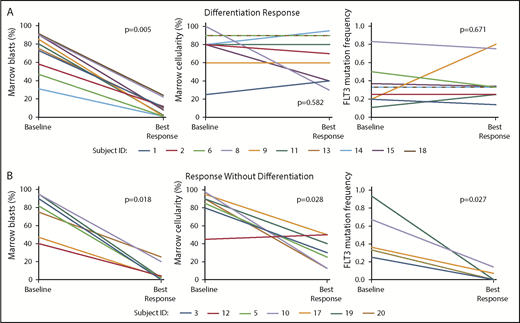
The first pattern, a differentiation response, occurred in 10 of 21 subjects (47.6%). In comparison with pretreatment specimens, bone marrows during response showed relatively stable total marrow cellularity with marked left-shifted granulocytic hyperplasia (without dysplasia) (Figure 1A, top panels, and Figure 1B). The percentage of erythroid and megakaryocytic cells was unaffected or showed relative hypoplasia. Flow cytometry performed during response confirmed the presence of discrete mature granulocytic and/or monocytic populations (supplemental Figure 2). Despite the observed cellular maturation and a significant reduction in marrow blast percentage (P = .005; Wilcoxon signed-rank test), FLT3 mutation burden remained largely stable (Figure 2A). Of note, 2 subjects developed clinical evidence of possible differentiation syndrome during response; both were treated with glucocorticoids with rapid resolution of symptoms. Details of these cases are outlined in supplemental Table 2. Two additional subjects developed a skin rash and fever. Biopsy of the rashes confirmed neutrophilic infiltrates (Sweet syndrome–like),12 consistent with a prior study in which neutrophils purified from a skin biopsy specimen after FLT3 inhibitor treatment were shown to harbor FLT3 mutations.13 Together, these data are supportive of gilteritinib-induced differentiation of leukemic blasts.
Gilteritinib induces 2 distinct morphologic patterns of response in R/R FLT3mut+AML: responses with and without differentiation. (A) Representative core biopsy marrow specimens from prior to initiation of gilteritinib and at the time of best marrow response show the effect of gilteritinib on total marrow cellularity in a subject with a differentiation response (top row) and in a subject with response without differentiation (second row). Differentiation responses are generally characterized by persistently hypercellular marrows, while responses without differentiation are associated with a reduction in total marrow cellularity. The core biopsy specimens are hematoxylin and eosin–stained at 400× objective magnification. (B) Bone marrow aspirate morphology at baseline and after treatment with gilteritinib in a subject with a differentiation response shows marked granulocytic hyperplasia with a left shift. The aspirate smears are Wright-Giemsa–stained at 500× objective magnification (oil immersion).
Gilteritinib induces 2 distinct morphologic patterns of response in R/R FLT3mut+AML: responses with and without differentiation. (A) Representative core biopsy marrow specimens from prior to initiation of gilteritinib and at the time of best marrow response show the effect of gilteritinib on total marrow cellularity in a subject with a differentiation response (top row) and in a subject with response without differentiation (second row). Differentiation responses are generally characterized by persistently hypercellular marrows, while responses without differentiation are associated with a reduction in total marrow cellularity. The core biopsy specimens are hematoxylin and eosin–stained at 400× objective magnification. (B) Bone marrow aspirate morphology at baseline and after treatment with gilteritinib in a subject with a differentiation response shows marked granulocytic hyperplasia with a left shift. The aspirate smears are Wright-Giemsa–stained at 500× objective magnification (oil immersion).
Differentiation responses to gilteritinib are associated with stable total marrow cellularity and FLT3 estimated mutant allele frequency despite significant reduction in marrow blast percentage. (A) Differentiation responses are characterized by a significant reduction in marrow blast percentage with a relatively stable total marrow cellularity and FLT3 mutation frequency at the time of best marrow response compared with baseline. (B) Responses without differentiation are associated with a reduction in marrow blast percentage with a concomitant significant reduction in marrow cellularity and FLT3 mutation frequency. Subjects 9, 13, and 18 had both FLT3-ITD and FLT3-D835 mutations. Only FLT3-ITD mutation frequencies are shown here. See supplemental Figure 3 for changes in FLT3-D835 frequencies in these subjects. Subject 12 had variably cellular (0% to 90%) total marrow cellularity at baseline; thus, mean baseline cellularity (45%) is shown on the graph. FLT3 mutation frequencies are not shown for subject 12 (specimens were suboptimal for analysis) or subject 13 (baseline FLT3 polymerase chain reaction was not performed, as the subject’s FLT3 mutation was confirmed by a next-generation sequencing panel).
Differentiation responses to gilteritinib are associated with stable total marrow cellularity and FLT3 estimated mutant allele frequency despite significant reduction in marrow blast percentage. (A) Differentiation responses are characterized by a significant reduction in marrow blast percentage with a relatively stable total marrow cellularity and FLT3 mutation frequency at the time of best marrow response compared with baseline. (B) Responses without differentiation are associated with a reduction in marrow blast percentage with a concomitant significant reduction in marrow cellularity and FLT3 mutation frequency. Subjects 9, 13, and 18 had both FLT3-ITD and FLT3-D835 mutations. Only FLT3-ITD mutation frequencies are shown here. See supplemental Figure 3 for changes in FLT3-D835 frequencies in these subjects. Subject 12 had variably cellular (0% to 90%) total marrow cellularity at baseline; thus, mean baseline cellularity (45%) is shown on the graph. FLT3 mutation frequencies are not shown for subject 12 (specimens were suboptimal for analysis) or subject 13 (baseline FLT3 polymerase chain reaction was not performed, as the subject’s FLT3 mutation was confirmed by a next-generation sequencing panel).
The second pattern of gilteritinib response, response without differentiation, occurred in 7 of 21 subjects (33.3%) and was notable for a significant reduction in total marrow cellularity (Figure 1A, bottom) and blast percentage (P = .028 and P = .018, respectively; Wilcoxon signed-rank test) without granulocytic hyperplasia. Subjects experiencing a response without differentiation also had a significant reduction in FLT3 estimated mutant allele frequency (P = .027; Wilcoxon signed-rank test) (Figure 2B).
Baseline FLT3 mutation allelic ratio or a history of allogeneic hematopoietic stem cell transplantation were not predictive of type of response. In contrast to our observations with quizartinib, where differentiation responses to quizartinib were enriched for the genotype FLT3-ITD+, NPM1mut+, and DNMT3Amut+ with a normal karyotype,10 a triple-mutant genotype and/or normal karyotype did not predict for type of response (supplemental Table 3). Of note, 1 of 2 subjects who had a FLT3-D835 mutation without a concomitant FLT3-ITD mutation at baseline had a differentiation response to gilteritinib. All 3 subjects with both FLT3-ITD and FLT3-D835 mutations at study entry had a differentiation response, and serial monitoring of the FLT3 mutations in these patients showed persistence of the FLT3-ITD with clearance of the D835 mutation (supplemental Figure 3).
Impact of response type on clonal hematopoiesis and clinical outcome
Evidence of persistent clonal hematopoiesis based upon the presence of either a clonal cytogenetic abnormality or somatic leukemia-associated mutations was seen throughout gilteritinib treatment in 14 of 17 (82.4%) responding subjects’ marrows, including subjects with both response types and those who achieved a morphologic remission (supplemental Table 4). Of note, the 3 subjects without evidence of persistent clonal hematopoiesis during gilteritinib response all had responses without differentiation, and 2 of 3 had received a prior allogeneic hematopoietic stem cell transplantation. In addition, cytogenetic clonal evolution was common during gilteritinib therapy. Nine of 15 (60%) subjects with available karyotypes from study entry and during gilteritinib treatment developed new or additional cytogenetic abnormalities during response (n = 4) or at disease progression (n = 5) (supplemental Table 4). This suggests that although gilteritinib monotherapy is capable of clearing and/or differentiating leukemic blasts, it does not eradicate the underlying leukemic clone.
Comparing the 2 response types, we observed a trend toward improved overall survival and complete morphologic remission rates among patients with response without differentiation (supplemental Figures 4 and 5; supplemental Table 5). While these differences are limited by our sample size and were not statistically robust, they are consistent with a recent correlative analysis from the Chrysalis study, which showed that patients with a marked reduction in allele burden or clearance of FLT3-ITD as measured by a quantitative next-generation sequencing assay experience longer survival than patients with a persistently elevated FLT3-ITD burden.14 While requiring independent confirmation, response without differentiation may yield clinically superior outcomes.
Conclusions
We show that gilteritinib therapy promotes differentiation of leukemic blasts in a sizeable subset of R/R FLT3mut+ AML. Additional studies are needed to assess the biologic mechanisms underlying type of marrow response to selective FLT3 inhibition in AML and understand whether differentiation responses reflect true terminal myeloid differentiation.
Although differentiation responses to gilteritinib are similar to the differentiation responses observed with quizartinib, responses without differentiation to gilteritinib are characterized by normocellular marrows with peripheral count recovery. In contrast, responses without differentiation to quizartinib are characterized by hypocellular marrows and incomplete peripheral count recovery.10 Gilteritinib may be less myelosuppressive than quizartinib as a result of its less potent Kit inhibitory activity.15 Of note, we also observed differentiation responses to gilteritinib in patients with FLT3-D835 mutations, which are not inhibited by quizartinib. Thus, although differentiation responses appear to be a class effect of the selective FLT3 inhibitors, differences in the kinase inhibitory profiles may lead to important differences in responses to these agents.
These data also show that responses to selective FLT3 inhibitors exhibit persistent clonal hematopoiesis and thus differ fundamentally from traditional cytotoxic agents. Clonal persistence and cytogenetic evolution despite marrow response may in part explain the incomplete hematopoietic reconstitution that is characteristic of response to selective FLT3 inhibitors1,2,4-6 ; it may also contribute to drug resistance, which requires further study. Our data also suggest that type of response to gilteritinib may impact clinical outcomes. Differentiation responses have also been reported in patients treated with IDH-mutant AML treated with the IDH inhibitors enasidenib and ivosidenib, including subsets with target mutation clearance.8,16,17 Alternative approaches to define clinical responses in patients treated with these novel targeted therapies therefore may be required, particularly as combination regimens are developed.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Rachel Astles for assistance with gathering patient records, Robin Blauser for assistance with sample acquisition, and Andrew Cucchiara for statistical guidance. They also acknowledge the many physicians, physician extenders, and nurses who provided clinical care to these patients.
Financial support for these studies was provided by the Biff Ruttenberg Foundation. C.M.M. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. This work was also supported by grants R21CA198621 (M.C. and A.E.P.) and R01CA198089 (M.C.) from the National Cancer Institute of the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: C.M.M. collected and analyzed data and wrote and edited the manuscript; J.C. enrolled and treated patients on the clinical trial, collected marrow specimens, and collected data; B.R. and R.L.S. analyzed the bone marrow histology and edited the manuscript; C.D.W. and J.J.D.M. performed the molecular genetic, sequencing, and cytogenetic studies and edited the manuscript; J.N.Q. performed the analysis of flow cytometry data and edited the manuscript; M.C. designed the study, analyzed data, and edited the manuscript; A.E.P. designed the study, enrolled and treated patients on the clinical trial, collected marrow specimens, analyzed data, and edited the manuscript; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: A.E.P. has served as a consultant or advisor to Astellas Pharmaceuticals, Arog Pharmaceuticals, Daiichi Sankyo, Pfizer, Novartis, Takeda, NewLink Genetics, Actinium Pharmaceuticals, and Agios; and has received research support from FujiFilm, Daiichi Sankyo, Astellas, and Novartis. M.C. receives research support from Astellas Pharmaceuticals. R.L.S. is now an employee of Janssen Research and Development but performed this work previously at the University of Pennsylvania. The remaining authors declare no competing financial interests.
Correspondence: Alexander E. Perl, Division of Hematology/Oncology, Perelman School of Medicine, University of Pennsylvania, 12-154 South Tower, Perelman Center for Advanced Medicine, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: alexander.perl@uphs.upenn.edu.
References
Author notes
C.M.M. and J.C. contributed equally to this work.
M.C. and A.E.P. contributed equally to this work.