Key Points
Annual rates of respiratory illness in sickle cell disease decreased by >50% during the second year of monthly doses of oral vitamin D3.
Reduction in rates was similar with high-dose (100 000 IU/mo) and standard-dose (12 000 IU/mo) treatment.
Abstract
In sickle cell disease, respiratory infection and asthma may lead to respiratory complications that are a leading cause of morbidity and mortality. Vitamin D has anti-infective and immunomodulatory effects that may decrease the risk for respiratory infections, asthma, and acute chest syndrome. We conducted a randomized double-blind active-controlled clinical trial to determine whether monthly oral vitamin D3 can reduce the rate of respiratory events in children with sickle cell disease. Seventy sickle cell subjects, ages 3-20 years, with baseline records of respiratory events over 1 year before randomization, underwent screening. Sixty-two subjects with 25-hydroxyvitamin D levels of 5-60 ng/mL were randomly assigned to oral vitamin D3 (100 000 IU or 12 000 IU, n = 31 each) under observed administration once monthly for 2 years. The primary outcome was the annual rate of respiratory events (respiratory infection, asthma exacerbation, or acute chest syndrome) ascertained by the use of a validated questionnaire administered biweekly. Analysis included 62 children (mean age of 9.9 years, 52% female, and predominantly with homozygous HbS disease [87%]) with mean baseline 25-hydroxyvitamin D of 14.3 ng/mL. The annual rates of respiratory events at baseline and intervention years 1 and 2 were 4.34 ± 0.35, 4.28 ± 0.36, and 1.49 ± 0.37 (high dose) and 3.91 ± 0.35, 3.34 ± 0.37, and 1.54 ± 0.37 (standard dose), respectively. In pediatric patients with sickle cell disease, 2-year monthly oral vitamin D3 was associated with a >50% reduction in the rate of respiratory illness during the second year (P = .0005), with similar decreases associated with high- and standard-dose treatment. This trial was registered at www.clinicaltrials.gov as #NCT01443728.
Introduction
Acute respiratory illness is a principal cause of morbidity and mortality worldwide1 and can be devastating for children with sickle cell disease, an inherited red blood cell disorder affecting an estimated 100 000 Americans,2 predominantly of African ancestry. Sickle cell disease is characterized by a shortened life expectancy, hemolytic anemia, acute episodes of vaso-occlusive pain, and recurrent chronic damage to vital organs.2-3 Pulmonary manifestations are common and often severe. Respiratory infections or asthma attacks that would have no lasting effects in individuals with no sickle hemoglobinopathy can trigger severe or even fatal manifestations in those with sickle cell disease.4 For instance, children with influenza and sickle cell disease are hospitalized at a rate that is >50-fold greater than children without sickle cell disease.5 Asthma is common, affecting 15-28% of children with sickling disorders.6 Unique to sickle cell disease is a respiratory complication that is characterized by fever, respiratory symptoms, and a new pulmonary infiltrate, known as acute chest syndrome (ACS).7 Commonly precipitated by respiratory infections and asthma, ACS is the leading cause of death in sickle cell disease.6-7 The pathogenesis of sickle cell lung disease is unclear but involves microvascular occlusion, hemolysis-induced endothelial dysfunction, and vasculopathy that produce a chronic inflammatory state, often exacerbated by an infectious trigger.2,8-9 Compromised immunity from functional asplenia may contribute to the risks for respiratory infections and pulmonary disease.10 Improved vaccination and penicillin prophylaxis have greatly reduced the risk of bacterial pathogens but are ineffective against viruses and atypical organisms that now predominate as risks for ACS.11
Vitamin D, in addition to its role in calcium and bone homeostasis, is a multifunctional regulator of innate and adaptive immune responses and of inflammation.12-16 Vitamin D acts, in part, through its metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D],17-18 which binds to the vitamin D receptor to function as a transcription factor, inducing vitamin D–responsive genes that are present in most, if not all, cells of the immune system.19 1,25(OH)2D mediates the innate immune host response against respiratory tract pathogens by stimulating expression of cathelicidin (hCAP18/LL37), an antimicrobial peptide with activity against viral, bacterial, and fungal pathogens.20-21 It also regulates the adaptive immune system by modulating T-lymphocyte proliferation and function and by downregulating the inflammatory response and cytokine expression.22 In addition to these functions of 1,25(OH)2D, the parent compound vitamin D itself is a potent and general mediator of endothelial stability and barrier function.23 A recent Cochrane review of randomized clinical trials identified high-quality evidence that vitamin D supplementation reduced the risk for asthma exacerbations, although too few pediatric trials were included to permit definitive conclusions for children.24-25 Although observational studies have consistently found significant associations between low vitamin D levels and increased susceptibility to respiratory infections, randomized clinical trials examining the effects of vitamin D supplementation on the risk for respiratory infections have had conflicting findings that are due, in part, to differences in the baseline status of the participants, the duration of the studies, and the amount and frequency of dosing.26-29
Children with sickle cell disease are at high risk for vitamin D deficiency due to limited sun exposure, dark skin color, and poor nutrition. Our own and other observational studies have found that most children with sickle cell disease have low vitamin D status.30-31 Contrary to an earlier hypothesis,32 the low levels of vitamin D in black Americans are not explained by differences in the concentration of vitamin D–binding protein.33-34 Considering that individuals with low vitamin D levels seem to derive the most benefit from supplementation29 and that children with sickle cell disease have a heightened susceptibility to respiratory infections and asthma, together with a greatly increased vulnerability to complications, we conducted a 2-year active-controlled double-blind randomized clinical trial comparing monthly high-dose oral vitamin D3 (100 000 IU, daily equivalent 3333 IU/d) with standard-dose oral vitamin D3 (12 000 IU, daily equivalent 400 IU/d)35 to determine whether oral vitamin D3 (cholecalciferol) can reduce the risk for respiratory complications in children and adolescents, 3-20 years old, with sickle cell disease (ViDAS trial). Because of the established risks for inadequate mineralization of the skeleton associated with vitamin D deficiency,14,35-37 a placebo group could not be included in the study design.
Patients and methods
Participants
Prior to the clinical trial, we prospectively collected baseline data of respiratory events, including respiratory infections, asthma exacerbations, and ACS, ascertained by the use of a respiratory events questionnaire (see supplemental Methods for a copy of the questionnaire). Participants were 99 children with sickle cell disease, ages 2-19 years, recruited from among ∼200 patients followed at the Columbia University Medical Center Pediatric Sickle Cell Program.
Participants in the clinical trial were recruited from among these 99 eligible patients, ages 3-20 years old, with baseline respiratory events data collected over an approximate period of 1 year prior to the trial that served as baseline for the primary outcome measure of the randomized trial. Subjects were to have been on a stable dose of hydroxyurea or chronic asthma medication for ≥3 months, not on chronic transfusion therapy, not on chronic steroids (except for inhaled steroids) or anticonvulsants, no diagnosis of rickets, and without history of hypercalcemia or diagnosis of any medical condition associated with hypercalcemia. The protocol was approved by the Institutional Review Board of Columbia University Medical Center and by the US Food and Drug Administration (IND Number 107584). Written informed consent was provided by participants, parents, and guardians, along with written informed assent when applicable. Participants received reimbursement for travel expenses.
Trial design
This study was a double-blind active-controlled parallel-group trial with random assignment in a 1:1 ratio to receive treatment once monthly for 24 months under observation with vitamin D3 (100 000 or 12 000 IU) in identical capsules (Tishcon Laboratories Inc., Salisbury, MD). An overview of the trial design is shown in Figure 1. Participants with screening 25–hydroxyvitamin D (OHD) <5 ng/mL were not randomized but were eligible to participate in an open-label manner. These patients were evaluated by a pediatric endocrinologist (I.F.) and treated with oral vitamin D3: 100 000 IU every other week for 2 months and then 100 000 IU monthly thereafter for the duration of the study. Patients with 25-OHD >60 ng/mL were also excluded; none of the participants had levels >60 ng/mL. Using computer-generated randomization, participants with 25-OHD concentrations of 5-60 ng/mL were randomly assigned using randomly permuted blocks of size 2, 4, or 6 assignments, with separate schema prepared for stratification by age (3-9 and 10-20 years), gender, and hydroxyurea therapy. Randomization was performed by the research pharmacy; all other research staff and participants were blinded to allocation. Participants were seen monthly for observed administration of study medication and interval history, including respiratory events, dietary vitamin D and calcium intake (Block Calcium/Vitamin D Screener; NutritionQuest, Berkeley, CA), sunlight exposure, and adverse events. Serum albumin–corrected calcium and spot urinary calcium/creatinine ratios were performed monthly for the first 6 months, every other month for the following 6 months, and every 3 months for the second year. Blood specimens for 25-OHD, obtained immediately before study drug administration, were collected at the same intervals, stored at −112°F (−80°C), and analyzed in a single batch at the end of the study using ultra-performance liquid chromatography–tandem mass spectrometry. A Clinical Trial Data and Safety Monitoring Board consisting of a Chair, a biostatistician, a pediatric hematologist, and a pediatric endocrinologist was organized to monitor the trial.
ViDAS trial schema. FENO, fractional exhaled NO; PFT, pulmonary function tests.
ViDAS trial schema. FENO, fractional exhaled NO; PFT, pulmonary function tests.
Outcome measures
The primary outcome was the annual rate of respiratory events, defined as episodes of respiratory infections, asthma exacerbations, or ACS, ascertained by the use of a validated questionnaire38 administered in person during monthly outpatient study visits and biweekly telephone interviews and confirmed by review of clinical records from outpatient visits and hospitalizations. The Respiratory Questionnaire had a sensitivity of 83% and a specificity of 77% in identifying the presence of 9 viral respiratory pathogens: adenovirus (B, C, and E), bocavirus, coronavirus (229E, NL63, and OC43), enterovirus, influenza virus (A and B), metapneumovirus, parainfluenza virus (1, 2, 3, 4a, and 4b), rhinovirus, and respiratory syncytial virus (A and B).38 The questionnaire queried whether the subject has had any of the following 6 symptoms in the past 2 weeks: difficulty breathing, wheezing, fever, cough, runny nose, and sore throat. If a symptom(s) was present, additional questions were asked about duration and severity of symptom(s), medication use, visit to a medical provider, hospitalization, and missed school day(s) due to the illness. A “respiratory event” was considered when ≥1 of the 6 symptoms occurred, except for fever alone without accompanying respiratory symptoms or a runny nose accompanied by itchy/watery eyes suggestive of allergy. A new event was counted if ≥7 days had elapsed since the last counted event. Patients with prolonged respiratory symptom(s) lasting >2 weeks were evaluated by the study pulmonologist (M.K.), whose final diagnosis determined whether the symptom(s) represented a respiratory event. All questionnaires were reviewed and adjudicated in a blinded fashion by a principal investigator (M.T.L.) to determine whether a respiratory event had occurred. A “respiratory infection” was defined as (1) runny nose, cough, or sore throat for ≥2 days or any of these symptoms for ≥1 day accompanied by fever, not otherwise attributed to allergy; (2) physician-diagnosed pharyngitis, otitis media, or influenza; or (3) physician-diagnosed croup, bronchiolitis, or pneumonia. An “asthma exacerbation” was defined as a physician-diagnosed acute asthma that required treatment with corticosteroids, emergency room treatment, or hospital admission. A standard definition was used for ACS as an acute illness characterized by fever and/or respiratory symptoms, accompanied by a new pulmonary infiltrate on a chest radiograph.39 In instances in which there was an overlap of these diagnoses within a single duration of symptoms, such as when respiratory infection preceded an asthma exacerbation or ACS or when infection or acute asthma occurred with an ACS, the separate diagnoses were counted as a single event. For secondary outcomes, pulmonary function tests (spirometry, lung volumes, and diffusion capacity) and fractional exhaled NO (FENO) were measured at baseline and at 12 and 24 months. Grip strength was measured by hand-held dynamometry at baseline and every 6 months for 24 months. Other prespecified secondary outcome measures included immune and bone turnover markers, which will be reported elsewhere.
Statistical methods
Our primary study analysis was an examination of the annual rate of respiratory events (infections, asthma exacerbations, or ACS) in comparison with the rate of respiratory events over a baseline period of 1 year, using an intention-to-treat analysis. The study sample size estimation, using the conditional efficient score method,40 and the statistical analysis plan are detailed in the supplemental Methods. The original sample size of 40 participants per group was estimated to provide power ≥0.9 with a 2-sided α of 0.05, using an effect size of a 40% reduction in respiratory events with allowance for 15% loss to follow-up. The actual trial sample size of 31 subjects per group was calculated to still provide >80% power with a 2-sided α of 0.05. Preliminary blinded review of the primary outcome data identified highly variable and nonnormally distributed baseline respiratory event rates. The statistical analysis plan of the primary outcome was then revised to use the proportional means model, which accommodates variability in the event rates between subjects. This modified plan was reviewed and accepted by the Clinical Trial Data and Safety Monitoring Board and approved by the sponsor prior to the final analysis. Crude estimates of group differences in the change in the annualized event rate from baseline to year 1 and year 2 were analyzed by linear mixed models. Further analysis of treatment group differences was assessed using variations of the Cox Proportional Hazards model.41-42 Additional analyses of treatment group differences are described in the supplemental data, including a per-protocol analysis of the subset of participants who completed the 2-year study (n = 56), as well as a responder analysis using stepwise logistic regression in which the entire per-protocol cohort was divided into 2 groups defined by improved (“responder” n = 36) or unimproved (“nonresponder” n = 20) annualized event rate in the treatment phase relative to baseline. Study group differences in continuous measures at baseline were compared using independent Student t tests, and differences in dichotomous categorical measures at baseline were compared using the Fisher’s exact test. Treatment group differences in adverse events of at least grade 2 severity were assessed with the χ2 or Fisher’s exact test. Statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc.). All tests were 2-sided, with statistical significance prespecified at P < .05.
Results
Enrollment and follow-up
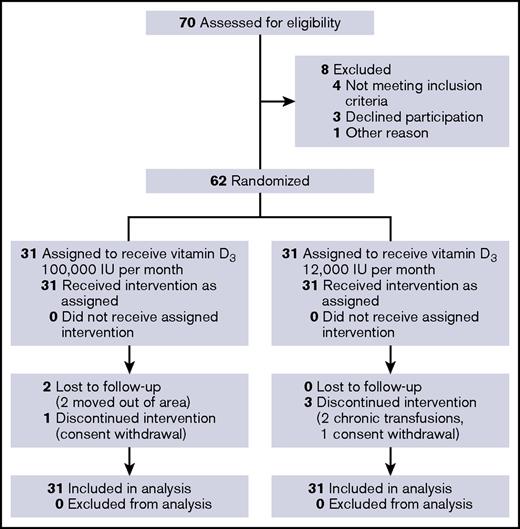
Between 13 December 2011 and 20 June 2013, 70 patients with baseline respiratory events data consented to participate and were assessed for eligibility. Of the 99 patients with baseline respiratory events data, no significant differences were found between the 70 patients who enrolled in the trial and the 29 patients who declined participation. Sixty-two participants (mean age 9.9 ± 3.9 [SD] years, 32 females [52%], predominantly with homozygous HbS disease [87%]) passed screening and were randomly assigned to monthly high-dose (n = 31) or standard-dose (n = 31) vitamin D3. Overall, 90.7% of the scheduled respiratory event questionnaires were completed. For the high-dose group, 96.5% of the expected person-months on study was completed compared with 93.4% in the standard-dose group. The study visit for the last participant was completed on 16 July 2015. Figure 2 shows the participant flow diagram.
Characteristics of trial participants
The baseline demographic, clinical, and laboratory characteristics of the trial participants were well-balanced between the treatment groups (Table 1). A majority of the participants (77%) had insufficient or deficient vitamin D levels (25-OHD <20 ng/mL),35-36 with overall mean baseline 25-OHD of 14.3 ng/mL (median 14.6, range 5-28), which was not significantly different between the groups (P = .48). The mean annual baseline respiratory event rate was 4.1, which was almost identical to our estimated 4 events per year used in the sample size and power calculations.
Primary outcome: respiratory events
The annual rate of respiratory events, predominantly respiratory infections, did not differ significantly between the groups receiving monthly high-dose and standard-dose oral vitamin D3 (P = .24), but it decreased significantly during year 2 in both groups (P = .0005; Figure 3). No significant difference was found between the study groups using proportional hazards estimates, including with adjustments for mean baseline event rates, mean baseline interevent interval, both mean baseline event rates and interevent interval, gender, age, and hydroxyurea therapy (supplemental Table 1). A per-protocol analysis (supplemental Table 2) and a responder analysis (supplemental Table 3) also showed no significant difference between the groups. Analysis of health care utilization (emergency department and hospitalization) and treatment (antibiotic use, oxygen requirement and transfusion) did not show significant differences between the groups over the 2-year treatment period (supplemental Table 4).
Annual rates of respiratory events by treatment group. Data are shown in a Tukey box plot. The horizontal line in the box represents the median. The bottom and top of the box indicate the first and third quartiles; the upper whisker shows the largest value from the top of the box that is no further than 1.5 times the interquartile range (IQR), the lower whisker shows the smallest value from the bottom of the box to within 1.5 times the IQR, and data beyond the ends of the whiskers are displayed as points. The mean (♦; ± standard error of the mean [SEM]) annual rates of respiratory events at baseline, intervention year 1, and intervention year 2 were 3.91 ± 0.35, 3.34 ± 0.37, and 1.54 ± 0.37 and 4.34 ± 0.35, 4.28 ± 0.36, and 1.49 ± 0.37, respectively, for monthly standard-dose and high-dose oral vitamin D3. The rates did not differ significantly between the groups (P = .24), but they decreased significantly during year 2 in both groups (P = .0005).
Annual rates of respiratory events by treatment group. Data are shown in a Tukey box plot. The horizontal line in the box represents the median. The bottom and top of the box indicate the first and third quartiles; the upper whisker shows the largest value from the top of the box that is no further than 1.5 times the interquartile range (IQR), the lower whisker shows the smallest value from the bottom of the box to within 1.5 times the IQR, and data beyond the ends of the whiskers are displayed as points. The mean (♦; ± standard error of the mean [SEM]) annual rates of respiratory events at baseline, intervention year 1, and intervention year 2 were 3.91 ± 0.35, 3.34 ± 0.37, and 1.54 ± 0.37 and 4.34 ± 0.35, 4.28 ± 0.36, and 1.49 ± 0.37, respectively, for monthly standard-dose and high-dose oral vitamin D3. The rates did not differ significantly between the groups (P = .24), but they decreased significantly during year 2 in both groups (P = .0005).
Serum 25-hydroxyvitamin D concentrations
As shown in Figure 4, the overall mean 25-OHD concentration during the 2-year treatment period was significantly higher in the high-dose group (mean 36.1 ng/mL) versus the standard-dose group (mean 19.1 ng/mL; P < .001). The 25-OHD concentrations of groups receiving 12 000 and 100 000 IU/mo of vitamin D3 did not differ statistically at baseline, but they plateaued at different levels at 3-4 months. For the final 20 months of the 24-month study, the standard-dose group stabilized at a mean concentration of 19.3 ng/mL, with 60% of the observations in the insufficient range (25-OHD, 12-20 ng/mL)36 and 15% in the deficient range (25-OHD <12 ng/mL).36 The high-dose group stabilized at a mean concentration of 37.0 ng/mL, with 98% in the sufficient range.14
Mean serum 25-hydroxyvitamin D concentrations by treatment group. The mean serum 25-OHD concentrations of the groups receiving standard-dose (12 000 IU/mo) and high-dose (100 000 IU/mo) vitamin D3 did not differ statistically at baseline and rose to plateau at different levels at 3-4 months; overall mean concentration during the 2-year treatment period was significantly higher in the high-dose group (36.1 ng/mL) compared with the standard-dose group (19.1 ng/mL; P < .001). Error bars indicate SEM.
Mean serum 25-hydroxyvitamin D concentrations by treatment group. The mean serum 25-OHD concentrations of the groups receiving standard-dose (12 000 IU/mo) and high-dose (100 000 IU/mo) vitamin D3 did not differ statistically at baseline and rose to plateau at different levels at 3-4 months; overall mean concentration during the 2-year treatment period was significantly higher in the high-dose group (36.1 ng/mL) compared with the standard-dose group (19.1 ng/mL; P < .001). Error bars indicate SEM.
Pulmonary function tests and hand dynamometry
Improvement in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were observed over time from baseline to year 2, without significant differences between the groups. However, there were no changes in % predicted FVC and %predicted FEV1 (Table 2). Neither treatment had a significant effect on FENO. Similarly, no significant differences in hand-grip strength were observed over time and between the 2 groups (Table 2).
Safety monitoring
A total of 291 adverse events was reported, including 55 serious adverse events in 20 subjects, most of which were expected for sickle cell disease; none were attributed to the study drug (Table 3). There were 17 abnormal safety laboratory values; all were determined to be clinically insignificant, without requiring the withholding or discontinuation of study treatment.
Discussion
In children and adolescents with sickle cell disease who received monthly bolus doses of oral vitamin D3 under observation for 2 years, the annual rates of respiratory events (respiratory infections, asthma exacerbations, or ACS) decreased by >50% during the second year of treatment, with similar reductions in the groups treated with 100 000 IU/mo or with 12 000 IU/mo. The treatment groups did not differ significantly with respect to pulmonary function and hand-grip strength. Both doses were safe, with no significant differences in adverse events.
This is the first randomized clinical trial to examine the potential benefit of vitamin D for preventing respiratory complications in sickle cell disease. We chose monthly dosing to allow for directly observed administration and avoid nonadherence with daily dosing. Treatment duration was sufficiently prolonged to allow for seasonal variation. Our primary outcome measure was collected biweekly using a validated questionnaire38 to adequately capture all respiratory events and was verified by a review of medical records. Because we could not include a placebo group, given the known risks for skeletal harm with vitamin D deficiency,14,35-37 the decrease in the annual rates of respiratory events during year 2 cannot be ascribed unequivocally to vitamin D supplementation. Nonetheless, no significant differences in the mean weekly rates of influenza-like illnesses in New York City during the baseline and study years (2012-2015) were found by the New York State Department of Health Influenza-like Illness Surveillance Network.43 Moreover, because recruitment for the trial extended over 18 months, overlap between study participants receiving supplementation during years 1 and 2 was considerable and would mitigate the impact of epidemiologic trends in respiratory illnesses that may have occurred during the course of the trial. For example, year-2 treatment of patients enrolled in months 1-6 of the trial coincided with year-1 treatment of patients enrolled in months 12-18.
In this study, the effects of standard- and high-dose vitamin D on respiratory events were statistically indistinguishable. The similarity of the response to the monthly standard- and high-dose vitamin D might be interpreted to suggest that monthly standard-dose vitamin D meets the requirement for respiratory health. In the standard-dose group, the estimated mean dietary vitamin D of 228.6 IU/d, together with the monthly supplement providing ∼400 IU/d, would meet the current Estimated Average Requirement for vitamin D of 600 IU/d.14 Nonetheless, 25-OHD concentrations were at insufficient or deficient levels for skeletal health in 75% of the standard-dose group, whereas they were in the sufficient range in 98% of the high-dose group. A recent Cochrane review of vitamin D supplementation for sickle cell disease has concluded that previous clinical studies were not of sufficient quality to guide clinical practice.44 Our result provides evidence that the current Estimated Average Requirement for vitamin D of 600 IU/d for skeletal health is inadequate for patients with sickle cell disease when administered as a monthly dose. Although the monthly high-dose vitamin D supplement in our study did raise 25-OHD concentrations into the sufficient range, bolus administration may fail to provide some extraskeletal benefits.29
The timing of the observed decreases in the annual rates of respiratory events and the uniformity in the magnitudes of the reductions with the standard- and high-dose treatments potentially could be explained by the monthly bolus dosing schedule for vitamin D used in the trial. Since the design of our study, evidence has accumulated that circulating concentrations of vitamin D3, the parent compound, rather than those of 25-OHD, may be vital determinants of the anti-infective, immunomodulatory, and other extraskeletal effects of supplementation.23,45-47 To be active, supplemental vitamin D3 must undergo 2 hydroxylations, first to 25-OHD and then to the functional metabolite, 1,25(OH)2D, which binds to the vitamin D receptor to function as a transcription factor inducing vitamin D–responsive genes. For the principal endocrine functions of vitamin D, bone mineralization, and calcium and phosphate homeostasis, the first hydroxylation takes place using a 25-hydroxylase in the liver,17-18,48 producing 25-OHD that enters the circulation. The second hydroxylation occurs in the kidney, using the 1-α-hydroxylase CYP27B1 to yield 1,25(OH)2D.17,19 By contrast, for extraskeletal autocrine and paracrine activities, vitamin D3, the parent compound, enters cells more easily than 25-OHD, and both hydroxylations may take place intracellularly.45-46,49 CYP27B1 and 25-hydroxylases are widely expressed and the vitamin D receptor is present in most, if not all, cells of the immune system.19
With a single oral bolus dose (12 000 IU) of vitamin D3, the half-life of 25-OHD is on the order of weeks, whereas that of vitamin D3 is ∼1 day. With a single oral bolus dose (100 000 IU) of vitamin D3, 25-OHD remains elevated for >3 months, whereas vitamin D3 returns to near baseline in <1 week.45-46,50-51 The repeated oral bolus doses of vitamin D3 resulted in the plateaus in 25-OHD shown in Figure 4. Although we did not measure vitamin D3 concentrations in our study, neither the standard- nor high-dose treatment would produce sustained increases in circulating vitamin D3, potentially explaining the uniformity of response to the 2 treatments. Vitamin D3 in blood seems to be in diffusional equilibrium with vitamin D3 in adipose tissue.45 With bolus doses, vitamin D3 in excess of use will accumulate in fat and gradually raise circulating vitamin D3 concentrations. A year of treatment with bolus dosing was potentially needed to permit sufficient amounts of vitamin D3 to circulate, accumulate within cells of the immune system, and produce the observed reductions in the rates of respiratory events. In shorter trials of bolus oral doses of vitamin D3, insufficient durations of treatment could explain the lack of effect on respiratory infections,29,52 perhaps in conjunction with other possible explanations for the lack of a protective effect of bolus doses of vitamin D.53-57 A study comparing the effects of daily vitamin D3 (3333 IU) with monthly bolus vitamin D3 (100 000 IU) on respiratory complications, together with measurements of serum vitamin D3, could test these hypotheses.
Our study had several limitations. As noted earlier, a placebo group could not be included in the trial design because of the established risks of inadequate mineralization of the skeleton associated with vitamin D deficiency.14,35-37 The primary outcome of the trial, the annual rate of respiratory events, relied upon parent/self-reported responses to the respiratory questionnaire. Although episodes of asthma exacerbations and ACS were reliably verified by review of documented medical and hospital visits, respiratory infections constituted 93% of the respiratory events. The respiratory questionnaire had a high sensitivity (88%) and specificity (77%) for the viral respiratory pathogens examined,38 but parent/self-reported responses are subject to misclassification and, potentially, unconscious response bias. When the study was designed, we relied upon serum 25-OHD as the indicator of vitamin D status and did not include measurement of the parent compound vitamin D3. Our sample size provided power to detect a difference between the study treatments >80%, whereas the numbers needed to detect a difference in individual events, such as asthma exacerbations or ACS, would be much larger. Finally, because the majority of our patients were treated with hydroxyurea, the prevalence of ACS was reduced.
Children with sickle cell disease typically have low vitamin D status,30-31 heightened susceptibility to respiratory infections and asthma, and a greatly increased vulnerability to potentially fatal complications of respiratory illness. Our study results provide evidence for a substantial protective effect of vitamin D against sickle cell respiratory complications that was manifest only after a year of bolus administration. Daily administration would produce sustained increases in the concentration of circulating vitamin D3 and could potentially show a more rapid protective effect, as well as differences in the response to standard- and high-dose treatments.45-46 Further studies using daily administration of vitamin D3 could provide a simple and low-cost intervention to help avert serious respiratory complications in sickle cell disease.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are deeply grateful to Millicent Sutton, Zhezhen Jin, and Aviva Sopher for serving as members of the trial Data and Safety Monitoring Board; Maureen Licursi for valuable contributions in the recruitment and coordination of trial participants; Ida Suen for skillful organization of the data collection and management; Susana Hernandez, Maria Debes, and Aurora Valones for study coordination and data collection; Genia Billote for research administrative support; and, most of all, to the patients and their families for their participation in the clinical trial.
This clinical trial was supported by Grant R01 FD003894 from the US Food and Drug Administration Orphan Product Development. Additional support for pilot data and research laboratory assays was provided by the Columbia University Irving Institute for Clinical and Translational Research and National Institutes of Health, National Center for Advancing Translational Sciences Grant UL1 TR000040.
Authorship
Contribution: M.T.L., G.M.B, and M.K. conceived the study; M.T.L., G.M.B, M.K., J.W.N., I.F., R.L.M., and S.C. designed the clinical trial; M.T.L., G.M.B, M.K., I.F., and S.C. acquired the data; D.J.M. analyzed the data; M.T.L., G.M.B, M.K., I.F., D.J.M., and J.W.N. interpreted the data; M.T.L. and G.M.B. wrote the manuscript; and M.K., I.F., S.M.A., R.L.M., S.C., D.J.M., and J.W.N. critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret T. Lee, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, Columbia University Medical Center, Children’s Hospital North 10th Floor, 3959 Broadway, New York, NY 10032; e-mail: ml653@cumc.columbia.edu.




![Figure 3. Annual rates of respiratory events by treatment group. Data are shown in a Tukey box plot. The horizontal line in the box represents the median. The bottom and top of the box indicate the first and third quartiles; the upper whisker shows the largest value from the top of the box that is no further than 1.5 times the interquartile range (IQR), the lower whisker shows the smallest value from the bottom of the box to within 1.5 times the IQR, and data beyond the ends of the whiskers are displayed as points. The mean (♦; ± standard error of the mean [SEM]) annual rates of respiratory events at baseline, intervention year 1, and intervention year 2 were 3.91 ± 0.35, 3.34 ± 0.37, and 1.54 ± 0.37 and 4.34 ± 0.35, 4.28 ± 0.36, and 1.49 ± 0.37, respectively, for monthly standard-dose and high-dose oral vitamin D3. The rates did not differ significantly between the groups (P = .24), but they decreased significantly during year 2 in both groups (P = .0005).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/9/10.1182_bloodadvances.2017013979/3/m_advances013979f3.jpeg?Expires=1768504732&Signature=A-6UXyVjSiUSJfsBzsyacBzceGtqM1ppJqsUJJKlwJvJvcdxWwY8fjXJ27X7lSS7NgZUd6ASmTporx-GyotpWqFFGNMBamFREiuHJkVfFzFMybBGX5VMvFs2sqhwzj2VZBSCKPDaXYARaBmfyqQz0CFmH~T~sX2q~tT20dtc4TE~WszQl-JLVX7rrkJDSmONCyzf9uKCQ5wxhpJbNW96EYvZ7NXWmcPOF4v9AJFk-PCdzRhP6dEt3B5rZ334F6-qsVHHwjyvDdNuGVx5JpqXo1Q9dhrOX3DMWWlN2ozEzFCw2o5UUz4bYFrRGtlO5iBAk~ZLr3ELw8NFlYkp5KuNbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 3. Annual rates of respiratory events by treatment group. Data are shown in a Tukey box plot. The horizontal line in the box represents the median. The bottom and top of the box indicate the first and third quartiles; the upper whisker shows the largest value from the top of the box that is no further than 1.5 times the interquartile range (IQR), the lower whisker shows the smallest value from the bottom of the box to within 1.5 times the IQR, and data beyond the ends of the whiskers are displayed as points. The mean (♦; ± standard error of the mean [SEM]) annual rates of respiratory events at baseline, intervention year 1, and intervention year 2 were 3.91 ± 0.35, 3.34 ± 0.37, and 1.54 ± 0.37 and 4.34 ± 0.35, 4.28 ± 0.36, and 1.49 ± 0.37, respectively, for monthly standard-dose and high-dose oral vitamin D3. The rates did not differ significantly between the groups (P = .24), but they decreased significantly during year 2 in both groups (P = .0005).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/9/10.1182_bloodadvances.2017013979/3/m_advances013979f3.jpeg?Expires=1768504733&Signature=rvNgiso6DzeCw3Avl-lH0jUi4BWkc2fzzjC-MvA~J69DrO8gkeksZjWAt1KYkbC7L5CuzPbGgziiY3MsjS-iiDHFjDTxWyEtWJgShZXclQgiMevs6bk03tlyn-VGGEaXUB80-nXiB-pmA6Zn9rU0ml3fvCEYVPa-8niIHVtP9CcPj2eZbVKaM4lU8YLe0jXdRmu4MLGR~KSdRuk-RjpsxvGt30xFmqMWYCHQDwk3uWojhT7iulJFJES2xJf5l2tyCOrPHm1~UwqgXuEnbOfSzEKaTG~2TzslE8-eLEzr6mB2NRtN6BnLxdZLHHJEGGc1pSLhJEUye8rNKwZjH9jZaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
