Key Points
IUHCT of human cord blood–derived CD34+ cells into fetal NSG mice results in systemic multilineage engraftment with human cells.
Preconditioning with in utero injection of an anti-c-Kit receptor antibody (ACK2) results in an improved rate of engraftment.
Introduction
In utero hematopoietic cell transplantation (IUHCT) is a potential therapy for the treatment of numerous genetic diseases such as hemoglobinopathies, immunodeficiencies, and inborn errors of metabolism.1 In utero therapy offers the benefit of avoiding host myeloablation and immunosuppression and has been shown to be successful in multiple animal models, including mice,2-5 dogs,6,7 pigs,8,9 and sheep.10-12 The timing of IUHCT exposes the transplanted human cells to the normal fetal migratory and developmental cues that facilitate proper stem cell distribution and differentiation.11,12 Clinically, IUHCT has been successful for fetuses with severe combined immunodeficiency (SCID),13,14 but therapeutic uses for other diseases, including hemoglobinopathies, have seen limited success.15 Further investigations identified multiple barriers to successful engraftment, including lack of space within the hematopoietic niche16,17 and the maternal immune system.2,18 Among available animal models of IUHCT, the fetal mouse remains an efficient and reproducible model to study the differentiation of stem cells in a nonirradiated host. NSG (NOD-SCID IL2Rγ-null) mice, which are developed with SCID and IL-2Rγ-null chain mutations, are a robust platform for the engraftment of human hematopoietic cells because they have no endogenous T, B, or natural killer cells.19-22 In this study, we used IUHCT of human CD34+ cells in NSG mice to create a reproducible mouse model to study stem cell engraftment, differentiation, and systemic repopulation after IUHCT.
Methods
Mice
NSG mice were obtained from The Jackson Laboratory. All procedures were performed according to a University of California, San Francisco Institutional Animal Care and Use Committee–approved protocol.
Study design
Human cord blood CD34+ cells were either purchased from AllCells (Alameda, CA) or purified from cord blood collected at time of delivery from normal-term infants. CD34+ cells were isolated and transplanted into fetal mice at embryonic day 13.5 (E13.5) or E14.5 as previously described23 with or without preconditioning with ACK2.17 Chimerism levels were checked at 4-week intervals and at harvest using flow cytometry and immunohistochemistry.
Please see supplemental Methods for a more detailed description of the methods used.
Results and discussion
Stable, multilineage chimerism in humanized mice after in utero transplantation of human cord blood CD34+ cells
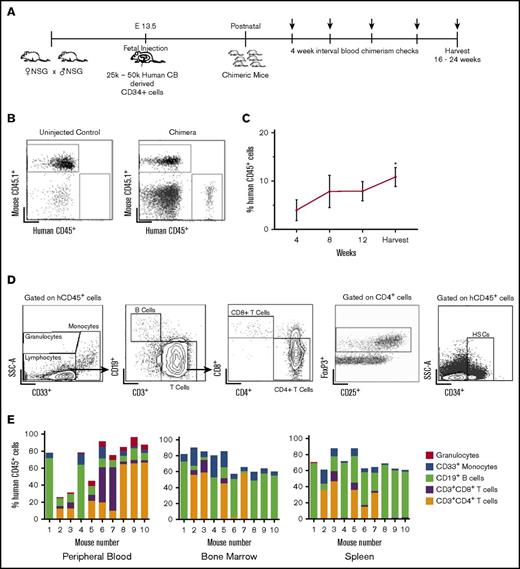
We first determined whether IUHCT in NSG mice would result in efficient engraftment and injected fetal NSG mice at E14.5 with 25 000 to 50 000 human cord blood CD34+ cells per pup using an intrahepatic injection method that we previously described utilizing murine hematopoietic stem cells23 (Figure 1A). A total of 57% of injected dams delivered viable pups; 74 of 206 transplanted pups survived to birth (36%), with 49 pups surviving to wean (24%). This survival rate is lower than we previously reported in wild-type mice24 and likely represents the fragility of the immunodeficient mouse model compared with BALB/c or B6 dams. Overall, 25 of 49 (51%) mice were found to be engrafted with human hematopoietic cells. The levels of human cells in peripheral blood (chimerism) were determined by flow cytometry every 4 weeks for 16 to 24 weeks (Figure 1A-B). Peripheral blood chimerism increased over time in 23 of 25 mice from the initial analysis at the time of 4 weeks after birth to the time of harvest (Figure 1C).
Stable, multilineage chimerism in humanized mice after in utero transplantation of human cord blood CD34+cells. (A) Experimental design for in utero transplantation timing and measurements of blood chimerism levels. Arrows indicate time points for peripheral blood chimerism checks. (B) Representative gating strategy of peripheral blood to detect human CD45+ cells. (C) Percentage of human CD45+ cells in peripheral blood over time (n = 25, *P < .05, **P < .01 by Student t test comparing chimerism levels each week to the initial 4-week level). (D) Representative gating strategy for lineage-specific chimerism, FoxP3+ cells, and confirmation of the presence of CD34+ cells within the bone marrow. Chimeric mice demonstrated different relative proportions of granulocytes, monocytes, B cells, and T cells. (E) Compiled lineage data in 10 individual mice with evidence of CD34+ cells in the bone marrow (n = 10). CB, cord blood; SSC-A, side scatter.
Stable, multilineage chimerism in humanized mice after in utero transplantation of human cord blood CD34+cells. (A) Experimental design for in utero transplantation timing and measurements of blood chimerism levels. Arrows indicate time points for peripheral blood chimerism checks. (B) Representative gating strategy of peripheral blood to detect human CD45+ cells. (C) Percentage of human CD45+ cells in peripheral blood over time (n = 25, *P < .05, **P < .01 by Student t test comparing chimerism levels each week to the initial 4-week level). (D) Representative gating strategy for lineage-specific chimerism, FoxP3+ cells, and confirmation of the presence of CD34+ cells within the bone marrow. Chimeric mice demonstrated different relative proportions of granulocytes, monocytes, B cells, and T cells. (E) Compiled lineage data in 10 individual mice with evidence of CD34+ cells in the bone marrow (n = 10). CB, cord blood; SSC-A, side scatter.
Flow cytometry of bone marrow, peripheral blood, and spleen demonstrated the presence of different hematopoietic lineages in individual litters (Figure 1D). The transplanted stem cells gave rise to both human B cells (CD19+) and T cells (both CD4 and CD8). Foxp3+ regulatory T cells were also detected in chimeric mice, particularly in the bone marrow (Figure 1D). Additionally, we established the presence of human CD34+ cells in the bone marrow of chimeric animals (Figure 1D). Compiled lineage data from 10 individual mice with bone marrow CD34+ cell engraftment demonstrate the range of human lineage distribution in this model (Figure 1E). We looked for the presence of human red blood cells in our mice but did not detect any. This finding is consistent with other reports in humanized mice, and the reason is thought to be secondary to destruction of human red blood cells by host murine macrophages and murine complement.25,26
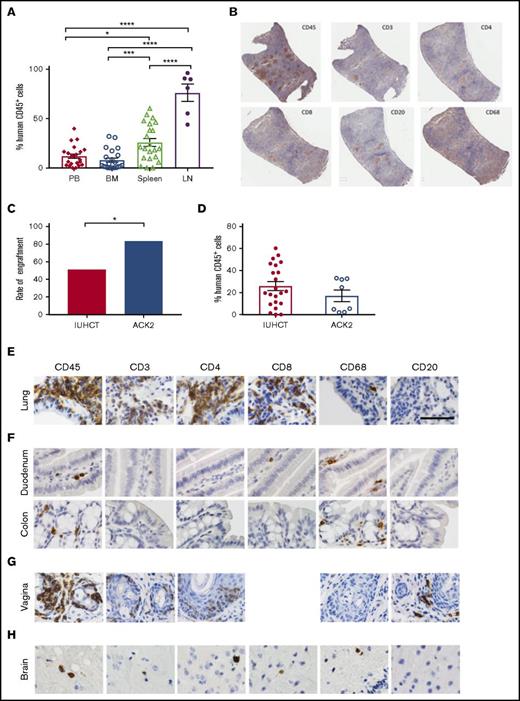
Circulating levels of human cells were lower than those seen in the lymphoid organs of chimeric animals (Figure 2A). The engraftment results determined by flow cytometry were supported by histological analysis. In the spleens, we detected T cells in organized structures similar to lymphoid follicles and a smaller portion of B cells in structures similar to germinal centers (Figure 2B). We found higher percentage of T-cell engraftment than typically noted in adult transplanted NSG mice, which may reflect homeostatic proliferation of T cells, as our CD34+ human samples were not completely pure and did contain a percentage of human T cells (3% to 17%). Earlier transplantation of human cells into NSG mice results in higher percentage of T cells when compared with adult transplantation,27 which may be taking place within our model. Although chronic graft-versus-host disease (GVHD) has been implicated in high T-cell numbers in NSG mice that receive human CD34+ cells,28 our mice showed no physical evidence of GVHD at 24 weeks. Our mice also had B cell percentages as high as 50% to 65%, which is further evidence of true engraftment rather than chronic GVHD. Our experience with in utero injections have also demonstrated that the fetal host is remarkably resistant to GVHD.29
Human CD45+cells show systemic multilineage engraftment with increased engraftment rates following ACK2 treatment. (A) Percentage of human CD45+ cells in lymphoid tissues of chimeric mice and peripheral blood. *P < .05, ***P < .001, ****P < .0001 (analysis of variance with Tukey’s honest significance test). (B) Immunohistochemistry of harvested spleen from a T-cell–dominant mouse demonstrating organized lymphoid follicles. (original magnification ×10; immunohistochemical analysis was performed on fixed/paraffin-embedded tissue sections for human CD45+, CD3+, CD4+, CD8+, CD20+, and CD68+ then counterstained with hematoxylin) (C) Effect of ACK2 treatment on the rates of engraftment (the proportion of surviving animals with ≥1% chimerism in any tissue). (D) Overall chimerism levels. (E-H) NSG mice treated with in utero transplantation of human CD34+ cells demonstrate populations of human cells, within the lung alveolar and bronchiole tissue (E), small and large bowel (F), female reproductive tract (G), and brain (H) (original magnification ×40; immunohistochemical analysis was performed on fixed/paraffin-embedded tissue sections for human CD45+, CD3+, CD4+, CD8+, CD68+, and CD20+ and counterstained with hematoxylin). BM, bone marrow; LN, lymph nodes; PB, peripheral blood.
Human CD45+cells show systemic multilineage engraftment with increased engraftment rates following ACK2 treatment. (A) Percentage of human CD45+ cells in lymphoid tissues of chimeric mice and peripheral blood. *P < .05, ***P < .001, ****P < .0001 (analysis of variance with Tukey’s honest significance test). (B) Immunohistochemistry of harvested spleen from a T-cell–dominant mouse demonstrating organized lymphoid follicles. (original magnification ×10; immunohistochemical analysis was performed on fixed/paraffin-embedded tissue sections for human CD45+, CD3+, CD4+, CD8+, CD20+, and CD68+ then counterstained with hematoxylin) (C) Effect of ACK2 treatment on the rates of engraftment (the proportion of surviving animals with ≥1% chimerism in any tissue). (D) Overall chimerism levels. (E-H) NSG mice treated with in utero transplantation of human CD34+ cells demonstrate populations of human cells, within the lung alveolar and bronchiole tissue (E), small and large bowel (F), female reproductive tract (G), and brain (H) (original magnification ×40; immunohistochemical analysis was performed on fixed/paraffin-embedded tissue sections for human CD45+, CD3+, CD4+, CD8+, CD68+, and CD20+ and counterstained with hematoxylin). BM, bone marrow; LN, lymph nodes; PB, peripheral blood.
Fetal host conditioning increases engraftment rate
Although the in utero transplantation model allows transplantation of allogeneic or xenogeneic cells without host conditioning, the levels of engraftment could be improved by using a nontoxic strategy to increase space in the hematopoietic niche. One such strategy is in utero treatment with ACK2, a monoclonal antibody that depletes hematopoietic stem cells (HSCs) by blocking the function of the murine c-Kit receptor.16 We have previously demonstrated that this antibody can be used in the fetal transplantation setting to improve engraftment of mouse fetal liver–derived HSCs.17 Because murine ACK2 does not inhibit human HSC survival, we next coinjected 2.5 µg/pup of ACK2 along with human cord blood–derived CD34+ cells at E14.5. Based on our previous work, human HSCs are only inhibited at doses 10× this dose, and this is the optimum dose for murine fetal HSC depletion.17 Survival of injected pups was 33% (16/49) to birth and 24% (12/49) survival to wean, with no significant difference in the survival outcomes compared with injection of cells alone. Ten of the 12 surviving pups were chimeric (83%), representing a significant increase in the rates of engraftment compared with no ACK2 treatment (P < .05) (Figure 2C). The overall levels of chimerism were not significantly different (Figure 2D).
We did not observe the significant human myeloid cell engraftment seen in other models of NSG mice that use irradiation. We believe this may be secondary to the less space created in the niche with ACK2 relative to irradiation. Additionally, stem cell factor expression is increased with irradiation. In a transgenic NSG model that expressed membrane-bound human stem cell factor, human myeloid cell engraftment is increased in both irradiated and nonirradiated hosts.27,30 Our levels of granulomonocytic hematopoiesis are comparable to those found in models of NSG mice in which there is no irradiation for preconditioning.27 However, because ACK2 increases the rate of chimerism, this strategy can be used as an adjunct to improve the efficiency of in utero transplantation.
Engraftment of human lymphocytes in critical mucosal sites after in utero transplantation
Given the importance of hematopoietic reconstitution of mucosal surfaces for the study of human disease, we assessed for human cells in respiratory, reproductive, and gastrointestinal mucosa using immunohistochemistry and flow cytometry. This analysis revealed a relatively high frequency of human cells in all examined mucosal sites. Local tissue environment appeared to guide HSC differentiation, as we found different ratios of human leukocytes in different tissues. In the lungs, the majority of human (CD45+) hematopoietic cells were hCD3+, hCD4+ and hCD8+ T lymphocytes (Figure 2E), with fewer macrophages. In the gastrointestinal tract, we observed human CD45+ cells in both the small and large intestine (Figure 2F). Donor-derived CD45+ human cells were also observed throughout the tissues of the female reproductive tract (including the vagina, cervix, and uterus) (Figures 1 and 2G). We did not observe any significant GVHD in transplanted mice (supplemental Figure 2).
Engraftment of human lymphocytes in the brain after in utero transplantation
The ability to transplant human cells prior to formation of the blood–brain barrier, which occurs at ∼E15.5 in mice,31 may present an opportunity to engraft human cells within the brain. We observed human hematopoietic cells (CD45+) in the brainstem and cerebral cortex of chimeric mice; serial sectioning revealed these cells to be T cells (hCD3+, hCD4+, and hCD8+) and hCD68+ macrophages (Figure 2H). The presence of human cells in the brain raises the possibility of using in utero transplantation to deliver cells into the brain for the treatment of neurological disorders.
In this study, we have demonstrated efficient systemic engraftment of human hematopoietic stem cells in NSG mice after in utero transplantation without the need for host irradiation. In future experiments, it will be important to determine whether repeated transplantation of human CD34+ further boosts engraftment and to investigate the ability of the stem cells in these mice to repopulate secondary recipients. This model will be useful for analysis of human stem cell plasticity, in vivo repopulation, and tissue-specific immunity during infection.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the members of the T.C.M., T. D. Burt, and J.V.G. labs for helpful discussions and technical assistance with different aspects of this work.
This work was supported by the Eli and Edythe Broad Stem Cell Fellowship Grant TG2-01153 (R.G.W.), California Institute of Regenerative Medicine New Physician Scientist Translational Research Award RN3-06532 (T.C.M), and National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases grants AI111899, AI073146, AI111899, and National Institute of Mental Health grant MH108179 (all J.V.G.). L.B.B. was supported by NIH, National Institute of Allergy and Infectious Diseases grant T32AI007151.
Authorship
Contribution: T.C.M., S.C.D., R.G.W., and E.M.K. designed the experiments; R.G.W., S.C.D., C.B., and P.W.M. performed the in utero injections, tissue harvesting, and flow cytometry; L.B.B., R.C., P.T.H., P.T., N.S., and J.V.C. performed immunohistochemistry of harvested tissues and data analysis; and R.G.W. wrote the manuscript with assistance from L.B.B., J.V.G., and T.C.M.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tippi C. MacKenzie, 35 Medical Center Way, Box 0665, Room 903D, San Francisco, CA 94143-0665; e-mail: tippi.mackenzie@ucsf.edu.