Key Points
Tungsten-induced rhEPO aggregates in clinical lots are associated with rhEPO-neutralizing antibodies and PRCA.
T-cell responses differentiate nonaggregated from aggregated rhEPO, confirming immunogenicity of tungsten-induced rhEPO aggregates.
Abstract
Immunogenicity of biotherapeutics and the elicitation of anti-drug antibodies are a key concern for their efficacy, pharmacokinetics, and safety. A particularly severe consequence of immunogenicity of a biotherapeutic is the rare development of antibody-mediated pure red cell aplasia (PRCA) in anemic patients treated with aggregated forms of recombinant human erythropoietin (rhEPO). Here, we investigated in vitro T-cell responses to experimentally heat-induced rhEPO aggregates, and to tungsten-induced rhEPO aggregates in clinical lots associated with rhEPO-neutralizing antibodies and PRCA. Heat-stressed rhEPO elicited T-cell responses only in blood obtained from healthy individuals identified as responders, whereas nonstressed rhEPO overall did not induce reactions neither in responders nor nonresponders. Tungsten-induced rhEPO aggregates in clinical lots associated with rhEPO-neutralizing antibodies and PRCA could induce in vitro T-cell responses in blood obtained from healthy donors, in contrast to rhEPO from low tungsten syringes. Importantly, ex vivo T-cell recall responses of patients treated with rhEPO without PRCA showed no T-cell responses, whereas T cells of a patient who developed PRCA after treatment with a clinical batch with elevated levels of tungsten and rhEPO aggregates showed a clear response to rhEPO from that clinical batch. To our knowledge, this is the first time that T-cell assays confirm the root cause of increased rhEPO immunogenicity associated with PRCA.
Introduction
Immunogenicity of biotherapeutics and the elicitation of anti-drug antibodies (ADA) are a key concern for their efficacy, pharmacokinetics, and safety.1,2 Prediction of clinical immunogenicity on the basis of quality attributes of biopharmaceuticals or by utilizing preclinical in vitro and in vivo screening remain challenging. Even fully human biotherapeutics have the potential for immunogenicity, and one important factor that might enhance potential immunogenicity is protein aggregation.1-9 A particularly severe consequence of immunogenicity of a biotherapeutic is the rare development of antibody-mediated pure red cell aplasia (PRCA) in anemic patients treated with increased levels of aggregated forms of recombinant human erythropoietin (rhEPO).10-12
In a clinical study of anemic predialysis patients, safety, immunogenicity, and efficacy of subcutaneous administration of rhEPO (HX575) were evaluated (www.clinicaltrials.gov, #NCT00701714) and 2 patients with rhEPO-neutralizing antibodies were observed, one of whom developed PRCA.12 Binding and neutralizing anti-rhEPO antibodies were determined by radioimmunoprecipitation (RIP) and an EPO-dependent cell line, and positive antibody results coincided with the development of PRCA in 1 patient (P1) (see Case 1 in Haag-Weber et al for a brief case report).12 Development of PRCA in this individual followed treatment with 1 specific clinical lot of HX575 (Table 1; clinical lot B). Tungsten, used in heat-resistant drilling pins for glass syringe manufacturing, was identified as the most likely root cause for rhEPO aggregation in prefilled syringes of this lot.13 To confirm the root cause hypothesis of tungsten-induced HX575 protein unfolding, aggregation, and possible immunogenicity, we performed various analytical and immunological investigations. Exceptionally, we were able to obtain limited volumes of blood samples from a subset of anemic patients enrolled in the aforementioned clinical study #NCT00701714, about 6 months after premature clinical study discontinuation and safety follow up, including patient P1 who developed rhEPO-neutralizing antibodies and PRCA.12
A low prevalence of preexisting nonneutralizing immunoglobulin M (IgM) and IgG1 anti-EPO antibodies has been reported across various clinical indications.14 Moreover, the presence of anti-EPO IgG4 antibodies has been associated with the development of PRCA.15 Specific high-affinity neutralizing IgG1 and IgG4 antibody responses to rhEPO indicate T-cell–dependent isotype switching.2,16 Because in vitro T-cell assays have been successfully used to evaluate the mechanism of immune responses to biotherapeutics,5-9 vaccines,17,18 and self-antigens in vitro,19,20 we investigated in vitro T-cell responses to experimentally heat-induced rhEPO aggregates, and tungsten-induced rhEPO aggregates in clinical lots associated with rhEPO-neutralizing antibodies and PRCA. Furthermore, we studied ex vivo T-cell recall responses of patients treated with rhEPO without PRCA, and of patient P1 who developed PRCA after treatment with a clinical batch with elevated levels of tungsten and aggregates.12,13 To our knowledge, this is the first time that T-cell assays confirm the root cause of increased rhEPO immunogenicity associated with PRCA.
Methods
Healthy volunteer and patient blood samples
Whole blood was obtained from healthy donors at the Red Cross, Basel, Switzerland, or at Keio University School of Medicine, Tokyo, Japan (supplemental Table 1). Blood collection from HX575-treated patients enrolled in clinical study 2007-22-INJ-17 (www.clinicaltrials.gov, #NCT00701714) at 2 clinical centers for the study was approved by the Ethics Committee of Technical University of Munich. All participants provided written informed consent, and procedures were conducted according to the principles of the Declaration of Helsinki.
Cell preparations
Heparinized blood samples were obtained from healthy volunteers or patients and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation. Coded PBMCs from HX575-treated patients were cryopreserved and shipped to Novartis AG in Basel.
Anti-rhEPO antibody isotype detection in patient serum
As described,12 blood samples for determination of anti-rhEPO antibodies were taken at screening and weeks 8, 16, 24, 40, and 52, and analyzed using a validated RIP assay. In case of a positive RIP assay, a second aliquot was analyzed using a validated neutralizing antibody assay, which determined the ability of anti-rhEPO antibodies found in patient sera to inhibit the rhEPO-dependent growth of UT-7 cells. The neutralizing antibody subclass of patient 1 from study 2007-22-INJ-17 (www.clinicaltrials.gov, #NCT00701714) reported here12 was characterized by a surface plasmon resonance (SPR)-based biosensor immunoassay, where anti-rhEPO antibody in serum is (1) detected by its binding to immobilized rhEPO, (2) confirmed via signal reduction in a competitive binding scenario, and (3) characterized using isotyping. For the assessment of antibody specificity, the positive clinical sample was spiked with rhEPO (at 330 nM). An aliquot spiked with sample buffer instead of rhEPO served as control. All samples were incubated for at least 15 minutes at room temperature before analysis. At a signal reduction of ≥70%,21 compared with the unspiked sample, the interaction was confirmed to be specific and the sample rated as truly positive. For the specific detection and isotyping of bound anti-rhEPO antibodies via signal enhancement, anti–human-IgG, -IgE, and -IgM and anti–human-IgG1, -IgG2, -IgG3, and -IgG4 antibodies were used. The antibody isotype of each positive sample was considered as confirmed when at least 100 RU of sample binding and an additional 100 RU of anti-human isotype confirmatory binding were observed.21 Samples not meeting the binding requirements were documented as “unable to determine”.
HLA-typing
For this analysis, EDTA blood was drawn and genomic DNA was prepared from whole blood. An HLA typing procedure was validated and performed with the NucleoSpin Blood Kit, (Macherey-Nagel) method. A polymerase chain reaction (PCR) process was used as an amplification step to acquire the needed target DNA. LABType SSO Typing Test (One Lambda, Inc.) was used as a DNA-based HLA-typing methodology, which required a post-amplification step to discriminate between the different alleles. Sequence-specific oligonucleotide probes bound to fluorescently coded microspheres to identify alleles encoded by the sample DNA. PCR, hybridization, and detection were performed in single reaction mixtures. First target DNA was PCR-amplified using group-specific primers for HLA-A*, -B*, -Cw*, -DRB1*, and -DQB1*. The PCR product was biotinylated, which allowed it to be detected using R-phycoerythrin (PE)-conjugated streptavidin. Fluorescent intensities of PE on each microsphere were identified using a LABScan 100-flow analyzer. The assignment of the HLA typing was based on the reaction pattern compared with patterns associated with published HLA gene sequence databases (http://hla.alleles.org/alleles/index.html).
Antigen preparations
The characteristics of required materials for expansion and restimulation of T-cell cocultures are described in Table 1 and supplemental Text 1.
Antigen-specific dendritic cell (DC)–T-cell coculture assays
To generate monocyte-derived DCs (MoDCs), freshly prepared PBMCs from healthy volunteers or thawed PBMCs from HX575-treated patients were plated out with a density of 5 × 106 cells/mL in RPMI-1640 medium supplemented with 10% fetal bovine serum, 50 U/mL penicillin, 50 μg/mL streptomycin, 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (all Life Technologies) and seeded in 24-well Primaria plates (BD Falcon) at 2-3 × 106 cells per well. After 24 hours, nonadherent cells were removed, and adherent cells were differentiated into MoDC for 6 to 7 days using complete RPMI-1640 medium supplemented additionally with 50 ng/mL recombinant human colony-stimulating factor (rhGM-CSF) and 50 ng/mL recombinant human interleukin 4 (rhIL-4) (both Novartis). Separately, CD4+ CD25− T cells were isolated from the nonadherent fraction using the CD4+ T Cell Isolation Kit II in combination with an anti-CD25 antibody (Miltenyi Biotec), and used for DC–T-cell coculture assays. To establish DC–T-cell cocultures, immature MoDCs were harvested and reseeded in complete RPMI-1640 medium in 24-well culture plates (Nunc) or 96-well round bottom plates (Costar) at 105 cells per well. The stimuli (placebo, 5 or 10 μg/mL HX575 batches, 10 limit of flocculation units (Lf) per mL tetanus toxoid (TT; batch 317430, Novartis Vaccines) with or without 1 or 10 ppm tungsten extracted from pins and 100 ng/mL lipopolysaccharide (LPS) were added to these cultures for maturation of MoDCs. After 24 hours, autologous CD4+ T cells (2 × 106/well) were added on top of the cells and incubated for further 10 days. Every third day, 30 ng/mL rhIL-2 (Novartis) was added to the culture. After 11 days, cells were harvested and seeded in 96-well flat bottom plates at 5 × 104 cells/well (Nunc). Cocultures were restimulated in the presence of 1 × 104 autologous MoDCs and stimuli (placebo, 5 or 10 μg/mL HX575 batches, and 10 Lf/mL TT with or without 1 or 10 ppm tungsten pin extract) for 48 hours. Afterward, cells were pulsed with 5 μCi 3H-thymidine (PerkinElmer) for an additional 16 hours and the cell proliferation was measured in a β-counter (PerkinElmer). A specific T-cell response shown as stimulation index (SI) was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control. Based on optimization experiments with various HX575 preparations, we defined an SI ≥1.5 as a positive response.
Generation and characterization of T-cell lines (TCLs)
Heparinized peripheral blood obtained from 2 healthy HLA-DRB1*09:01-negative or positive donors (donor 2 and donor 8, respectively; supplemental Table 1) was used to generate TCLs, by incubating T cells with heat-stressed HX575. PBMCs were isolated and cultured (2-3 × 106 cells/well) in RPMI-1640 supplemented with 10% fetal bovine serum, 50 ng/mL rhIL-4, and 50 ng/mL rhGM-CSF on Primaria 24-well culture plates for 7 days. Heat-stressed HX575 (5 μg/mL) was added to immature MoDCs and incubated with 100 ng/mL LPS overnight to induce maturation before being used in the assay. CD4-positive cells were isolated (2.5 × 105/well) using a MACS system and cultured with autologous mature MoDCs (5 × 104/well) in the presence of anti-CD29 monoclonal antibody (2.5 μg/mL; BD Biosciences) on 24-well culture plates for 14 days, with 30 U/mL rhIL-2 added every 3 days. Recovered T cells (5 × 105) were restimulated in complete RPMI-1640 medium with heat-stressed HX575 (5 μg/mL) with irradiated autologous Epstein-Barr virus (EBV)-transformed B-cell lines (106/well) and 100 U/mL rhIL-2 on 24-well round-bottomed culture plates for 10 days. Viable T cells were isolated and subjected to limiting dilution assays (3.125 to 100 cells/ per well). T-cell clones were collected in new tubes and centrifuged at 400 × g for 10 minutes. Supernatant was aspirated and the pellet resuspended in RPMI-1640 medium. An autologous EBV-transformed B-cell line was irradiated at 150 Gy. TCLs (2 × 104/well) were cultured with irradiated EBV-B cells (2 × 104/well) in complete RPMI-1640 medium with individual antigens (5 μg/mL) (Table 1) or EPO synthetic peptides (5 μg/mL; dissolved in dimethylsulfoxide) (Table 2) in 96-well round-bottom plates for 3 days. Cells were incubated with 0.5 μCi/well of 3H-thymidine for an additional 16 hours. Cells were harvested and 3H-thymidine incorporation determined in a Top-Count. Three HX575-reactive TCLs could be confirmed for each donor. Antigen-specific TCLs were characterized by phenotype and T-cell receptor (TCR) Vβ usage.
Flow cytometric TCR Vβ determination
Cultured cells were harvested, centrifuged, and washed once with fluorescence-activated cell sorter (FACS) buffer. Supernatants were discarded, pellets resuspended in FACS buffer, and transferred to a 96-well V-bottom plate for staining. All samples were stained for 30 minutes at 4°C with an anti CD4-antibody (BD BioSciences) and antibody cocktails staining for 24 TCR-Vβ families in 8 separate tubes. Anti–TCR-Vβ antibodies were labeled with PE, fluorescein isothiocyanate, and PE plus fluorescein isothiocyanate (IOTest β Mark, Beckman Coulter). Samples were acquired on an LSRII flow cytometer and analyzed according to the manufacturer’s specifications. Instrument settings were done with manual compensation by the FACS Diva 6 software and analysis of the data were done with FlowJo software.
Short-term PBMC differentiation assay
On day 1, cryopreserved PBMCs from HX575-treated patients were plated (0.2 × 106/well) in a 96-well flat-bottom plate (Costar) in complete RPMI-1640 medium supplemented with 100 ng/mL rhGM-CSF, 80 ng/mL rhIL-4, and different antigens (vehicle control, 5 μg/mL HX575 of clinical lots A and B, or 10 Lf/mL TT). After 24 hours, 100 ng/mL LPS was added to the different conditions as a maturation stimulus. On day 3, cells were pulsed with 5 μCi 3H-thymidine for an additional 16 hours and T-cell proliferation was measured in a β-counter (PerkinElmer) on day 4.22 A specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control. Based on optimization experiments with T cells isolated from PBMCs from HX575-naïve healthy donors and various HX575 preparations, we defined an SI ≥1.5 as a positive response.
Results
IgG1 and IgG4 anti-rhEPO antibody response in a PRCA patient
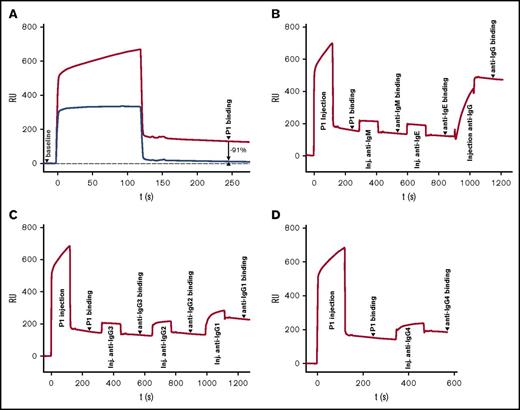
We determined rhEPO specificity (Figure 1A) and antibody subclass of the neutralizing antibodies detected in the HX575-treated patient with PRCA (patient P1; case 1)12 by using surface plasmon resonance. The anti-rhEPO antibody response of this patient consisted of IgG-class antibodies, with IgG1 (61%) and IgG4 (25%) as the major subclasses, and IgG2 (8%) as a minor fraction (Figure 1B-D). High-affinity IgG1 and IgG4 antibody responses to rhEPO indicate T-cell–dependent isotype switching.2,16
Anti-rhEPO antibody response: specificity and IgG subclasses. The baseline on each SPR-based biosensor immunoassay sensorgram represents a signal derived from EPO immobilized to the SPR chip surface, prior to injection of patient sample P1 at t = 0 seconds. “P1 binding” represents material remaining bound after injection of patient sample (as indicated). (A) Verification of rhEPO specificity of patient P1 serum. Injection of 1:10 diluted patient serum (upper line) compared with patient serum spiked with 10 μg/mL rhEPO (lower line), which resulted in 91% relative signal decrease compared to 2 minutes after end of serum injection, demonstrating rhEPO specificity of the P1 patient serum sample. (B) Antibody isotyping: injection of patient sample followed by serial injection of anti-IgM, anti-IgE, and anti-IgG. Only IgG shows significant signal increase (>300%, suggesting multiple binding of the polyclonal anti–human-IgG antibody), confirming ADA as an IgG class molecule. (C) Antibody subclass determination: injection of patient sample followed by serial injection of anti-IgG3: no signal increase (9% signal decrease 2 minutes after end of injection); anti-IgG2: minor increase (8% increase); and anti-IgG1 (strong signal increase of 61% confirming the presence of IgG1 in sample P1). (D) Antibody subclass determination: injection of patient sample followed by injection of anti-IgG4 (25% increase, confirming the presence of IgG4 in sample P1). inj, injection; RU, resonance units; s, seconds; t, time.
Anti-rhEPO antibody response: specificity and IgG subclasses. The baseline on each SPR-based biosensor immunoassay sensorgram represents a signal derived from EPO immobilized to the SPR chip surface, prior to injection of patient sample P1 at t = 0 seconds. “P1 binding” represents material remaining bound after injection of patient sample (as indicated). (A) Verification of rhEPO specificity of patient P1 serum. Injection of 1:10 diluted patient serum (upper line) compared with patient serum spiked with 10 μg/mL rhEPO (lower line), which resulted in 91% relative signal decrease compared to 2 minutes after end of serum injection, demonstrating rhEPO specificity of the P1 patient serum sample. (B) Antibody isotyping: injection of patient sample followed by serial injection of anti-IgM, anti-IgE, and anti-IgG. Only IgG shows significant signal increase (>300%, suggesting multiple binding of the polyclonal anti–human-IgG antibody), confirming ADA as an IgG class molecule. (C) Antibody subclass determination: injection of patient sample followed by serial injection of anti-IgG3: no signal increase (9% signal decrease 2 minutes after end of injection); anti-IgG2: minor increase (8% increase); and anti-IgG1 (strong signal increase of 61% confirming the presence of IgG1 in sample P1). (D) Antibody subclass determination: injection of patient sample followed by injection of anti-IgG4 (25% increase, confirming the presence of IgG4 in sample P1). inj, injection; RU, resonance units; s, seconds; t, time.
T-cell responses differentiate nonaggregated from heat-aggregated rhEPO
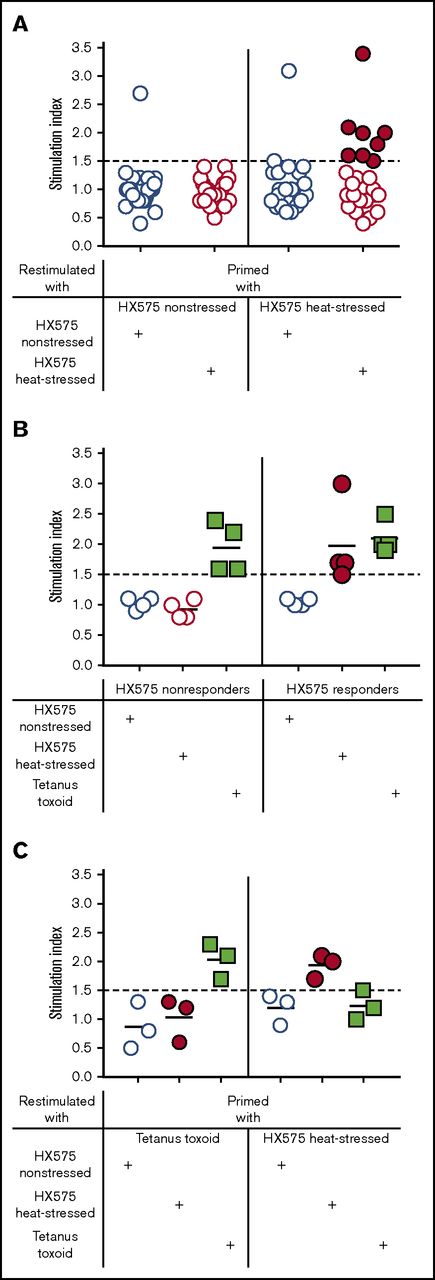
Prior to performing studies with PBMCs from HX575-treated subjects, we first established and optimized an in vitro DC–T-cell coculture assay to detect T-cell responses to aggregated HX575 utilizing PBMCs from healthy volunteers. Based on preliminary results and published data,19 we chose 5 μg HX575/mL (595 IU/mL) as the antigen concentration throughout most of our investigations, which is below EPO concentrations that significantly inhibit the proliferation of T cells (1500 IU/mL).23 We focused our studies on T-cell responses to nonaggregated and experimentally heat-aggregated batches of HX575 (Table 1). PBMCs from HLA-typed HX575-naïve healthy donors were primed with either nonstressed or heat-stressed HX575, and restimulated with either nonstressed or heat-stressed HX575, respectively. Responses to TT were used as positive control in these DC–T-cell cocultures, and as an exclusion criterion, when SI to this recall-antigen were below 1.5. Under these conditions, PBMCs from several healthy donors responded to heat-stressed HX575 (categorized as “responders”), compared with vehicle controls (Figure 2A). PBMCs from those donors who did not respond to heat-stressed HX575 were categorized as “nonresponders.” Two individuals showed responses to nonstressed rhEPO (Figure 2A), possibly due to epitope spreading. Responders used in subsequent experiments repeatedly showed T-cell responses when stimulated with heat-stressed HX575, whereas nonstressed HX575 did not induce reactions neither in responders nor nonresponders (Figure 2B). Both HX575-responders and nonresponders showed reactions to the recall antigen TT, which we consistently found to be relatively moderate across various independent experiments in 2 independent laboratories. This might be due to the immunization history of the blood-donating individuals and/or assay conditions.24-27 Specificity of anti-HX575 T-cell responses was demonstrated by the lack of response of heat-stressed HX575-primed T cells following restimulation with TT as an unrelated antigen, and vice versa (Figure 2C).
T-cell responses differentiate nonaggregated from heat-aggregated rhEPO. (A) T cells from HLA-typed HX575-naïve healthy donors (n = 24) were primed with either nonstressed or heat-stressed HX575, and restimulated with either nonstressed or heat-stressed HX575, respectively (for individual SI data and HLA-haplotypes, see supplemental Table 1). Donors whose T cells responded to heat-stressed HX575 were categorized as “responders” (closed red circles), whereas those donors whose T cells did not respond to heat-stressed HX575 were categorized as “nonresponders” (open red circles). Two individuals showed responses to nonstressed rhEPO, possibly due to epitope spreading. (B) Representative experiment showing T-cell responses to heat-stressed HX575 (closed red circles), nonstressed HX575 (open blue circles), and TT (green squares). (C) Specificity of anti-HX575 T-cell responses was demonstrated by priming PBMCs from HX575-naïve healthy donors with either TT or heat-stressed HX575, and restimulation with either nonstressed, heat-stressed HX575, or TT.
T-cell responses differentiate nonaggregated from heat-aggregated rhEPO. (A) T cells from HLA-typed HX575-naïve healthy donors (n = 24) were primed with either nonstressed or heat-stressed HX575, and restimulated with either nonstressed or heat-stressed HX575, respectively (for individual SI data and HLA-haplotypes, see supplemental Table 1). Donors whose T cells responded to heat-stressed HX575 were categorized as “responders” (closed red circles), whereas those donors whose T cells did not respond to heat-stressed HX575 were categorized as “nonresponders” (open red circles). Two individuals showed responses to nonstressed rhEPO, possibly due to epitope spreading. (B) Representative experiment showing T-cell responses to heat-stressed HX575 (closed red circles), nonstressed HX575 (open blue circles), and TT (green squares). (C) Specificity of anti-HX575 T-cell responses was demonstrated by priming PBMCs from HX575-naïve healthy donors with either TT or heat-stressed HX575, and restimulation with either nonstressed, heat-stressed HX575, or TT.
EPO epitopes and skewed TCR Vβ usage
To identify the epitope(s) responsible for promoting the (autoimmune) T-cell responses to (rh)EPO, we generated TCLs reactive to heat-stressed HX575 from 2 HX575-naïve healthy HLA-DRB1*09:01-negative or -positive donors (donor 2 and donor 8, respectively). Three HX575-reactive TCLs were derived from each of these 2 donors. Figure 3A shows the pronounced proliferative responses of the TCLs from these 2 donors to heat-stressed but not to nonstressed HX575. We then tested the reactivity of these TCLs to 31 synthetic EPO peptides (15- or 16-mers) with 10-residue overlaps (Table 2). TCLs of the HLA-DRB1*09:01-negative donor 2 responded mainly to peptide 27 (Figure 3B), whereas the TCLs from the HLA-DRB1*09:01-positive donor 8 responded mainly to peptide 7 (Figure 3C). These human EPO T-cell epitopes have been described previously.19 Analysis of the TCR Vβ usage of the EPO peptide responsive TCLs showed limited variability within each donor’s lines, but different usage between donors. Single TCR Vβ usage (TCR Vβ2, Vβ8, Vβ13.2, and Vβ22), found in 4 of 6 EPO-responsive TCLs, indicates likely clonality (Figure 3D-G), an observation also reported in antigen-specific T-cell responses and autoimmune disease.28
TCL responses to heat-stressed but not to nonstressed HX575, EPO epitopes, and skewed TCR Vβ usage. (A) TCLs reactive to heat-stressed HX575 were generated from 2 HX575-naïve healthy HLA-DRB1*09:01-negative or -positive donors (donor 2 and donor 8, respectively). Three HX575-reactive TCLs were derived from each of these 2 donors (triangle: donor 2; rhombus: donor 8), which showed pronounced proliferative responses of the TCLs to heat-stressed but not to nonstressed HX575. (B-C) Identification of HX575 T-cell epitopes in donors 2 and 8, by testing TCL reactivity to 31 synthetic EPO peptides (15- or 16-mers) with 10-residue overlaps (Table 2); ctrl = heat-stressed HX575; a specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control; data presented as mean ± SEM; average responses of 3 heat-stressed HX575-specific TCLs from HLA-DRB1*09:01-negative donor 2 (B); average responses of 3 heat-stressed HX575-specific TCLs from HLA-DRB1*09:01-positive donor 8 (C). (D-G) Representative flow cytometric plots showing gating of CD4 T cells, and showing staining for 24 TCR-Vβ families; (D) TCL YS21 derived from HLA-DRB1*09:01-negative donor 2, showing single TCR Vβ22+ usage (to peptide 27); (E) TCL YS34 derived from HLA-DRB1*09:01-negative donor 2, showing single TCR Vβ8+ usage (to peptide 27); (F) TCL TH38 derived from HLA-DRB1*09:01-positive donor 8, showing single TCR Vβ2+ usage (to peptide 7); (G) TCL TH39 derived from HLA-DRB1*09:01-positive donor 8, showing single TCR Vβ13.2+ usage (to peptide 10). FSC, forward scatter; SEM, standard error of the mean; SSC, side scatter.
TCL responses to heat-stressed but not to nonstressed HX575, EPO epitopes, and skewed TCR Vβ usage. (A) TCLs reactive to heat-stressed HX575 were generated from 2 HX575-naïve healthy HLA-DRB1*09:01-negative or -positive donors (donor 2 and donor 8, respectively). Three HX575-reactive TCLs were derived from each of these 2 donors (triangle: donor 2; rhombus: donor 8), which showed pronounced proliferative responses of the TCLs to heat-stressed but not to nonstressed HX575. (B-C) Identification of HX575 T-cell epitopes in donors 2 and 8, by testing TCL reactivity to 31 synthetic EPO peptides (15- or 16-mers) with 10-residue overlaps (Table 2); ctrl = heat-stressed HX575; a specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control; data presented as mean ± SEM; average responses of 3 heat-stressed HX575-specific TCLs from HLA-DRB1*09:01-negative donor 2 (B); average responses of 3 heat-stressed HX575-specific TCLs from HLA-DRB1*09:01-positive donor 8 (C). (D-G) Representative flow cytometric plots showing gating of CD4 T cells, and showing staining for 24 TCR-Vβ families; (D) TCL YS21 derived from HLA-DRB1*09:01-negative donor 2, showing single TCR Vβ22+ usage (to peptide 27); (E) TCL YS34 derived from HLA-DRB1*09:01-negative donor 2, showing single TCR Vβ8+ usage (to peptide 27); (F) TCL TH38 derived from HLA-DRB1*09:01-positive donor 8, showing single TCR Vβ2+ usage (to peptide 7); (G) TCL TH39 derived from HLA-DRB1*09:01-positive donor 8, showing single TCR Vβ13.2+ usage (to peptide 10). FSC, forward scatter; SEM, standard error of the mean; SSC, side scatter.
T-cell responses to rhEPO are HLA-DR or HLA-DQ restricted
The HLA restriction of PBMC-derived T-cell responses from healthy donors to heat-stressed HX575 was investigated using anti–HLA-DR and anti–HLA-DQ blocking antibodies (Figure 4). Responses detected in DRB1*09:01-positive carriers were restricted by HLA-DR, whereas responses in DRB1*09:01-negative individuals (carriers of DQB1*05/06 haplotypes) were HLA-DQ restricted (Figure 4A). Peptide 7–reactive TCLs from HLA-DRB1*09:01-positive healthy donor 8 showed HLA-DR–restricted responses to heat-stressed HX575 (Figure 4B). Peptide 27–reactive TCLs from HLA-DRB1*09:01-negative healthy donor 2 showed HLA-DQ–restricted responses to heat-stressed HX575 (Figure 4C), and peptide 27 (Figure 4D). HLA-DRB1*09 alleles have been reported to be linked to EPO-induced PRCA in Asian populations,29 whereas additional HLA alleles have been identified in non-Asian populations, including HLA-DQB1*03 and HLA-DQB1*06.30
T-cell responses to rhEPO are HLA-DR or HLA-DQ restricted. (A) T cells from responders were primed and restimulated with heat-stressed HX575, and cultured in the presence (+) of isotype control, anti–HLA-DR, or anti–HLA-DQ antibody. HLA-DR and HLA-DQ restriction of T-cell responses to heat-stressed HX575 is shown in HLA-DRB1*09:01-positive (n = 2) and HLA-DRB1*09:01-negative (n = 3) healthy donors, respectively. (B) HLA-DR restriction of TCL responses to heat-stressed HX575 in HLA-DRB1*09:01-positive donor 8. (C) HLA-DQ restriction of TCL responses to heat-stressed HX575 in HLA-DRB1*09:01-negative donor 2. (D) HLA-DQ restriction of TCL responses to peptide 27 in HLA-DRB1*09:01-negative donor 2. A specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control.
T-cell responses to rhEPO are HLA-DR or HLA-DQ restricted. (A) T cells from responders were primed and restimulated with heat-stressed HX575, and cultured in the presence (+) of isotype control, anti–HLA-DR, or anti–HLA-DQ antibody. HLA-DR and HLA-DQ restriction of T-cell responses to heat-stressed HX575 is shown in HLA-DRB1*09:01-positive (n = 2) and HLA-DRB1*09:01-negative (n = 3) healthy donors, respectively. (B) HLA-DR restriction of TCL responses to heat-stressed HX575 in HLA-DRB1*09:01-positive donor 8. (C) HLA-DQ restriction of TCL responses to heat-stressed HX575 in HLA-DRB1*09:01-negative donor 2. (D) HLA-DQ restriction of TCL responses to peptide 27 in HLA-DRB1*09:01-negative donor 2. A specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control.
T-cell responses differentiate nonaggregated from tungsten-aggregated rhEPO
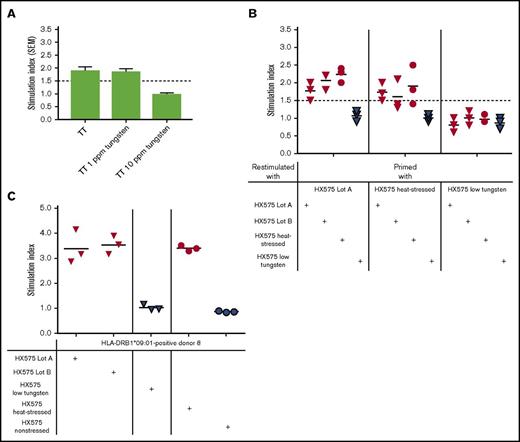
Having identified healthy volunteers (HX575-naïve), whose PBMCs responded to heat-stressed HX575, we next investigated the relationship between tungsten content of suspected clinical lots A and B, the presence of increased HX575 aggregates, and in vitro T-cell activation. Concentrations of tungsten and HX575 aggregates were determined in clinical and experimental samples subsequently used for DC–T-cell coculture assays (Table 1). Clinical lots analyzed in the DC–T-cell coculture assays were characterized as follows: HX575 drug product solutions from individual prefilled syringes were screened for the presence of dimers and higher-order aggregates, and the residual content of those syringes with aggregation levels >2% was pooled (separately for lots A and B) without any further preparatory manipulation to avoid the introduction of any unknown factors, possibly influencing the read-out of the DC–T-cell assays. Tungsten levels in pooled syringes were 2.4 ppm and 2.9 ppm and the sum of dimers and higher-order aggregates present in clinical lots A and B was 3.1% and 2.2%, respectively (Table 1). Dimers and higher-order aggregates were found at an ∼1:1 ratio (Table 1). Both clinical lots were associated clinically with the development of neutralizing antibodies to rhEPO.12,13 Lot B was associated with the development of PRCA.12,13 We assessed the potential toxicity of soluble tungstate (Na2WO4)31 on TT-specific T-cell responses in the presence of 0, 1, or 10 ppm tungsten pin extract, and observed a tungsten-dependent inhibition of T-cell proliferation at 10 ppm tungsten pin extract (Figure 5A). This is above the levels found in various HX575 preparations (Table 1). PBMC-derived T cells from 3 HX575-naïve healthy donors (responders 2, 4, and 6; supplemental Table 1) primed with clinical lot A showed a marked response upon re-stimulation with clinical lot A (SI 1.5, 2.0, and 1.8), clinical lot B (SI 1.8, 2.2, and 2.2), and heat-stressed HX575 (SI 2.0, 2.3, and 2.4), but not to HX575 obtained from low tungsten syringes (Figure 5B, left). T cells primed with heat-stressed HX575 proliferated in response to incubation with both clinical lots, however, these T cells did not respond to HX575 obtained from low tungsten syringes (Figure 5B, middle). T cells primed with HX575 obtained from low tungsten syringes failed to respond to any of HX575 lots examined (SI between 0.6 and 1.2) (Figure 5B, right), suggesting that T-cell epitope(s) were not generated in DC–T-cell cocultures containing HX575 from low-tungsten syringes. No dimers or aggregates were detected in HX575 from low-tungsten syringes (Table 1). These syringes have a specified low median level of not more than 0.05 ppm soluble tungsten, based on an improved syringe manufacturing process. Furthermore, TCLs reactive to heat-stressed HX575, established from healthy HLA-DRB1*09:01-positive donor 8 (Figure 5C), showed marked responses to preparations of experimentally heat-stressed HX575 as well as to clinical lot A and B, but not to nonstressed or low-tungsten preparations (Figure 5C). These findings indicated that the suspect clinical lots induce in vitro immunogenic responses and are comparable to heat-stressed HX575, whereas HX575 obtained from low-tungsten syringes was not immunogenic in the DC–T-cell coculture assay, or using TCLs.
T-cell responses differentiate nonaggregated from tungsten-aggregated rhEPO. (A) PBMCs from healthy donors were primed with TT, and restimulated with TT in the presence of 0, 1, or 10 ppm tungsten pin extracts; data presented as mean ± SEM. (B) T cells from 3 normal HX575-naïve healthy donors were primed with clinical lot A, a batch of heat-stressed HX575, or low-tungsten HX575, and restimulated with clinical lot A or B (red triangles), heat-stressed HX575 (red circles), or low-tungsten HX575 (blue triangles). (C) TCLs reactive with heat-stressed HX575, established from healthy HLA-DRB1*09:01-positive donor 8, were restimulated with clinical lot A or B (red triangles), low-tungsten HX575 (blue triangles), nonstressed HX575 (blue circles), or heat-stressed HX575 (red circles). A specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control. SEM, standard error of the mean.
T-cell responses differentiate nonaggregated from tungsten-aggregated rhEPO. (A) PBMCs from healthy donors were primed with TT, and restimulated with TT in the presence of 0, 1, or 10 ppm tungsten pin extracts; data presented as mean ± SEM. (B) T cells from 3 normal HX575-naïve healthy donors were primed with clinical lot A, a batch of heat-stressed HX575, or low-tungsten HX575, and restimulated with clinical lot A or B (red triangles), heat-stressed HX575 (red circles), or low-tungsten HX575 (blue triangles). (C) TCLs reactive with heat-stressed HX575, established from healthy HLA-DRB1*09:01-positive donor 8, were restimulated with clinical lot A or B (red triangles), low-tungsten HX575 (blue triangles), nonstressed HX575 (blue circles), or heat-stressed HX575 (red circles). A specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control. SEM, standard error of the mean.
Recall T-cell response confirms immunogenicity of tungsten-induced rhEPO aggregates associated with PRCA
Importantly, we had the opportunity to investigate whether PBMCs from HX575-treated anemic PRCA patient P1 would show an antigen-specific recall response to heat-stressed or tungsten-induced HX575 aggregates in clinical lot B, compared with 2 additional HX575-treated patients P2 and P3, who did not develop neutralizing antibodies nor PRCA. Patient P1 who was treated with lot B shortly before having developed the first signs of IgG1 and IgG4 rhEPO neutralizing antibodies (Figure 1), subsequently developed PRCA.12 PBMCs from all patients showed recall responses to TT, indicating the validity of the assays (Figure 6). Heat-stressed HX575 induced a slight increase in T-cell responses in cells of the patient P1 (SI = 1.5), but not in T cells of the other 2 patients (SI = 1.2 and SI = 1.3, respectively) (Figure 6A). Furthermore, using an accelerated DC–T-cell coculture assay,22 only patient P1 showed a robust antigen-specific T-cell response to a pooled preparation of clinical lot B (SI = 3.2), but not to clinical lot A (SI = 1.1), after a single antigen stimulation (Figure 6B). T cells from the other 2 HX575-treated individuals (patients P2 and P3) did not respond to either clinical lot. The prompt T-cell response of patient P1 after 72 hours without restimulation indicated memory to aggregated HX575 in clinical lot B. Table 3 shows that PRCA patient P1 carries HLA-DQB1*06:03, whereas HX575-treated patients P2 and P3, who did not develop neutralizing antibodies, share HLA-DQB1*03.
Recall T-cell response confirms immunogenicity of tungsten-induced rhEPO aggregates associated with PRCA. (A) T cells obtained from 3 patients treated with HX575 during the clinical trial INJ-17 were incubated with nonstressed or heat-stressed HX575, or TT as control. (B) PBMCs obtained from 3 patients treated with HX575 during the clinical trial INJ-17 were incubated with clinical lot A or B, or TT as control in a short-term PBMC differentiation assay22 ; a specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control; treatments were performed in an anonymized manner.
Recall T-cell response confirms immunogenicity of tungsten-induced rhEPO aggregates associated with PRCA. (A) T cells obtained from 3 patients treated with HX575 during the clinical trial INJ-17 were incubated with nonstressed or heat-stressed HX575, or TT as control. (B) PBMCs obtained from 3 patients treated with HX575 during the clinical trial INJ-17 were incubated with clinical lot A or B, or TT as control in a short-term PBMC differentiation assay22 ; a specific T-cell response shown as SI was calculated by a ratio of cell proliferation with individual stimuli divided by the cell proliferation with vehicle control; treatments were performed in an anonymized manner.
Discussion
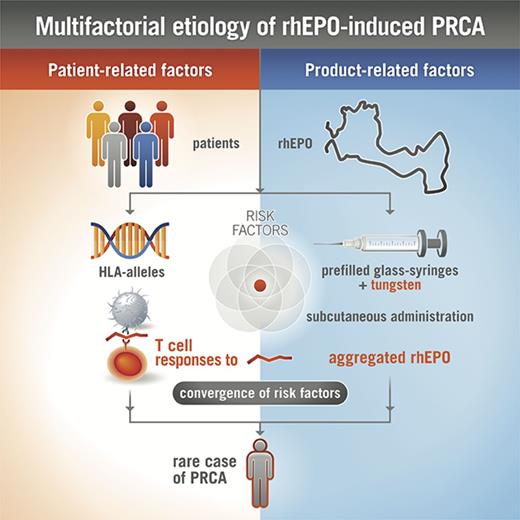
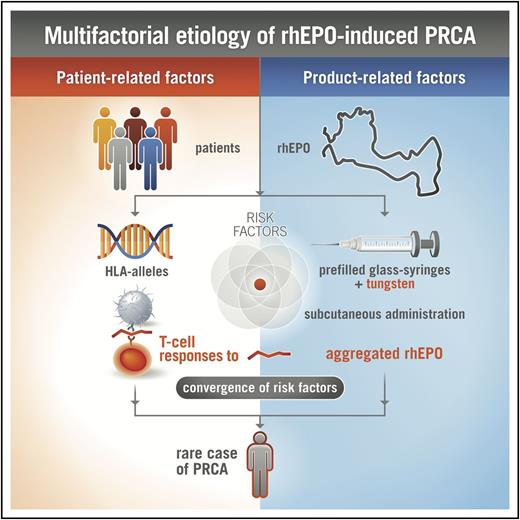
rhEPO has been used as treatment of anemia for many years, and is generally well tolerated in the majority of treated subjects. However, in rare cases, when rhEPO elicits a neutralizing ADA response cross-reacting with endogenous EPO, antibody-mediated PRCA develops.10-12 The reasons for increased immunogenicity of rhEPO leading to antibody-mediated PRCA have not been satisfactorily clarified. In particular, the impairments to central and peripheral immunological tolerance that lead to neutralizing antibody formation against endogenous EPO are not well understood. Most likely product-related (eg, route of administration, aggregation state) and host-related factors (eg, HLA alleles) influence the development of rare adverse events, such as antibody-mediated PRCA (Figure 7).11
Multifactorial etiology of rhEPO-induced PRCA. The complex interaction between various product-related and patient-related factors, including the formation of rhEPO aggregates, antigenic processing, and presentation of immunodominant epitopes by antigen-presenting cells, presence of specific HLA class II proteins capable of binding certain rhEPO peptides, and potentially other characteristics of EPO, together influence the immune response to rhEPO after subcutaneous injection, leading to rare cases of PRCA in susceptible patients.
Multifactorial etiology of rhEPO-induced PRCA. The complex interaction between various product-related and patient-related factors, including the formation of rhEPO aggregates, antigenic processing, and presentation of immunodominant epitopes by antigen-presenting cells, presence of specific HLA class II proteins capable of binding certain rhEPO peptides, and potentially other characteristics of EPO, together influence the immune response to rhEPO after subcutaneous injection, leading to rare cases of PRCA in susceptible patients.
Here, we retrospectively link quality data of rhEPO study medication to the observed immunogenicity results of a phase 3 clinical trial (www.clinicaltrials.gov, #NCT00701714),12 with 1 case of antibody-mediated PRCA. Development of PRCA in patient P1 followed subcutaneous treatment with 1 specific clinical lot of HX575 (lot B). Anti-rhEPO antibody responses in this PRCA patient showed an IgG1 and IgG4 subclass profile. This finding is consistent with previously published reports of mixed IgG subclass patterns found in antibody-mediated PRCA patients.2,14,15 Tungsten, used in heat-resistant drilling pins for glass syringe manufacturing was identified as the most likely root cause for aggregation in prefilled syringes of this lot.13 Polytungstates leaching from prefilled syringes may elicit protein aggregation, dependent on the pH of the solution, the tungsten species, and concentration.32 Protein aggregation is likely driven by electrostatic interactions of polytungstate and the α-helical protein.32 We have previously demonstrated that sodium polytungstate can bind rapidly and reversibly to rhEPO, and has a strong denaturing effect on the secondary structure of rhEPO, inducing rapid unfolding of the glycoprotein.13 Sodium polytungstate, as well as an extract of the tungsten pins used to manufacture the syringes, induced dimerization/aggregation of rhEPO. These aggregates were similar to those detected in the two suspect batches of the study medication from the clinical trial. A proportion of the dimers could not be dissociated by organic solvents and were most likely covalently linked via disulphide bonds.13 However, the precise polytungstate interaction with rhEPO, leading to specific T-cell epitope exposure resulting in clinically relevant immunogenicity, remains to be explored.
Here, we report various analytical and immunological investigations linking tungsten-induced HX575 protein aggregation and clinical immunogenicity, as part of a larger root cause analysis.13 Because specific high-affinity IgG1 and IgG4 antibody responses to rhEPO point to T-cell–dependent isotype switching,2,16 we focused our investigations on the question whether T-cell responses can differentiate nonaggregated from aggregated rhEPO. In vitro T-cell assays have been successfully used to evaluate the mechanism of immune responses to biotherapeutics,5-9 vaccines,17,18 and self-antigens.19,20 Besides preexisting nonneutralizing IgM and IgG1 anti-EPO antibodies across various clinical indications,14,15 EPO-specific T cells are part of the T-cell repertoire in healthy individuals.33 Hence, T cells obtained from healthy donors may enable investigations of the immunogenic potential of exogenous rhEPO.33 In this way, T-cell assays may help to establish a link between analytical findings of specific HX575 clinical batches and associated T-cell–dependent antibody-mediated PRCA, and thus may provide an opportunity to confirm the aforementioned likely root cause.
Indeed, we were able to demonstrate that both T-cell and TCL responses could differentiate nonaggregated from heat- or tungsten-aggregated rhEPO. T-cell responses to rhEPO were either HLA-DR or HLA-DQ restricted. Epitopes responsible for promoting T-cell responses to rhEPO could be identified by generating TCLs reactive to heat-stressed HX575 from 2 HX575-naïve healthy HLA-DRB1*09:01-negative or -positive donors. Tungsten-induced rhEPO aggregates in clinical lots associated with rhEPO-neutralizing antibodies and PRCA, elicited a positive response similar to heat-induced rhEPO aggregates in a DC–T-cell assay, utilizing cells from rhEPO-naïve healthy donors. Furthermore, TCLs reactive with heat-stressed HX575, established from a healthy HLA-DRB1*09:01-positive donor, showed marked responses to preparations of experimentally heat-stressed HX575 as well as to clinical lot A and B, but not to nonstressed or low-tungsten preparations. These findings indicated that the suspect clinical lots induce in vitro immunogenic responses and are comparable to heat-stressed HX575, whereas HX575 obtained from low-tungsten syringes was not immunogenic in the DC–T-cell coculture assay, or using TCLs.
Exceptionally, we could obtain PBMCs from limited blood samples of anemic patients treated with rhEPO without PRCA, and of patient P1 who developed PRCA after treatment with clinical batch B with elevated levels of tungsten and aggregates. Only rhEPO-treated patient P1 with PRCA showed an ex vivo recall response after incubation with that clinical batch. To our knowledge, this is the first time that DC–T-cell assays have been used in this manner to retrospectively link quality data of study medication to the observed immunogenicity results of a clinical study.
In summary, despite inherent limitations to obtain additional blood samples from subjects enrolled in the HX575 clinical trial INJ17 for further analysis, these data support the conclusion that tungsten species in clinical syringe suspect lots A and B induced aggregation of HX575,12,13 causing increased clinically relevant immunogenicity. Hence, the complex interaction between various exogenous and endogenous factors including the formation of rhEPO aggregates, antigenic processing, and presentation of immunodominant epitopes by antigen-presenting cells,19 the presence of specific HLA class II proteins capable of binding certain rhEPO peptides,29,30 dose-dependent inhibition of naïve CD4+ T cells and skewed T-cell subsets,23 and potentially other characteristics of EPO,34 together influence the immune response to rhEPO after subcutaneous injection, leading to PRCA in a subset of susceptible patients (Figure 7). Our investigations demonstrate the value of T-cell assays to establish a link between analytical data of well-defined clinical batches and clinical immunogenicity.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients, healthy donors, and supporting physicians for blood donations, as well as Mark McCamish and Jörg Windisch for support and guidance, and Takako Hamano for facilitating logistics between Basel (Switzerland) and Tokyo (Japan).
Authorship
Contribution: M. Kammüller, T.R.-S., M. Kuwana, S.-D.C., T.M.W., and A.S. designed the research; T.R.-S., M. Kuwana, B.C., M.A., O.H., F.Z., R.F., and V.K. performed the research; M. Kammüller, T.R.-S., M. Kuwana, and A.S. analyzed data; M. Kammüller and A.S. are responsible for the overall design and coordination of the project, and integration of the results; M. Kammüller wrote the paper; and all of the authors read and contributed to the preparation of the final manuscript.
Conflict-of-interest disclosure: M. Kuwana has received a research grant from Novartis; T.R.-S., B.C., S.-D.C., and M. Kammüller are full-time employees of Novartis; T.M.W. has been a former full-time employee of Novartis; and M.A., O.H., F.Z., R.F., V.K., and A.S. are full-time employees of Hexal AG.
The current affiliation for M. Kuwana is Nippon Medical School, Department of Allergy and Rheumatology, Tokyo, Japan.
The current affiliation for T.M.W. is Regulus Therapeutics, San Diego, CA.
Manuela Aßenmacher died on 14 November 2015.
Correspondence: Michael Kammüller, Novartis Institutes for Biomedical Research, Klybeckstr 141, 4057 Basel, Switzerland; e-mail: michael.kammueller@novartis.com; and Andreas Seidl, Technical Development Biosimilars, Novartis Biologics Technical Development and Manufacturing, Keltenring 1+3, 82041 Oberhaching, Germany; e-mail: andreas.seidl@novartis.com.