Key Points
Functional health of transplanted HS patients was significantly diminished. Patients with higher enzyme levels showed favorable results.
Psychosocial health appeared unaffected compared with healthy peers.
Abstract
Hurler syndrome (HS) is a lysosomal storage disease characterized by multisystem morbidity and death in early childhood. Hematopoietic stem cell transplantation (HSCT) results in long-term survival, although with significant residual disease burden. How this residual disease affects the health-related quality of life is unknown. Therefore, we conducted a multicenter cohort study on functional and psychosocial health and compared the outcomes to normative data using the Child Health Questionnaire and Pediatric Outcomes Data Collection Instrument. Perception of care was evaluated by the Measure of Processes of Care questionnaire. Sixty-three HS patients receiving HSCT with at least 3 years of follow-up after HSCT were included. The influence of potential predictors was analyzed using linear regression analysis, and correlation analysis was performed using Spearman rank correlation. Functional health of transplanted HS patients was significantly diminished compared with normative data (median physical summary z score, −2.4 [range, −3.5 to −1.6]; median global functioning z score, −3.2 [range, −4.8 to −1.8]). Psychosocial health was comparable or only slightly reduced compared with healthy peers (median psychosocial summary z score, 0.15 [range, −0.7 to 0.8]). A higher obtained lysosomal enzyme level post-HSCT predicted for superior functional health. Overall, parents were satisfied with the care received. Functional health of transplanted HS patients appeared significantly more affected than psychosocial health. To improve functional health, the use of only noncarrier donors and striving to achieve full-donor chimerism, both resulting in higher enzyme levels, is advised. Assessing the health-related quality of life could play an important role in evaluating outcomes of HS patients receiving novel (cell) therapies, including autologous gene-transduced HSCT.
Introduction
Hurler syndrome (HS) is a lysosomal storage disorder caused by a deficiency of the lysosomal enzyme α-l-iduronidase (IDUA). Without hematopoietic stem cell transplantation (HSCT), this devastating disease is characterized by progressive multisystem morbidity, including severe orthopedic complications, neurodevelopmental deterioration, and cardiopulmonary complications leading to death in early childhood (median survival, 7.8 years).1,2 With more than 500 patients transplanted so far, HS is the most extensively transplanted inborn error of metabolism and therefore often serves as a paradigm disorder for HSCT in these conditions. Although enzyme replacement therapy has become available for mucopolysaccharidosis type I, HSCT remains the preferred treatment option in HS patients, because it is the only treatment that delivers the deficient enzyme to the central nervous system, which is necessary to prevent progressive neurodegeneration.
HSCT dramatically attenuates the clinical course of HS and allows affected individuals to achieve long-term survival. However, significant residual disease burden is observed in almost all transplanted patients, with striking variability between patients.3 One of the main issues are orthopedic complications, which require surgical intervention in the majority of the patients despite successful HSCT.4 To what extent this disease burden impacts the health-related quality of life of transplanted HS patients remains unknown. Furthermore, due to the large number of complications that can be encountered in the years following HSCT, all patients require long-term care from a large number of medical specialists. Until now, little was known about the parents’ perception of care of transplanted HS patients.
This multicenter cohort study was conducted to provide a broader context of the burden of disease of HS patients, addressing the health-related quality of life and processes of care of transplanted HS patients from the parents’ perspective.
Patients and methods
Inclusion criteria
HS patients receiving an allogeneic HSCT in one of the 7 participating European transplant centers of expertise and present during a multidisciplinary follow-up day at the local center were evaluated for inclusion in the study. Assays of leukocyte IDUA activity at presentation in combination with the clinical phenotype confirmed the diagnosis in all patients. For inclusion, patients had to be between 5 and 18 years of age with >10% donor chimerism and a minimum of 3 years follow-up post-HSCT. Only patients with at least one completed questionnaire were included.
Data collection
One member of the study team (M.A.) visited all participating study centers during multidisciplinary follow-up days. The parents of eligible patients were asked to complete 3 questionnaires, which are described in more detail below. The medical records of all included patients were retrospectively evaluated according to a standardized set of potential patient-, donor-, transplantation-, and disease-related predictors. Cognitive developmental impairment (intelligence quotient <70), growth retardation (<−2 standard deviation, according to the World Health Organization growth curves),5 and in receipt of medical intervention at time of transplant were considered potential disease-related predictors.
This study was approved by the institutional review boards of the University Medical Center Utrecht (center of the principal investigator) and all other participating centers thereafter. Written informed consent was obtained from the parents or legal guardians of the patients.
Questionnaires
Child Health Questionnaire.
The 28-item parent form of the Child Health Questionnaire (CHQ-PF28) is a validated questionnaire designed and normed for children aged 5 to 18 years.6 All subscale scores were generated using the scoring algorithm provided by the authors of the instrument. The various subscale scores were aggregated as appropriate to derive 2 summary component scores: the physical and psychosocial summary scores. The subscale and summary scores were compared with a normative sample to derive z scores.6
Pediatric Outcomes Data Collection Instrument.
The Pediatric Outcomes Data Collection Instrument (PODCI) is a validated questionnaire designed by the American Academy of Orthopedic Surgeons and the Pediatric Orthopedic Society of North America for children 2 to 18 years of age with moderate to severe orthopedic disease.7 All subscale scores were generated using the scoring algorithm provided by the authors of the instrument. A global functioning score was calculated, combining 4 of the subscales, and z scores were derived by comparison with a normative sample.8
Measure of Processes of Care questionnaire.
The 20-item Measure of Processes of Care (MPOC-20) questionnaire was used to assess the parents' perception of the care their child received from their treatment center. This questionnaire is validated for children aged 0 to 18 years.9,10 All scores were generated using the scoring algorithm provided by the authors of the instrument.
End points
Functional health.
Functional health domains assessed using the PODCI included the “upper extremity function,” “transfers and mobility,” “sports and physical function,” and “pain and comfort” subscales as well as the “global functioning” summary score. The items of the CHQ attributing to the functional health were the “physical functioning,” “role/social limitations-physical,” “general health,” “bodily pain,” and “parental impact-time” subscales as well as the “physical summary score.”
Psychosocial health.
Psychosocial health domains assessed using the PODCI included the “pain and comfort” and “happiness” subscales as well as the “global functioning” summary score. Regarding the CHQ, the “role/social limitations-emotional/behavioral,” “general health,” “bodily pain,” “parental impact-emotional,” “self esteem,” “mental health,” and “behavior” subscales as well as the “psychosocial summary score” were attributed to the psychosocial health.
Perception of care.
Perception of care was assessed using the five subscales of the MPOC questionnaire with scores ranging from 1 (“not at all”) to 7 (“to a very great extent”).
Statistical analysis
To identify differences concerning patient-, donor-, transplantation-, and disease-related characteristics between the eligible patients included in the study and those eligible but not included, χ2 and analysis of variance tests were used. The association between the various potential patient-, donor-, transplantation-, and disease-related predictors mentioned in Table 1 and the end points were analyzed using univariate and multivariate linear regression analysis. Univariate predictors of outcome parameters that were statistically significant (P < .10) were selected for multivariate analysis. Results were expressed as odds ratios and corresponding 95% confidence intervals. P< .05 was considered statistically significant. Spearman rank correlations, expressed as a correlation coefficient (r), were used to compare correlations between the individual functional and psychosocial PODCI and CHQ subscale and summary scores. Statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL) and RStudio 0.99.441 (RStudio Inc., Boston, MA). Graphs were created using RStudio 0.99.441.
Results
Study population
The questionnaires were distributed among the parents of 81 eligible patients, of whom 63 (78%) completed at least one of the questionnaires. The inability of parents to complete the questionnaires due to language barriers was the main reason not to be included in the study.
The included HS patients were transplanted at a median age of 15 months (range, 3-42 months). The median age at assessment was 8 years (range, 5-18 years). The stem cell source consisted of a bone marrow (63%), unrelated cord blood (24%), or peripheral blood stem cell (13%) graft. Thirty-two percent of the patients obtained enzyme levels below the local lower reference limit because of mixed-donor chimerism (in 24%) and/or the use of a carrier donor (19%). Enzyme levels obtained ≥1 year after HSCT showed minimal change over time. The patient-, donor-, and transplantation-related characteristics of the 63 included patients are shown in Table 1. Only ethnicity (P = .008) and relationship of donor (P = .02) differed between the eligible patients included in the study and those not included (data not shown).
Of the included patients, 46% suffered from cognitive developmental impairment, while 51% had growth retardation. Medical interventions were frequently performed in the patients included in this study. Data regarding the disease-related characteristics, including cognitive development, growth, and the various medical interventions, are shown in supplemental Table 1. No significant differences in cognitive development, growth, or occurrence of a medical intervention were found between the included patients and those not included in the study (data not shown).
The CHQ, PODCI, and MPOC questionnaire were completed by 92%, 90%, and 97% of the 63 included patients in the study, respectively.
Health-related quality of life
Functional health.
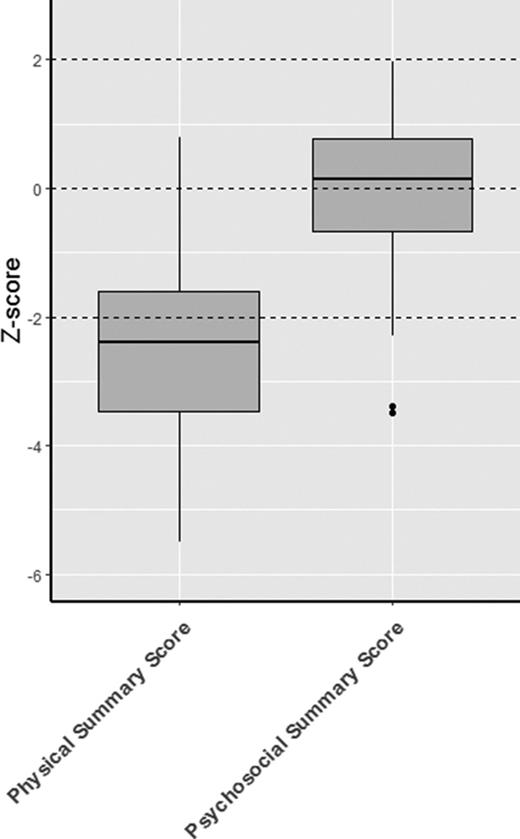
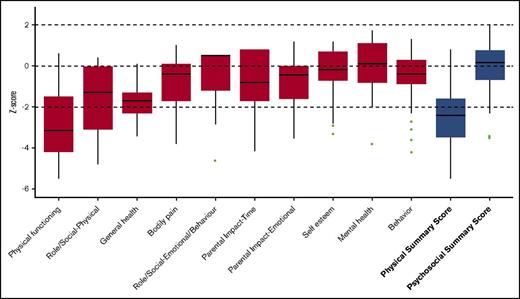
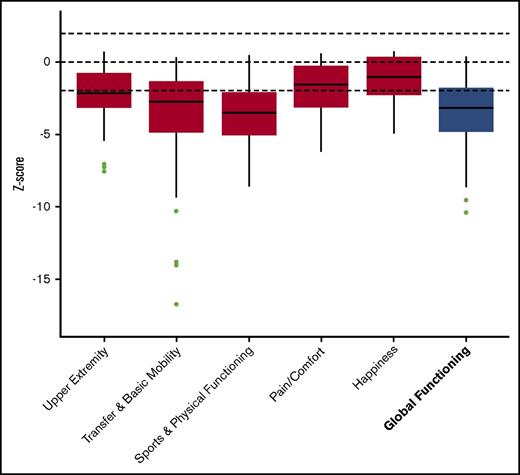
Significantly reduced scores were observed regarding the functional health subscales of both the CHQ and PODCI when compared with normative data. This is also demonstrated by the low summary scores in both questionnaires (median physical summary z score, −2.4 [range, −3.5 to −1.6]; mean global functioning z score, −3.2 [range, −4.8 to −1.8]) (Figures 1 and 2). The reduced summary scores were largely determined by the low “physical functioning” subscale of the CHQ and the “transfer & basic mobility” and “sports & physical function” subscales of the PODCI.
CHQ. Boxplot showing the z scores, with the median and interquartile range. The results of the subscales are depicted in red and the summary scores are depicted in blue.
CHQ. Boxplot showing the z scores, with the median and interquartile range. The results of the subscales are depicted in red and the summary scores are depicted in blue.
PODCI. Boxplot showing the z scores, with the median and interquartile range. The results of the subscales are depicted in red and the summary score is depicted in blue.
PODCI. Boxplot showing the z scores, with the median and interquartile range. The results of the subscales are depicted in red and the summary score is depicted in blue.
Psychosocial health.
The psychosocial health appeared to be normal to only slightly reduced compared with normative data, as demonstrated by the psychosocial summary score of the CHQ (median, 0.15 (range, −0.7; 0.8). The “self esteem”, “mental health”, and “behavior” subscales of the CHQ showed to be comparable to the healthy population sample (Figure 1).
Predictors.
A lower obtained IDUA enzyme level, expressed as percentage of the mean, predicted for a significantly lower functional health concerning both the CHQ and PODCI (physical summary score, P = .004; global functioning score P < .001) (Table 2). No significant association was found between any of the other potential patient-, donor-, transplant-, or disease-related predictors and the various functional or psychosocial health scores.
Correlation between CHQ and PODCI.
The scores of all functional domains in the CHQ did significantly correlate with those in the PODCI, except for “bodily pain” in the CHQ and “upper extremity” in the PODCI (supplemental Table 2). The physical summary score of the CHQ and the global functioning scale of the PODCI showed excellent correlation (r = 0.772, P < .001). Regarding psychosocial function, the scores of “general health,” “bodily pain,” and “mental health” in the CHQ did highly correlate with all psychosocial domains in the PODCI, except for that of “general health” and “happiness.”
Processes of care
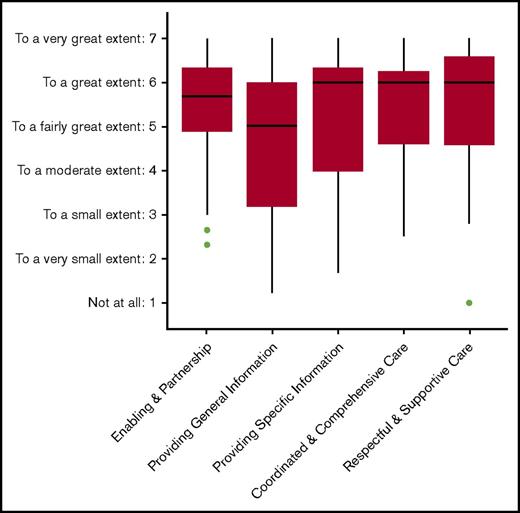
The parents were satisfied overall with the care they received from the transplantation center, with median scores ranging from 5.0 (“providing general information”) to 6.0 (“respectful and supportive care”) (Figure 3).
MPOC questionnaire. Boxplot showing the scores of the subscales, with the median and interquartile range.
MPOC questionnaire. Boxplot showing the scores of the subscales, with the median and interquartile range.
Discussion
This cross-sectional survey confirms that the functional health of HS patients post-HSCT is significantly diminished compared with a normative population. This is presumably due to the residual disease burden observed in transplanted patients, especially the frequently occurring orthopedic complications. The psychosocial health showed a much more favorable outcome, with normal or only slightly reduced scores compared with their healthy peers.
Due to the collaborative effort, a substantial population size was achieved despite the relative rarity of the disease. Although there was a large age range of the patients assessed, this did not have a significant impact on any of the functional or psychosocial health measures. In previous research, age was found to be a predictor mainly for neurocognitive outcomes, which was not a studied outcome in this study.4 Interestingly, none of the disease-related characteristics were found to be a predictor for either functional or psychosocial health. This was similar for patients receiving enzyme replacement therapy prior to HSCT. The only predictor found in this study was the IDUA enzyme level obtained after HSCT, which significantly influenced functional health. Previously, this same predictor also appeared to be a significant predictor for the prognosis in HS patients after HSCT, including orthopedic outcome measures.4 Superior functional health might therefore be achieved in this patient population if higher IDUA enzyme levels are obtained after HSCT, although in this study, as in a previous study, we did not find a threshold effect in the normal IDUA level range.4 Higher IDUA levels can be realized by using exclusively noncarrier donors and transplantation techniques promoting complete donor chimerism. Previous studies have showed that the chance of full-donor chimerism is higher after cord blood transplantation.11 Our analyses did not find cord blood to be a predictor for better outcomes, which might be due to a relatively low number (n = 15) of cord blood transplantations. Whether supranormal enzyme levels, potentially obtained by new treatment approaches such as autologous gene-transduced HSCT resulting in overexpression of the enzyme, will further improve functional health remains an interesting question that needs to be elucidated.12 The first trials of gene therapy in HS patients are close to clinic.
A potential limitation in our evaluation of functional and psychosocial health in HS patients was the fact that these outcome measures were evaluated by their parents. Most of our included patients were too young to complete the questionnaires or lacked the necessary cognitive abilities to effectively interpret and answer the questions. Therefore, the number of patient reports that could potentially be used in the analysis was too low, and only parent forms were included. Interestingly, a previous study in children with a wide range of musculoskeletal problems showed an overall strong level of agreement between the child and parent responses for most domains in both the CHQ and PODCI, although parents had a tendency to report more physical limitations and pain than these children acknowledged themselves.13 It is therefore likely that the responses of the parents in this study reliably represent those of their children as well. Nevertheless, it would be of great interest and importance to continue to evaluate the functional and psychosocial health in this group of patients in the upcoming decade(s).
Although assessment of the various organ systems by medical specialists during follow-up is an important aspect in evaluating treatment outcome, we believe that this should be accompanied by evaluation of the health-related quality of life from the patients’ or parents’ perspective. Furthermore, because the residual burden of the disease has a complex effect on health status, including the physical and psychosocial health, it is important to assess both health modalities. This might help to identify specific areas of difficulties these patients might encounter, enabling the most appropriate support. Importantly, there appeared to be a modest to strong correlation between the various functional and psychosocial domain scores of the CHQ and PODCI questionnaires, indicating that both questionnaires comparably measure these outcomes in this patient population. The CHQ offers a broader scope of health and can therefore examine functional as well as psychosocial attributes. The PODCI, however, offers a more detailed picture of physical functioning, with the possibility to differentiate between the various physical problems.14,15
The findings of the MPOC questionnaire indicate that parents of patients with HS overall positively rate the care they receive. Most improvement can be achieved by addressing the relatively low scores on the provision of general information. All centers participating in this study have developed multidisciplinary settings with highly experienced specialists to evaluate these patients and provide medical intervention where required. This might have been contributed to the high level of satisfaction observed in this study.
Taken together, the functional health of transplanted HS patients was significantly more affected than psychosocial health, as demonstrated by both the CHQ and the PODCI. A higher IDUA enzyme level obtained after HSCT predicted for superior functional health, supporting the use of only noncarrier donors and striving to achieve full-donor chimerism. Assessing functional and psychosocial health should play an important role in the evaluation of outcomes in HS patients receiving HSCT as well as upcoming new treatment approaches, such as autologous gene-transduced HSCT aimed at overexpression of the enzyme. Furthermore, the parents were satisfied overall with the care provided. Multidisciplinary settings involving specialists experienced in HS for long-term follow-up and medical interventions are recommended.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all parents and patients for completion of the questionnaires and all participating centers for sharing patient information.
This work was supported by a research grant from the Netherlands Organisation for Scientific Research (92003535) (M.A.) and fellowship grant from the European group for Blood and Marrow Transplantation (M.A.). B.T.A.v.d.B. was supported by a research grant from the Sylvia Toth Charity Foundation while working on this study.
The sponsors of this study are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: M.A. conceptualized, designed, and supervised the study, visited the centers for data collection, carried out the initial analyses, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted; B.T.A.v.d.B. critically reviewed and revised the manuscript, performed additional analyses, and approved the final manuscript as submitted; R.F.W. conceptualized, designed, and supervised the study, contributed to the acquisition of the data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted; J.J.B. conceptualized, designed, and supervised the study, contributed to the acquisition of the data, carried out the initial analyses, drafted the initial manuscript, and approved the final manuscript as submitted; and A.O., P.V., A.R., S.A.J., R.P., P.M.v.H., M.R., V.B., and T.J.d.K. contributed to the acquisition of the data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Conflict-of-interest disclosure: S.A.J. receives non–study-related funding from Genzyme and BioMarin. R.P. receives non–study-related personal fees and nonfinancial support from Genzyme, Shire, and BioMarin. The remaining authors declare no competing financial interests.
Correspondence: Jaap Jan Boelens, Department of Pediatrics, Pediatric Blood and Marrow Transplantation Program, Room KC03.063.0, University Medical Center, Utrecht, Lundlaan 6, 3584 EA Utrecht, The Netherlands; e-mail: j.j.boelens@umcutrecht.nl.
References
Author notes
M.A. and B.T.A.v.d.B. contributed equally to this study.