Key Points
This study lays the foundation for the use of nonmyeloablative GVHD-free haploidentical HSCT.
This approach offers treatment of hematological diseases and a safe potential platform for cell therapy and organ transplantation.
Abstract
The establishment of safe approaches to attain durable donor-type chimerism and immune tolerance toward donor antigens represents a major challenge in transplantation biology. Haploidentical hematopoietic stem cell transplantation (HSCT) is currently used for cancer therapy either as a T-cell–depleted megadose HSCT following myeloablative conditioning or with T-cell–replete HSCT following nonmyeloablative conditioning (NMAC) and high-dose posttransplant cyclophosphamide (PTCY). The latter approach suffers from a significant rate of chronic graft-versus-host disease (GVHD), despite prolonged immunosuppression. The use of T-depleted grafts, although free of GVHD risk, is not effective after NMAC because of graft rejection. We now demonstrate in mice conditioned with NMAC that combining the power of high-dose PTCY with T-cell–depleted megadose HSCT can overcome this barrier. This approach was evaluated in 2 patients with multiple myeloma and 1 patient with Hodgkin lymphoma. The first myeloma patient now followed for 25 months, exhibited full donor-type chimerism in the myeloid and B-cell lineages and mixed chimerism in the T-cell compartment. The second myeloma patient failed to attain chimerism. Notably, the low toxicity of this protocol enabled a subsequent successful fully myeloablative haploidentical HSCT in this patient. The third patients was conditioned with slightly higher total body irradiation and engrafted promptly. All patients remain in remission without GVHD. Both engrafted patients were able to control cytomegalovirus reactivation. Enzyme-linked immunospot analysis revealed immune tolerance toward donor cells. Our results demonstrate a novel and safer nonmyeloablative haplo-HSCT offering a platform for immune tolerance induction as a prelude to cell therapy and organ transplantation.
Introduction
The use of full-haplotype mismatched donors as an alternative source for hematopoietic stem cell transplantation (HSCT) is highly attractive, because virtually all patients have a readily available haploidentical family member who can serve as an HSCT donor. Over the past few years, the clinical use of haploidentical donors has gained great momentum, either through the use of megadose T-cell–depleted (TCD) peripheral blood progenitor cells (PBPCs) or unmanipulated transplants followed by high-dose posttransplant cyclophosphamide (PTCY).1,2 The former approach is more time consuming and requires costly graft processing but offers better prevention of graft-versus-host disease (GVHD). Notably, although some risk of GVHD is tolerable in the treatment of patients with hematologic malignancies, this is unacceptable in the case of haploidentical transplantation for nonmalignant diseases. Furthermore, the use of myeloablative conditioning for such patients is unnecessary and should be avoided.
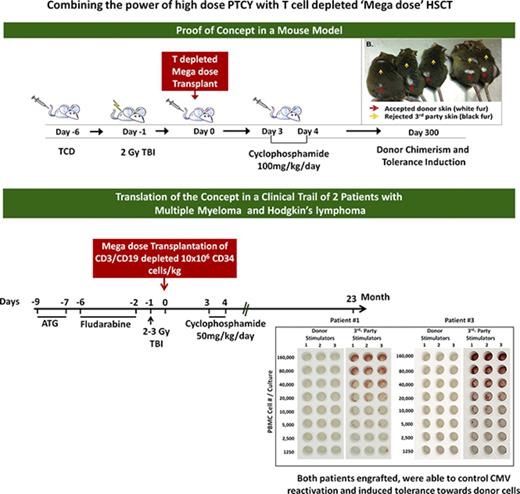
Thus, TCD haplo-HSCT, with its minimal risk of GVHD, if successfully applied in combination with nonmyeloablative conditioning (NMAC) could potentially offer a highly attractive and safer treatment modality for elderly or immunocompromised patients with hematological malignancies who cannot tolerate harsh conditioning and for treatment of nonmalignant hematological diseases. Moreover, allogeneic HSCT can also serve as a platform for subsequent cell therapy or engraftment of organ transplants from the same donor, without the need for continuous immunosuppressive therapy. However, the major advantage of NMAC is also its main caveat; although sparing host immunity improves immune reconstitution, it also allows a robust host-versus-graft response that increases the chances of graft rejection. Thus, transplantation of HSCT following an NMAC regimen has been largely limited over the past 2 decades to T-cell–replete transplants, attaining engraftment by virtue of the large number of donor alloreactive T cells at the expense of the substantial risk of acute and chronic GVHD, even with extensive posttransplant GVHD prophylaxis.3-5 As described above, the use of high-dose PTCY has reduced the risk of GVHD, but not enough to justify such transplants as a platform for organ transplantation or in the treatment of nonmalignant diseases1,6-9 or high-risk hematological malignancies in which posttransplant immune suppression can adversely impact antitumor immunity. On the other hand, attaining engraftment following TCD bone marrow (BM) transplants, especially in haploidentical patients conditioned with a nonmyeloablative regimen, still represents a major challenge. In the present study, we demonstrate in a stringent mouse model that the combination of 2 current clinical approaches in haplo-HSCT, TCD megadose BM transplantation and PTCY, enables engraftment and durable chimerism induction without the risk of GVHD under very mild NMAC and in the absence of continued posttransplant immune suppression. Furthermore, we present initial clinical results showing the translation of this approach to the treatment of 2 patients with high-risk multiple myeloma (MM) and 1 patient with Hodgkin lymphoma (HL).
Materials and methods
Murine studies
Mice were maintained under sterile conditions in the Weizmann Institute animal facility. Studies complied with a protocol approved by the Institutional Animal Care and Use Committee using littermate controlled mice of the same age (8-12 weeks) and sex. BALB/c (H-2Dd), BALB/c-nude (H-2Dd), C57BL/6 (H-2Kb), and C3H (H-2Kk) mice were purchased from Envigo Israel.
BM transplantation.
In these experiments, C3H/Hen mice served as recipients, with 6 or 7 mice in each group (the maximum allowed by the Institutional Animal Care and Use Committee) to reach statistical significance; BALB/c-nude mice served as megadose TCD BM donors. Most of the experiments were performed at least twice. All data, including outliers, were included in all analyses; randomization or blinding was not relevant.
T-cell debulking.
C3H/Hen female mice (9-10 weeks of age) were each infused with 300 µg anti-CD4 (BioXCell clone GK1.5) and anti-CD8 antibodies (BioXCell clone YTS169.4) on day −6 of the transplantation protocol.
Transplantation.
On day −1, the recipients were irradiated with 2 Gy total body irradiation (TBI), and BALB/c-nude BM cells were inoculated on day 0. Cyclophosphamide (10 mg/kg) was injected on days +3 and +4 posttransplant, and chimerism analysis was performed 30 days posttransplant.
Chimerism analysis.
Cell origin (host vs donor) was determined by flow cytometry, as described in the Supplemental Methods.
Flow cytometric analysis.
Fluorescence-activated cell sorting (FACS) analysis was performed using a BD FACSCanto II system running BD FACS DIVA software. Cells were stained with labeled antibodies specific for CD3 phycoerythrin (PE)/fluorescein isothiocyanate (FITC)/allophycocyanin (APC), CD8-PE/FITC/APC, CD11b-PE/FITC/APC, CD4-PE/FITC/APC, and CD45B220-PE/FITC/APC from BioLegend and BD Biosciences; H-2Kb-PE/FITC, H-2Dd-PE/FITC, and H-2Kk-PE/FITC from BD Pharmingen; and streptavidin-APC from Jackson ImmunoResearch Laboratories.
Skin grafting.
Skin grafting was performed as previously reported10 and as described in the Supplemental Methods.
Bulk culture for the analysis of residual antidonor activity.
Antidonor immune activity was performed by bulk mixed lymphocyte reaction followed by a 35S-methionine release cytotoxicity assay (Supplemental Methods).
Frequency calculation of cytotoxic T lymphocyte precursors (CTL-p).
To calculate the frequency from the limiting dilution culture readout, we used the equation ln y = −fx + ln a (which represents the zero-order term of the Poisson distribution11 ), where y is the percentage of nonresponding cultures, x is the number of responding cells per culture, f is the frequency of responding precursors, and a is the y-intercept (theoretically equal to 100%). Microwell cultures were considered positive for a cytolytic response when values exceeded the mean spontaneous release value by at least 3 SDs of the mean. The percentage of responding cultures was defined by calculating the percentage of positive cultures.11
IFN-γ ELISpot analysis.
The enzyme-linked immunospot (ELISpot) assay,12 a highly sensitive immunoassay that measures the frequency of interferon-γ (IFN-γ)–secreting cells at the single-cell level, was used to evaluate the frequency of residual antidonor reactive cells in patient peripheral blood mononuclear cells (PBMCs) following transplantation, as described in Supplemental Methods.
Statistical analysis.
Laboratory data were analyzed with the Mann-Whitney test when comparing percent chimerism or χ2 when comparing percent chimera in different treatment groups. For all comparisons, P values were used, and P < .05 was considered statistically significant.
Clinical studies were performed in 3 patients using a protocol that was approved for clinical use by the Parma Ethics Committee and the AIFA-Italian Medicine Agency (EudraCT; registration number 2013-003628-35) in high-risk MM and HM. This study was conducted in accordance with the Declaration of Helsinki. There was no randomization or blinding.
Inclusion and exclusion criteria are provided in Supplemental Methods.
Donors.
Selection of donor was based on typing of HLA-A, B, C, DR loci. The donor was required to be at least HLA-A, B, C, DR haploidentical to the patient but could differ for 2 or 3 HLA alleles on the unshared haplotype. Donors were prioritized on the basis of younger age, better health, and being cytomegalovirus (CMV) negative for CMV-negative recipients. Donor eligibility was evaluated according to Italian Bone Marrow Donor Registry criteria.
Transplantation protocol.
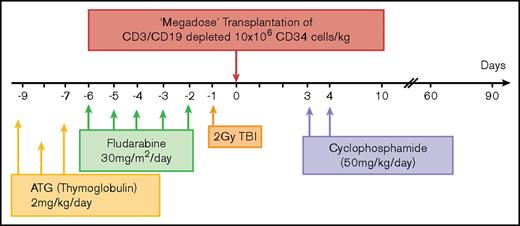
The pretransplant conditioning regimen consisted of antithymocyte globulin (2 mg/kg per day) from day −9 to day −7, fludarabine (30 mg/m2) from day −6 to −2, and single-fraction TBI (2-3 Gy) on day −1. Cyclophosphamide (CY) (50 mg/kg) was given on days +3 and +4. Additional pharmacologic prophylaxis for GVHD or granulocyte colony-stimulating factor was not administered after transplantation. Prophylaxis against infection consisted of either micafungin (50 mg/day) or liposomal amphotericin B (3 mg/kg every other day) from the first day of the conditioning regimen until neutropenia resolved. Ganciclovir (10 mg/kg) was used for CMV prophylaxis from day −9 to day −2. The decision whether to treat the patient once CMV polymerase chain reaction positive was based on the patient’s clinical condition.
Preparation of PBPCs and T-cell depletion are described in Supplemental Methods.
Chimerism analysis.
Quantification of donor chimerism was performed by polymerase chain reaction–based procedures using short tandem repeat analysis and evaluated using the PowerPlex Fusion System. This procedure allows coamplification and 4-color detection of 24 loci, including all Combined DNA Index System core loci and European Standard Set loci, 23 short tandem repeat loci, and amelogenin (D3S1358, D1S1656, D2S441, D10S1248, D13S317, D16S539, D18S51, D2S1338, CSF1PO, TH01, vWA, D21S11, D7S820, D5S818, TPOX, D8S1179, D12S391, D19S433, D22S1045, and FGA plus Penta E, Penta D, and DYS391). Size fractionation of the fluorochrome labeled amplicons was performed by capillary electrophoresis on a 3500Dx Genetic Analyzer (Applied Biosystems, Foster City, CA), and size calling of the alleles at individual loci was performed using both GeneMapper (version 4.1, Applied Biosystems) and ChimerMarker (SoftGenetics) analysis software by size comparison with 500-ROX (ABI). ChimerMarker software was employed to assign the allelic profile and calculate the donor-to-patient ratio of DNA extracted from blood or BM cells; the result was expressed as percent donor chimerism.
Determination of CMV- and EBV-specific T cells and percentage of specific T cells.
For detection of CMV or Epstein Barr virus (EBV)–specific cytotoxic memory T lymphocytes, multimeric major histocompatibility complex (MHC) Dextramer reagents (Immudex 2002) for flow cytometry were used as described in Supplemental Results.
Results
Synergism between megadose TCD BM and high-dose PTCY
To test the potential synergy between megadose TCD BM transplantation and high-dose PTCY in overcoming rejection, we transplanted, on day 0, standard (5 × 106) or high-dose (25 × 106) BALB/c-nude BM cells (naturally depleted of T cells by virtue of the “nude” phenotype) into fully allogeneic recipients (C3H/Hen) (supplemental Figure 1). The recipients were conditioned by T-cell debulking with anti-CD4 and anti-CD8 antibodies (300 µg each) delivered on day −6 and exposure to 2 Gy TBI on day −1. High-dose CY (100 mg/kg) was administered on days +3 and +4.
Chimerism within PBMCs and splenocytes was assessed by staining for donor and host MHC antigens and myeloid cell (CD11b), B-cell (CD45B220), and T-cell (CD3, CD4/CD8) markers.
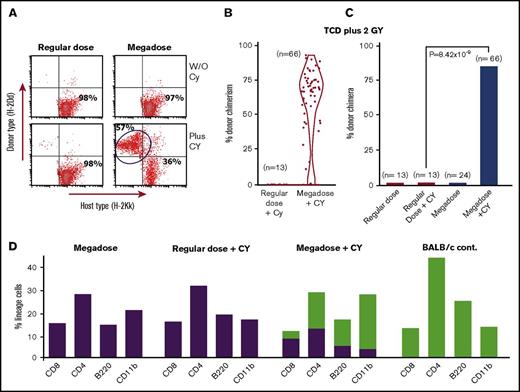
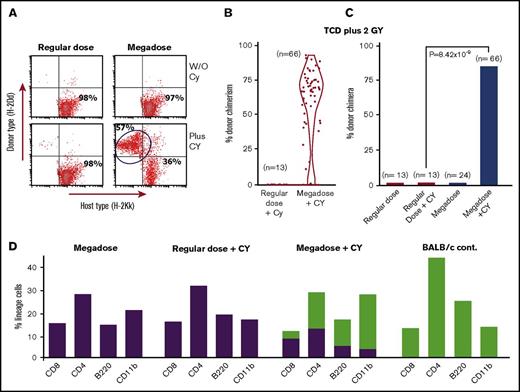
In our initial experiment, chimerism analysis on days 35, 95, 180, and 225 revealed that none of the BM recipients that were transplanted with a regular dose of 5 × 106 TCD BM in the presence or absence of CY expressed donor type chimerism. In contrast, 4 out of 7 mice receiving 25 × 106 cells and CY on days +3 and +4 exhibited more than 47.7% ± 9% donor-type multilineage chimerism on day 225. These findings were subsequently confirmed in 9 independent experiments including a total of 66 mice treated with a megadose of 25 × 106 TCD BM cells plus CY (percent chimeric mice, 84.8%; average chimerism, 53.9 ± 27.4) compared with a total of 13 mice receiving a regular dose of 5 × 106 BM cells plus CY (percent chimeric mice, 0%; P = 8.42 × 10−9) (Figure 1A-C).
Marked enhancement of chimerism induction upon combining megadose TCD BM transplantation with PTCY. (A) Typical mixed chimerism detected by FACS analysis at day 180. (B-C) Cumulative results of 9 experiments including a total of 66 recipient mice treated by megadose TCD BM plus PTCY, measured at 210 to 300 days posttransplant. Data are presented by violin plot representing the distribution of percent donor chimerism (B) and percent donor chimera (C). Statistical analysis was performed by χ2 analysis. (D) Chimeric mice exhibit multilineage chimerism (green bars, donor; purple bars, host).
Marked enhancement of chimerism induction upon combining megadose TCD BM transplantation with PTCY. (A) Typical mixed chimerism detected by FACS analysis at day 180. (B-C) Cumulative results of 9 experiments including a total of 66 recipient mice treated by megadose TCD BM plus PTCY, measured at 210 to 300 days posttransplant. Data are presented by violin plot representing the distribution of percent donor chimerism (B) and percent donor chimera (C). Statistical analysis was performed by χ2 analysis. (D) Chimeric mice exhibit multilineage chimerism (green bars, donor; purple bars, host).
Notably, significant split chimerism was detected in all lineages tested, including CD4 and CD8 T cells, CD220 B cells, and CD11b myeloid cells (Figure 1D).
To determine the optimal radiation dose required for chimerism induction, the experiments described above were repeated using different doses of TBI. Notably, chimerism induction was significantly lower in mice receiving 1 Gy TBI (n = 7; Mann-Whitney, 0.01 > P > .001) (Figure 2A) than in mice receiving 2 Gy TBI (n = 15), a further increase of TBI to 2.5 Gy (n = 6), 3 Gy (n = 5), or 3.5 Gy (n = 5) did not significantly enhance chimerism (Mann-Whitney, 0.2 > P > .1) (Figure 2A). In addition, increasing the CY dose to 125 or 150 mg/kg did not significantly enhance donor chimerism compared with that attained by the CY dose of 100 mg/kg per day used above (Mann-Whitney 0.5 > P > .1) (Figure 2B).
Chimerism induction following different doses of TBI or PTCY. The effect of different doses of irradiation (A) or increased doses of cyclophosphamide (B) on donor-type chimerism in recipients of megadose (25 × 106) TCD Nude-BM (Nu/BM) treated with high-dose PTCY. Statistical comparisons were performed using the Mann-Whitney U test.
Chimerism induction following different doses of TBI or PTCY. The effect of different doses of irradiation (A) or increased doses of cyclophosphamide (B) on donor-type chimerism in recipients of megadose (25 × 106) TCD Nude-BM (Nu/BM) treated with high-dose PTCY. Statistical comparisons were performed using the Mann-Whitney U test.
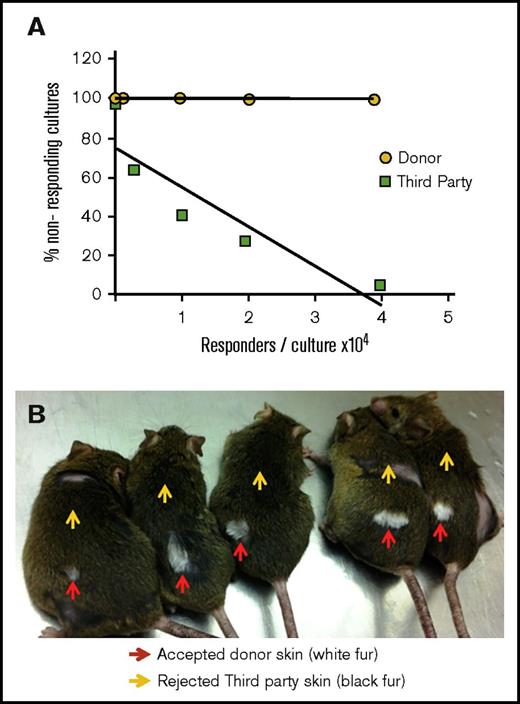
The stability of chimerism and the absence of signs of GVHD (weight loss or poor general appearance) strongly suggested that tolerance was likely achieved. To verify tolerance induction, a limiting dilution analysis of CTL-p in the spleen of chimeric mice was performed. As shown in Figure 3, tolerance induction was confirmed by the lack of detectable CTL-p against donor-type cells (BALB/c) in contrast to the significant CTL-p frequency against third-party (C57BL/6) stimulators (f = 1/6499) (Figure 3A). Further confirmation of central tolerance to donor MHC was obtained by skin grafting, showing that donor-type skin was accepted whereas third-party skin grafts were uniformly rejected (Figure 3B).
Tolerance induction of allogeneic TCD donor BM following transplantation of megadose BM and PTCY. (A) Limiting dilution analysis of CTL-p performed against donor (yellow) or third-party (green) splenocytes. A typical experiment assessing 1 chimeric mouse out of 3 tested is shown. (B) Acceptance of donor-type (BALB/c, white fur, red arrows) skin implanted at 7 months after BM transplantation, with rejection of third-party grafts (C57BL/6, black fur, yellow arrows).
Tolerance induction of allogeneic TCD donor BM following transplantation of megadose BM and PTCY. (A) Limiting dilution analysis of CTL-p performed against donor (yellow) or third-party (green) splenocytes. A typical experiment assessing 1 chimeric mouse out of 3 tested is shown. (B) Acceptance of donor-type (BALB/c, white fur, red arrows) skin implanted at 7 months after BM transplantation, with rejection of third-party grafts (C57BL/6, black fur, yellow arrows).
Putative tolerogenic CD8+ cells in the BM are not responsible for chimerism induction by megadose TCD BM combined with PTCY
Considering previous suggestions by Ildstad et al13 that a subset of CD8+ T-cell receptor α/β–negative (TCRα/β−) BM cells is critical for achieving donor-type chimerism, we next determined whether such cells are indeed essential for engraftment of megadose TCD BM transplants when combined with PTCY. To that end, CD8+ cells were depleted from the BALB/c-nude megadose BM preparation (supplemental Figure 2), and chimerism induction was compared with control nondepleted nude BM cells. The results of 2 independent experiments revealed no significant difference (Mann-Whitney, P ≥ 1) in chimerism levels between mice transplanted in the presence (47.3% ± 31% chimerism in 75% chimera, n = 16) or absence (47.3% ± 29% chimerism in 80% chimera, n = 15) of CD8+TCR− cells (supplemental Figure 3), suggesting that such cells are not essential when using megadose TCD transplants in conjunction with CY.
Transplantation of haploidentical megadose TCD HSCT in high-risk MM and HL patients
Based on the above proof-of-concept studies in the mouse model, a similar protocol was developed (Figure 4) and approved for clinical use by the Parma Ethics Committee and the AIFA-Italian Medicine Agency (EudraCT; registration number 2013-003628-35) in high-risk MM (see eligibility criteria in “Materials and methods”).
Scheme summarizing the haploidentical transplantation protocol combining megadose TCD HSCT and high-dose PTCY in high-risk MM patients. ATG, antithymocyte globulin.
Scheme summarizing the haploidentical transplantation protocol combining megadose TCD HSCT and high-dose PTCY in high-risk MM patients. ATG, antithymocyte globulin.
The first patient, a 54-year-old male, exhibited unfavorable cytogenetics (tetraploidy t(4:14);ampl.(1q21)) with light-chain myeloma and compromised renal function at the time of diagnosis (creatinine, 3.1 mg/dL, free light chain [FLC] ratio, 0.0012; serum protein electrophoresis [SPEP], 0.6 g/dL; urine protein electrophoresis [UPEP], 7 g/24 hours; immunofluorescence [IF] positive for both serum and urinary λ.) From March to June 2014, he was treated with 4 courses of Velcade-thalidomide-dexamethasone and attained a stringent clinical remission (CR). On December 2014, he underwent autologous BM transplantation, attaining complete remission at disease revaluation and good performance status with normalized renal function (FLC ratio, 0.89; SPEP, negative; UPEP, negative; IF, negative; BM progenitor cells [PCs], <5%; stringent CR).
On 9 June 2015, the patient received megadose haploidentical TCD (following CD3/CD19 depletion) HSCT (containing 15.4 × 106 CD34+ cells/kg, 1.17 × 105 CD3+ T cells/kg, and 27.7 × 106 CD56+ natural killer [NK] cells/kg) from his haploidentical sister. (For details of HLA typing of donor and recipient, see supplemental Table 1.) The patient engrafted promptly with polymorphonuclear neutrophils (>1 × 109/L), and platelets (>25 × 109/L) on days 15 and 17 posttransplant, respectively. As shown in supplemental Table 2, prompt lymphocyte recovery of CD4 T cells (134/µL on day 52), CD8 T cells (898/µL on day 45), and NK cells (572/µL on day 31) was exhibited.
Chimerism analysis at different time points after transplantation showed >97% donor-type chimerism in the myeloid and B-cell lineages throughout the 23 months of follow-up. T cells were initially predominantly of host type (10% to 23% donor type), gradually increasing to 63% to 72% donor type at 9 to 23 months posttransplant (Figure 5). Notably, the observed T cells predominantly expressed CD45RO during the first 6 months posttransplant. CD45RA+ T cells began to emerge by the end of the first year, in parallel to the increasing proportion of donor-type T cells, indicating the emergence of donor-derived naive T cells from the thymus between the first and second year (supplemental Table 3). Notably, the majority of these CD45RA+CD62L+ T cells also expressed CD95, indicating possible differentiation of naive T cells into memory stem cells, as previously suggested for immune reconstitution after CYPT (supplemental Figure 4).14,15
Chimerism analysis at different time points (percentage of donor [D] cells out of total PBMCs and BM).
Chimerism analysis at different time points (percentage of donor [D] cells out of total PBMCs and BM).
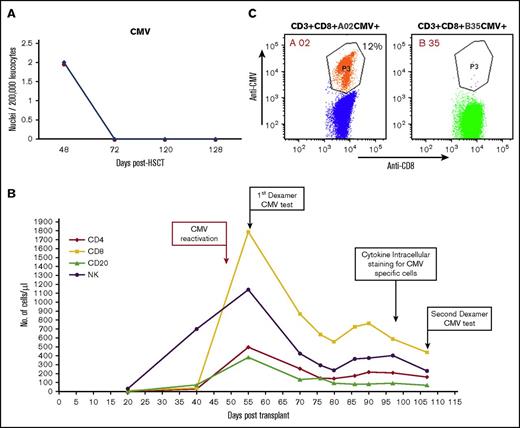
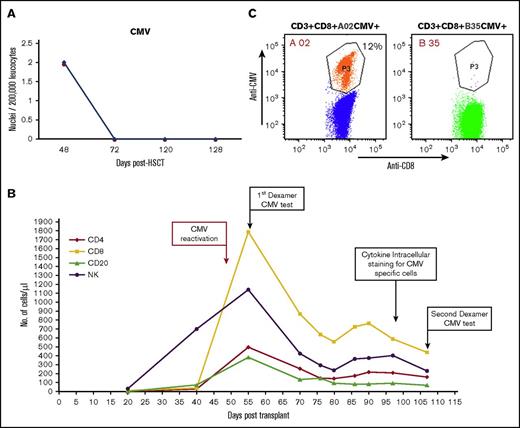
The patient was able to overcome CMV (Figure 6A) reactivation. Based on his good clinical condition (no sign or symptoms of CMV disease), and because we observed a spike in the CMV-specific CD8+ host T lymphocyte count, no further antiviral treatment was used. Notably, Dextramer analysis for CMV-specific CD8 T cells, performed at several time points after CMV reactivation (Figure 6B), revealed that the expending CMV-specific CD8 T cells were exclusively of host type (Figure 6C). FACS analysis of IFN-γ–positive cells in response to different CMV peptides revealed a cumulative percentage of 6.7% CMV-specific T cells among total CD4+ T cells and 8.7% among total CD8+ T cells (supplemental Figure 5). A proportion of the CMV-specific T-cell responses were also positive for CD107a, suggesting normal functionality (supplemental Figure 6).
CMV reactivation following megadose haploidentical TCD (following CD3/CD19 depletion) HSCT. (A) Immune resolution of CMV reactivation as indicated by viral DNA titer at different time points. (B) Lymphocyte subset reconstitution in peripheral blood, and time points of FACS analysis of CMV-specific T cells. (C) Enumeration of CMV-specific CD8+ T cells with the Dextramer CMV kit 55 days posttransplant. The host-type anti-CMV CD3+/CD8+/HLA-A02 was present at 214 cells/µL (vs 0 for donor-type CD3+/CD8+/HLA-B35/µL).
CMV reactivation following megadose haploidentical TCD (following CD3/CD19 depletion) HSCT. (A) Immune resolution of CMV reactivation as indicated by viral DNA titer at different time points. (B) Lymphocyte subset reconstitution in peripheral blood, and time points of FACS analysis of CMV-specific T cells. (C) Enumeration of CMV-specific CD8+ T cells with the Dextramer CMV kit 55 days posttransplant. The host-type anti-CMV CD3+/CD8+/HLA-A02 was present at 214 cells/µL (vs 0 for donor-type CD3+/CD8+/HLA-B35/µL).
A similar expansion of EBV-specific T cells upon reactivation, which took place after resolution of CMV reactivation (supplemental Figure 7A), was also associated with marked expansion of EBV-specific CD8 T cells (supplemental Figure 7B); these cells were also exclusively of host-type origin (supplemental Figure 7C).
At the last evaluation (+25 months), complete hematological remission and normal FLC ratio were confirmed. No clonal plasma cells were detected in the bone marrow (FLC ratio, 0.80; SPEP, negative; UPEP, negative; IF, negative, BM PCs, <5%; stringent CR).
The second patient was a 50-year-old male with high-risk MM (unfavorable cytogenetics: complex karyotype, 50 XY, +5, +7, +9, −13, +19, +21, and a 13q14 monosomy on fluorescence in situ hybridization analysis; FLC, not available; SPEP, 7.25 g/dL; UPEP, negative; IF, positive; immunoglobulin Gk serum, urinary negative).
He achieved a partial remission after 4 courses of lenalidomide-dexamethasone. He subsequently underwent tandem autologous HSCT and was later (July 2011) put on a maintenance regimen with lenalidomide until disease relapse in September 2014. Salvage therapy consisted of 8 courses of bortezomib-dexamethasone, achieving CR (FLC, not available; SPEP, negative; UPEP, negative; IF, negative; CR).
On November 2015, the patient underwent a megadose TCD HSCT from his haploidentical cousin (HLA disparity shown in supplemental Table 1), fractionated by the same procedure described above (10.8 × 106 CD34+ cells/kg, 1.2 × 105 CD3+ T cells/kg). Despite transient engraftment (50% donor cells on day +17), graft failure (0.04% donor-type chimerism) was documented on day +30. It is likely that this patient exhibited graft failure as opposed to graft rejection based on the observation that lactate dehydrogenase levels were not increased, and no lymphocyte spike or phagocytosis in the BM was detected. Furthermore, the patient exhibited rapid autologous hematopoietic recovery. However, because we cannot completely rule out the possibility that some form of graft rejection took place (lenalidomide is known to activate NK cells), we opted to increase TBI from 2 Gy to 3 Gy for the subsequent patient. After 5 months, this patient tolerated a second haploidentical HSCT (different donor) after standard myeloablative conditioning (antithymocyte globulin, treosulfan, thiotepa and fludarabine) and α/β TCR/CD19-depleted PBPCs. At 17 months after the second HSCT, he shows no sign of GVHD, very good immunological reconstitution, and excellent quality of life and remains in complete remission.
For the third patient, we opted to increase TBI from 2 Gy to 3 Gy. The patient was a 49-year-old female with advanced-stage HL resistant to 2 cycles of Adriamycin, bleomycin, vinblastine, and dacarbazine. Because of progression of disease, she received 4 courses of escalated bleomycin, etoposide, Adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone followed by 19 doses of the antibody brentuximab vedotin. A complete remission was achieved (positron emission tomography/computed tomography scan negative). As a very poor mobilizer, she was not eligible for autologous stem cell transplantation. Because no well-matched unrelated donor was available, she underwent haplo-HSCT on December 2016, as described above, except that TBI was increased from 2 Gy to 3 Gy. The graft contained 10.6 × 106 CD34+ cells/kg, 8.7 × 104 CD3+ T cells/kg, and 30.6 × 106 CD56+ NK cells/kg. As shown in supplemental Figure 8, engraftment was rapid, with a neutrophil count >0.5 × 109/L on day +17 and a platelet count >20 × 109/L on day +12.
On day 40 after HSCT, chimerism analysis showed 100% donor-type engraftment of the myeloid (CD33+) and B lymphoid (CD19+) cells and 95% in T cells (CD3+). A first CMV reactivation in blood occurred on day 48 with 3986 copies/μL. Rapid reduction of the viral load was observed in the absence of any anti-CMV treatment, with a clearance in 15 days. As in the first patient, the CMV reactivation was associated with marked elevation of host CD3+ T cells increasing up to 60% (day +58) (supplemental Figure 8).
Both CD4+ and CD8+ T cells exhibited a memory phenotype, and CD8+ T cells were predominantly effector memory cells displaying a CD45RA−CCR7− phenotype (data not shown). Dextramer FACS analysis using HLA-A02 to identify the origin of CMV-specific CD8 T cells showed that these cells were predominantly of host origin (data not shown). All B and NK cells were of donor type (data not shown).
The CMV-specific CD4+ and CD8+ T-cell response was also assessed by intracellular cytokine staining following overnight stimulation with specific CMV peptides. The cumulative percentages of CMV-specific T cells were 12.03% and 64.8% of total CD4+ and CD8+ T cells, respectively.
At a follow-up of 10 months, the patient is alive in complete hematologic remission and in very good clinical condition.
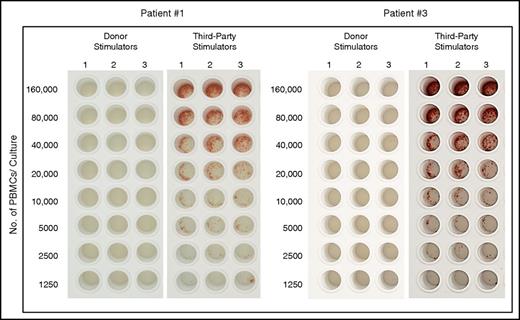
Notably, despite the robust functionality of host T cells both in patients 1 and 3, during the early post transplant period, donor-type chimerism of the myeloid of B-cell lineages remained stable, indicating establishment of immune tolerance toward donor-type antigens. Indeed, this was confirmed by IFN-γ ELISpot analysis of CTL-p against donor and third-party target cells, similar to our findings in the mouse model (Figure 7).
Tolerance induction of donor cells following haploidentical transplantation combining megadose TCD HSCT and high-dose PTCY. Analysis of CTL-p against donor and third-party target cells by IFN-γ ELISpot assay, which measures the frequency of IFN-γ–secreting cells following specific activation at the single-cell level, in PBMCs of patients 1 and 3 at 144 and 117 days following transplantation, respectively (for details, see Materials and methods).
Tolerance induction of donor cells following haploidentical transplantation combining megadose TCD HSCT and high-dose PTCY. Analysis of CTL-p against donor and third-party target cells by IFN-γ ELISpot assay, which measures the frequency of IFN-γ–secreting cells following specific activation at the single-cell level, in PBMCs of patients 1 and 3 at 144 and 117 days following transplantation, respectively (for details, see Materials and methods).
Discussion
Our proof-of-concept studies in a stringent fully allogeneic mouse model strongly suggest that combining megadose TCD mismatched HSCT with PTCY can offer a novel approach for durable chimerism induction following NMAC, providing complete immune tolerance to subsequent skin grafting. A major attribute of this approach is associated with its very low anticipated transplant-related mortality, as this protocol is free of GVHD risk and utilizes low-toxicity conditioning, which spares a substantial portion of host T cells. These cells provide critical antipathogen immunity during the early posttransplant period. In addition, as this approach does not require the use of any posttransplant immune suppression after CY administration, the full graft versus leukemia capacity of the newly formed immune system can be harnessed. This important attribute cannot be fully used in T-cell–replete HSCT, which requires strong GVHD prophylaxis. Furthermore, the occurrence of GVHD in T-cell–replete transplants also affects thymic function, thereby further impacting GVL reactivity. However, while the feasibility of this approach has now been established in 2 patients, further extensive clinical studies are required to evaluate its benefit in comparison with PTCY T-cell–replete transplants.
It could be suggested that the only likely benefit of Cytoxan therapy is in promoting engraftment by interfering with rejection and that pretransplant Cytoxan could potentially lead to successful engraftment similar to that obtained with PTCY. However, it should be noted that although human TCD transplants (containing ∼1 × 105 T cells/kg) pose a very low risk of GVHD, this risk does exist because of imperfect separation, as sometimes occurs, or other individual variables. Accordingly, the administration of CY following transplant can offer an additional level of safety, further ensuring that GVHD will not occur. Thus, even if pretransplant CY can provide similar engraftment rates, we still believe that it would be less attractive for clinical use.
Our preliminary clinical results in 2 patients with high-risk MM and HL highlighted the advantages of this approach. Rapid and durable engraftment was attained with almost complete chimerism in the myeloid and B-cell lineages, while a marked level of host T cells persisted during the first 6 months posttransplant, providing active cellular immune protection until sufficient donor-derived T cells were generated. Indeed, these bridging host T cells enabled the patient to overcome CMV and EBV reactivation without any antiviral treatment. Notably, the first patient with MM remains in complete remission at 25 months posttransplant.
Nevertheless, the rejection experienced by the second patient suggests that further fine-tuning of the conditioning might be required to ensure universal engraftment. Importantly, thanks to the mild conditioning used, the patient could tolerate a second full myeloablative haploidentical HSCT and is now, 12 months posttransplant, in complete remission and in excellent condition.
Based on this experience, a slight enhancement of the conditioning protocol, by increasing TBI from 2 Gy to 3 Gy, was used in the third patient with high-risk HL. As in patient 1, engraftment and full donor-type chimerism were promptly attained in the myeloid and B-cell lineages, whereas split chimerism with a substantial level of host T cells was observed in the early posttransplant period. In her case, host-type T cells spared by the mild conditioning in the absence of GVHD, which can potentially ablate host T cells, enabled the patient to overcome CMV reactivation without any need for treatment with antiviral agents. CD45RO+ T cells are found in the peripheral blood until ∼6 months posttransplant, indicating possible expansion of host and donor-type memory T cells, possibly following activation against viral antigens or as a result of homeostatic expansion. However, between 6 and 12 months, we observed in patient 1 a steady rise of naive CD45RA+CD62L+CD4 and CD45+CD62L+CD8 T cells alongside an increasing shift toward donor-type T cells (supplemental Table 3). Notably, FACS analysis of CD95 expression of these CD45RA+ T cells (supplemental Figure 4) suggest that they largely comprise memory stem cells, consistent with previous suggestions that following T-cell–replete PTCY, the majority of naive T cells seem to differentiate into CD45RA+CD62L+CD95+ memory stem cells.14,15 Furthermore, our ability to avoid the use of immune-suppressive drugs after Cy administration might help control relapse through the activity of the host and donor T cells, as well as the substantial level of NK cells. Clearly, while no statement regarding relapse can be made based on our limited data, if these patients or future patients treated by this protocol do relapse, they would remain eligible for further treatment with immune cells from the original donor, including chimeric antigen receptor T cells, NK cells, or simply donor lymphocyte infusion.
Apart from the obvious application of this approach to the treatment of nonmalignant or malignant hematopoietic disease, our results in the mouse model showing specific tolerance to donor-type skin grafts indicate that it could potentially serve as a platform for immune tolerance induction followed by solid organ transplantation or cell therapies. Notably, both in the mouse studies and in patients 1 and 3, limiting dilution analysis of alloreactive T cells revealed complete immune tolerance toward donor stimulators while maintaining a marked response against third-party cells.
An early study by Sykes et al16 indicated that chimerism can be partially attained in nonmyeloablative haploidentical transplants without GVHD using in vivo anti-CD2 antibody combined with purified CD34 cells selected by magnetic beads, albeit requiring posttransplant immune suppression for 35 days. Two of 4 patients exhibited graft rejection, whereas the other 2 patients were successfully engrafted, although follow-up was relatively short due to disease progression on days 95 and 119. Recently, Strober’s group17 found that megadose TCD HSCT can induce immune tolerance for subsequent kidney transplants from the same donor in the setting of HLA-identical allogeneic nonmyeloablative transplantation. However, this transplantation strategy using the same conditioning and graft composition in haploidentical recipients failed to induce chimerism. This approach is currently being re-evaluated using a substantially higher number of donor T cells in the graft, with increased risk for GVHD. Our results strongly suggest that instead of using alloreactive donor T cells for chimerism induction, it might be safer to combine megadose TCD stem cells transplants with PTCY. Ildstad’s group13 suggested that immune tolerance for kidney transplantation can be attained by using hematopoietic grafts enriched for CD8+ TCRα/β− “facilitating cells” residing in the BM. However, their protocol also makes use of a substantial number of alloreactive T cells in the BM inoculum combined with PTCY, and it requires additional immunosuppressive drugs administered for a prolonged period. Our present results in mice suggest that prior depletion of the CD8+TCRα/β− population from the megadose TCD BM preparation does not affect its ability to induce marked chimerism when combined with PTCY, in the absence of any further posttransplant immune suppression. Our finding of an additive impact of megadose transplants and PTCY can be explained by their different mechanisms of action. Whereas the former was shown to operate via veto activity (ie, deletion of cognate alloreactive host T cells),18 it was recently demonstrated that the role of PTCY might be mediated in part by induction of regulatory T cells.19 Thus, these 2 modalities can complement each other in overcoming host antidonor T cells without fully ablating the host immune system.
Our results combining extensive long-term analysis in a mouse model and the outcomes of 3 patients offer a proof of concept for a safer platform for haploidentical HSCT in which GVHD is prevented by rigorous T-cell depletion of the graft while rejection is overcome by initial transient host T-cell debulking with anti–T-cell antibodies and the use of megadose stem cells combined with PTCY. Further clinical trials to optimize this approach in MM, HL, or other hematological malignancies, as well as in nonmalignant hematopoietic diseases or as a preparatory regimen for organ transplantation and cell therapy, are warranted.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by Cell Source Ltd. and by a research grant from Roberto and Renata Ruhman. Y.R. holds the Henry Drake Professorial Chair in Transplantation Immunology.
Authorship
Contribution: E.B.-L. and Y.R. designed the preclinical studies and wrote the manuscript; F.A. and Y.R. designed the clinical study and wrote the manuscript; E.B.-L., N.O.-G., and Y.Z.-K. performed the preclinical experiments; F.A., L.P., S.B., I.M., A.M., C.S., G.S., M. Sassi, M. Soli, S.G., M.B., N.G., F.L., and S.P. performed the clinical study; and M.F.M. reviewed the manuscript.
Conflict-of-interest disclosure: Y.R. serves as a consultant and shareholder of Cell Source Ltd., which supported part of this work. The remaining authors declare no competing financial interests.
Correspondence: Yair Reisner, Weizmann Institute of Science, 234 Herzl St, Rehovot 7610001, Israel; e-mail: yair.reisner@weizmann.ac.il.
References
Author notes
F.A. and E.B.-L. contributed equally to this study.





![Figure 5. Chimerism analysis at different time points (percentage of donor [D] cells out of total PBMCs and BM).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/24/10.1182_bloodadvances.2017009423/8/m_advances009423f5.jpeg?Expires=1769121793&Signature=oEZKNoqDCt112nq9XKrXNvxGMDx7ZItB5GOZ9vfW~NfpCD5FI6RW5OUJAIF7LBq2TwYzwSprRqusMvqAtCa~92ViGY7b229d9rq6UGBMqZuxcI-gLTmnfrS20lnNtUlzelKuYflRsHBSqRViV-KPbFHCvG6VhWlXb76br8V~6tUNkGsteFqMwGOKZJ~VSvBpyUG8Oh~Xh5yYvfexZLE2Y7p91L0CWF7W9VW~UOaemJ4owqDGYOv26ET~XkhC~oGPtR5HranJiA6deyfRsjS0V5Tz1EmE5RYPnpPtqIP1kNm76JwOuFN0mP7PjgF9L3rA6TumFTOmcRfndm01MQFvgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. Chimerism analysis at different time points (percentage of donor [D] cells out of total PBMCs and BM).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/24/10.1182_bloodadvances.2017009423/8/m_advances009423f5.jpeg?Expires=1769252743&Signature=18Oz9K6GmPr25NA0QcA6pVGGVdRLjuzZN~cOSq7-10eM68huMa5~ztC11EIAe8b6u1HUrIUv3wCgezcSYIIfv16Ia8wwzxJVU-Uzp9~o50DC8vvKB3ICgiFVJPqkRxMm4M7aBBcFZRvmxh17-n2H~wLxqR-aY1-65KR-r7KGrekykuo-Vrr8lQbqm3eNi69vdh5~bza99fuvV6i~gwXoGad8JCCsm2cy-maGCm5xDvg-ZQ6~D6nfdgMO5alYkX8sP-M1zxXDCeO7mwXCKWwCx8ZP~gqzHgoReM3y6nEVVXW1HDee2tyunDesaNPOuA5oVn-sB2YyW2F9Apm2MviblA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)