Key Points
AT1413 is a monoclonal antibody isolated from a cured patient with AML that recognizes CD43s, a novel epitope expressed by AML and MDS blasts.
AT1413 eliminates CD43s-expressing leukemic blasts in vitro and in vivo and may have potential as a therapeutic antibody.
Abstract
Immunotherapy has proven beneficial in many hematologic and nonhematologic malignancies, but immunotherapy for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) is hampered by the lack of tumor-specific targets. We took advantage of the tumor-immunotherapeutic effect of allogeneic hematopoietic stem cell transplantation and searched the B-cell repertoire of a patient with a lasting and potent graft-versus-AML response for the presence of AML-specific antibodies. We identified an antibody, AT1413, that was of donor origin and that specifically recognizes a novel sialylated epitope on CD43 (CD43s). Strikingly, CD43s is expressed on all World Health Organization 2008 types of AML and MDS. AT1413 induced antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity of AML cells in vitro. Of note, AT1413 was highly efficacious against AML cells in a humanized mouse model without affecting nonmalignant human myeloid cells, suggesting AT1413 has potential as a therapeutic antibody.
Introduction
Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are high-risk hematologic malignancies with long-term disease-free survival obtained in only 20% to 40% of patients.1,2 AML occurs at all ages, and outcome is dismal in particular for elderly patients, who generally have more aggressive disease, and for whom only a minority qualify for high-dose chemotherapy.3-5 For patients younger than 60 years fit enough to be treated aggressively with chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT), the prognosis is better, with 5-year survival rates of 40% to 50%.4,5 A significant proportion of allogeneic HSCT recipients die, however, as a result of transplantation-related complications such as graft-versus-host disease and infections, whereas the lives of allogeneic HSCT survivors are often significantly affected by the detrimental effects of acute and chronic graft-versus-host disease.6 Hence, alternative and less harmful treatment approaches that can also be applied to elderly or less-fit younger patients are highly needed.
New modalities such as treatment of AML and MDS with monoclonal antibodies are being explored. In nonmyeloid malignancies, antibodies directed against CD20 (rituximab, ofatumumab) and CD38 (daratumumab), antibody-drug conjugates such as brentuximab-vedotin (CD30), and chimeric antigen receptor T cells and chimeric proteins (bispecific T-cell engagers) that redirect T cells to CD19-expressing malignant cells have significantly improved the prognosis of patients.7-14 In myeloid malignancies, CD33, CD123 (interleukin 3 receptor), CLEC12A/CCL-1 (C-type lectin), and CD25 are being explored as immunotherapeutic targets.15-18 However, although, for example, the antibody–drug conjugate vadastuximab–talirine (SGN-CD33A) was effective and safely applied in combination with hypomethylating agents or cytarabine in a small series of patients, these myeloid targets are not exclusively expressed by AML/MDS, and off-target adverse effects are a concern. Vadastuximab–talarine and CD33 as a bispecific T-cell engager antibody showed significant toxicity, and clinical studies with these agents are currently on hold. These observations have made it clear there is a need for the identification of novel tumor antigens, specifically for AML and MDS.
The only form of immunotherapy with proven efficacy in AML and MDS so far is allogeneic HSCT. Potent graft-versus-leukemia (GVL) responses are generated via the induction of T-cell, NK cell, and antibody responses and are associated with tumor clearance and survival.19 Targets of GVL antibodies as identified by serologic screening of leukemia-derived cDNA libraries or protein microarrays include both intracellular and membrane-expressed proteins.20-23 Although these data suggest antibody responses contribute to the GVL effect of allogeneic HSCT, the antibody-producing B-cell clones of these patients were not retrieved in a monoclonal format, and the actual contribution of these antibodies to GVL responses could not be verified. Nevertheless, the ability of the donor immune system to elicit antibodies directed against malignant cells via allogeneic HSCT is important, as it can be employed to identify novel tumor antigens, expressed on AML and MDS cells.
Here, we examined the antibody repertoire of an allogeneic HSCT patient with high-risk AML who remained disease free as the result of a potent GVL response. We obtained 5 monoclonal antibodies from this patient that bound to AML cells and only weakly or not at all to nontransformed cells. One of these antibodies in particular, AT1413, bound to all AML cell lines and leukemic blasts isolated from patients with newly diagnosed AML and MDS tested. The target of this antibody is a sialylated epitope on CD43 (CD43s), which is overexpressed by malignant myeloid cells. AT1413 induced antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) on AML cells in vitro and in vivo without affecting nonmalignant cells.
Materials and methods
Patient and healthy human materials
Study protocols were approved by the Medical Ethical Committee of the Academic Medical Centre. All participants signed informed consent. Freshly isolated blasts were obtained from blood or bone marrow of patients with AML/MDS. Healthy bone marrow was acquired from the sternum of patients undergoing thoracotomy for cardiac surgery. Healthy peripheral blood mononuclear cells were isolated from buffy coats from blood donations (Sanquin, the Netherlands).
Cells and cell lines
The following cells and cell lines were used: THP-1, HL-60, HepG2, Huh7, CaCo-2, DLD-1, Colo-205, BJ fibroblasts, Jurkat, RPMI 8226, MM1.s, U266 and SKBR-3 (ATCC), Molm13, Kasumi3, SH-2 and Mono-Mac6, normal human adult dermal fibroblast, human aortic endothelial cells (HAEC), mouse aortic endothelial cells (Cell Biologics), and human umbilical vein endothelial cells (HUVEC) (Lonza). Blood outgrowth endothelial cells were a kind gift of Sanquin (the Netherlands). The cholangiocyte cell line H69 was kindly provided by Douglas M. Jefferson (Tufts University School of Medicine, Boston, MA).24 Cells were maintained according to manufacturer’s instructions. All culture medium was acquired from Gibco or Lonza, the penicillin/streptomycin was obtained from Roche, and the FBS from Hyclone. All cultures were routinely tested for the presence of mycoplasma by polymerase chain reaction.
B-cell cloning, antibody selection, and recombinant antibody production
Memory CD27+ immunoglobulin G-positive (IgG+) B cells were sorted and transduced as described previously, using a fluorescence-activated cell sorter (FACS) ARIA3 from BD25 ; seeded at a concentration of 20 cells per well; and expanded with interleukin 21 and CD40L. Supernatants of expanded B-cell minicultures were screened for antibody binding to AML cell lines and to nonhematopoietic cells by FACS, using goat-anti-human IgG H+L AF647 (Life Technologies) as a secondary antibody. Samples were measured with a FACSCanto or a FACS LSR Fortessa X20 (Becton Dickinson) and analyzed using FlowJo software (Tree Star). The in-house-generated influenza-specific antibody AT1002 (AT10-002)26 was used as a negative control. To produce recombinant antibody, we isolated total RNA with the RNeasy mini kit (Qiagen), generated cDNA, performed polymerase chain reaction, and cloned the heavy and light chain variable regions into the pCR2.1 TA cloning vector (Invitrogen). Several independent cloning experiments were performed to rule out reverse transcriptase or DNA polymerase-induced mutations. Heavy and light variable regions of each antibody were cloned in frame with human IgG1 and κ constant regions into a pcDNA3.1 (Invitrogen)-based vector and transiently transfected 293T cells; recombinant antibodies were purified from the culture supernatant with protein A columns.
AT1413 target identification and validation
THP-1 lysate (0.5% Triton X114 [Sigma], 150 mM NaCl, 10 mM Tris⋅HCL at pH 7.4, 1.5 mM MgCl2 plus protease and phosphatase inhibitors [Roche]) was precleared with palivizumab (antibody against respiratory syncytial virus F), Protein-G, and streptavidin beads (Pierce) and incubated with bead-bound AT1413 or control antibody AT1002 (3 hours at 4°C). After washing, proteins were eluted from the beads (0.1 M Glycine at pH 10.5, 150 mM NaCl, 1% Triton X100, 1 mM EDTA), run on an SDS-PAGE gel, and incubated with Imperial protein stain (Pierce). Some immunoprecipitation (IP) samples were transferred to polyvinylidene difluoride membrane (Bio-RAD) for immunoblotting with Ponseau S, blocked with bovine serum albumin, and incubated with CD43 (clone MEM-59, Abcam) for western blot analysis.
Trypsin digestion and mass spectrometry analysis
Protein bands were digested overnight with trypsin (12.5 ng/μL in 25 mM NH4HCO3, sequencing-grade modified trypsin; Promega) after reduction and alkylation with dithiothreitol (10 mM) and iodoacetamide (55 mM), respectively. Tryptic peptides were analyzed by liquid chromatography–tandem mass spectrometry analysis, using an Ultimate 3000 RSLCnano system (Thermo Fisher Scientific) coupled to an amaZon electron transfer dissociation ion trap (Bruker Daltonics). Proteins were subsequently identified by searching the mass spectrometry data against the human Uniprot database, using the Mascot algorithm (Mascot 2.4.1, Matrix Science). A MS tolerance of 0.3 Da and a MS/MS tolerance of 0.5 Da were used. Trypsin was designated as the enzyme of choice, and up to 2 missed cleavage sites were allowed. Carbamidomethylcysteine was selected as a fixed modification, and oxidation of methionine as a variable modification.
Generation of CD43 truncated variants
CD43 cDNA (Geneart, Life Technologies) with a 3×FLAG tag in-frame on either C or N terminus was cloned into the pHEF-TIG third-generation lentiviral vector containing an internal ribosome entry site–green fluorescent protein 3′ of the CD43 cDNA; vesicular stomatitis virus G lentiviral particles were produced in HEK293T cells. THP1, MOLM, and other cells were transduced with these viruses in the presence of retronectin and sorted for GFP expression to obtain a pure population of CD43 overexpressing cells. Truncated CD43 variants were constructed by polymerase chain reaction cloning of the CD43 C-terminal FLAG-tagged cDNA to contain the signal peptide (amino acid 1-19) followed by the wild-type full-length extracellular sequence (variant A: S20-P400, followed by 3×FLAG: DYKDHDGDYKDHDIDYKDDDDK) or truncated extracellular sequences (variant B-J: B, 31-400; C, 59-400; D, 82-400; E, 112-400; F, 133-400; G, 148-400; H, 166-400; I, 184-400; J, 202-400; K, 220-400). These variants were expressed in THP1 cells by lentiviral transduction and sorted for GFP expression. Sorted cells were lysed and immunoprecipitated with AT1413 and control CD43 antibodies, as described earlier. Eluted IP samples were run on SDS-PAGE and immunoblotted with anti-FLAG-HRP (Sigma).
Flow cytometry analyses
In all experiments with 2-step staining procedures, Fc receptors were blocked by incubating cells on ice for 20 minutes with 30% normal goat serum (Sigma), diluted in phosphate-buffered saline +1% bovine serum albumin (Roche). The following antibodies were used: IgG H+L AF647 (Life Technologies), IgG Fcy AF647 (Jackson), Dapi (Sigma), the CD43 antibodies 84-3C1 (-PE; Ebioscience), L10 (-FITC; Invitrogen), MEM-59 (unlabeled or -FITC; Abcam), DF-T1 (unlabeled; Thermo Scientific), CD4, CD8, CD14, CD19, CD34, CD38, CD45, and CD66b (Biolegend). AT1413 was directly labeled with AF647 (Thermo Fisher). For competition experiments, THP-1 cells were incubated with AT1413 and CD43 antibodies at a maximum concentration of 10 μg/mL (60 minutes, on ice). In all experiments, antibodies were used at a concentration of 1 μg/mL. Samples were measured with a FACSCanto or a FACS LSR Fortessa ×20 (Becton Dickinson) and analyzed with FlowJo software (Tree Star).
Immunohistochemistry
Tissue sections were pretreated in a PT-link module (PT-link, Dako) in citrate buffer (pH 6) and baked for 30 minutes at 60°C. Tissue sections were sequentially blocked using 10 min incubation steps with 0.3% H2O2, serum-free protein block (Dako) and Avidin-Biotin Kit (Biocare), incubated with AT1413-biotin or AT1002-biotin, washed and incubated and detected with 4plus streptavidin-HRP (Biocare) and diaminobenzidine. Nuclei were visualized with hematoxylin, and after dehydration with alcohol and xylene, tissue sections were mounted under glass coverslips with pertex mounting medium.
AML mouse model
Study protocols were approved by the animal experimental committee Amsterdam (DEC) and the central committee for animal experiments (CCD). Sublethally irradiated (1 Gy) newborn NSG mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) were reconstituted with ∼105 human hematopoietic progenitor cells (CD34+CD38−lineage−) derived from fetal liver (human immune system [HIS] mice).27 After confirmation of human hematopoietic progenitor cell engraftment, mice were inoculated 5 months later with 1×107 luciferase-labeled cells of the human AML cell line SH2 through tail vein injection. From day 19 after inoculation, mice received biweekly treatment with 15 mg/kg antibody (IV). AML progression was measured noninvasively weekly by bioluminescence imaging, using a Photon Imager (Biospace laboratory). Mice were injected intraperitoneally with VivoGlo Luciferin (Promega, 3.75 mg per mouse), and images were acquired 15 minutes later. Total photon flux (photons/s) of the whole body was quantified. All mice were killed at day 39.
Results
Identification of AML-specific B-cell clones
We selected a 49-year-old patient with relapsed acute monoblastic leukemia (patient 101), who has remained disease free for more than 6 years after receiving a nonmyeloablative, allogeneic HSCT from a matched, unrelated donor. From this patient, we isolated peripheral blood B cells 2 years after his transplantation. B cells were transduced with BCL-xL and BCL-6, as described previously,25 expanded by culturing them on CD40-ligand-expressing fibroblasts in the presence of interleukin 21 and deposited at 20 cells per well in a 96-well microtiter plate. Supernatants of these minicultures contained the antibodies produced by the cultures, and screening of the supernatants identified 5 of 5500 cultures that bound very well to the AML cell lines THP-1, Molm13, and MonoMac6, but not to primary skin fibroblasts and the colon cell line CaCo2 (supplemental Figure 1). By subcloning (1 cell per well) of these minicultures, we retrieved 5 clonal B-cell lines that produced antibodies binding to AML cell lines (Table 1). Two of these antibodies, AT1413 and AT1508, had an IgG1 isotype and more than 10 somatic hypermutations in the heavy and light chains, indicating antigen-induced class-switching and affinity maturation. Using microchimerism analysis of genomic polymorphisms through profiling of the short-tandem repeat DNA loci of the parental B-cell clone, we confirmed that all antibodies were of donor-origin.
AT1413 interacts with malignant myeloid cells
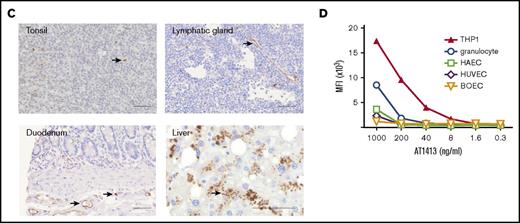
To further evaluate the breadth and specificity of AT1413, we tested binding of the recombinant antibody to a wide variety of cell lines and cells obtained from healthy individuals. AT1413 interacted with all AML cell lines tested, representing most French-American-British classification AML types (Figure 1A). The antibody also bound to a subset of nonmalignant CD34+ and CD38+ hematopoietic progenitor cells obtained from healthy bone marrow, and to peripheral blood monocytes and granulocytes derived from healthy individuals (Figure 1B). AT1413 did not bind to healthy lymphoid cells from blood and tonsil, or lymphoid progenitors obtained from thymus, to the T-ALL cell line Jurkat, or with tissue cell lines or patient-derived cells of liver, cholangiocytes, colon, fibroblasts, breast cancer, or multiple myeloma (Figure 1B; supplemental Figure 2). We performed a tissue microarray immunohistochemistry screen on a large variety of healthy tissues (177 tissue cores including, among others, the small and large intestines, muscle, kidney, liver, gallbladder, pancreas, and lung). Membrane staining of a few mononuclear cells in tonsils was confirmed. We observed scattered intracellular staining of macrophage-type cells throughout the tissues, most prominently in the liver (Kupffer cells), and we noted staining of endothelial cells in blood vessels (Figure 1C). FACS analysis of AT1413 binding to the endothelial cell lines HUVEC and HAEC demonstrated this occurred only at relatively high antibody concentrations (Figure 1D). In the same experiment, we observed binding of AT1413 to granulocytes to be much weaker than binding to AML cells.
Identification of AML-specific B-cell clones. (A) Subcloning of miniculture 2K23 yielded an AML-specific clone, producing the antibody AT1413 that binds to AML cell lines (French-American-British classification M0-M5). In all experiments, the recombinant antibody was used. (B) AT1413 also bound to a subset of nonmalignant hematopoietic progenitor cells and to peripheral blood-derived nonmalignant monocytes and granulocytes. AT1413 did not bind to blood-, tonsil-, or thymus-derived mature and immature lymphoid cells, nor did it bind to the tissue cell lines HepG2 (liver), Huh7 (liver), H69 (cholangiocytes), Caco2 (colon), or BJ (foreskin fibroblasts), and primary cultured fibroblasts (normal human adult dermal fibroblasts). (C) Immunohistochemistry of AT1413-biotin with streptavidin-HRP as a secondary antibody confirmed binding to mononuclear cells in tonsil and demonstrated binding to endothelial cells in blood vessels and a punctuate staining pattern of macrophage-type cells in the liver. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate diaminobenzidine (DAB). Scale bars, 50 μm. (D) Comparison of AT1413 staining to THP-1 cells (black triangles), granulocytes (white circles), and endothelial cells with FACS analysis. HUVEC, white diamonds; HAEC, white squares; BOEC, blood outgrowth endothelial cells, white triangles. The in-house generated influenza-specific antibody AT1002 was used as a negative control in A-C.
Identification of AML-specific B-cell clones. (A) Subcloning of miniculture 2K23 yielded an AML-specific clone, producing the antibody AT1413 that binds to AML cell lines (French-American-British classification M0-M5). In all experiments, the recombinant antibody was used. (B) AT1413 also bound to a subset of nonmalignant hematopoietic progenitor cells and to peripheral blood-derived nonmalignant monocytes and granulocytes. AT1413 did not bind to blood-, tonsil-, or thymus-derived mature and immature lymphoid cells, nor did it bind to the tissue cell lines HepG2 (liver), Huh7 (liver), H69 (cholangiocytes), Caco2 (colon), or BJ (foreskin fibroblasts), and primary cultured fibroblasts (normal human adult dermal fibroblasts). (C) Immunohistochemistry of AT1413-biotin with streptavidin-HRP as a secondary antibody confirmed binding to mononuclear cells in tonsil and demonstrated binding to endothelial cells in blood vessels and a punctuate staining pattern of macrophage-type cells in the liver. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate diaminobenzidine (DAB). Scale bars, 50 μm. (D) Comparison of AT1413 staining to THP-1 cells (black triangles), granulocytes (white circles), and endothelial cells with FACS analysis. HUVEC, white diamonds; HAEC, white squares; BOEC, blood outgrowth endothelial cells, white triangles. The in-house generated influenza-specific antibody AT1002 was used as a negative control in A-C.
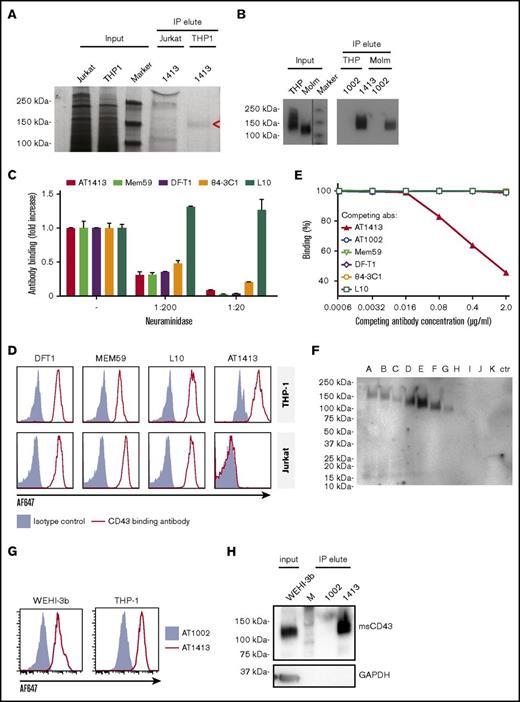
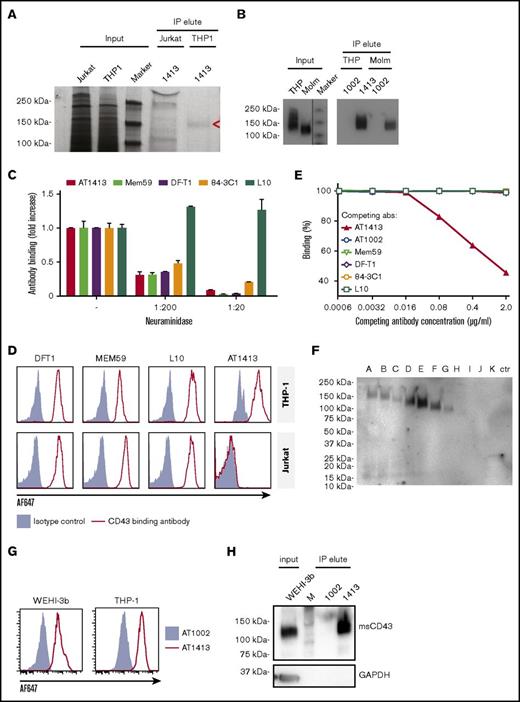
The target of AT1413 is a novel sialylated CD43 epitope
IP of lysate of the AML cell line THP-1 with biotin-labeled sortase-tagged AT1413 yielded a ∼140-kDa band that was not precipitated with lysate of the lymphocyte cell line Jurkat (Figure 2A). Mass spectrometry analysis of the immunoprecipitated band revealed CD43 as the target protein. Three intracellular (nonglycosylated) tryptic peptides (RTGALVLSR, GSGFPDGEGSSR, QGSLAMEELK) were identified in the material precipitated from THP-1 cells. We confirmed CD43 as the target protein of AT1413 by western blot analysis, using Mem59, a commercially available CD43 antibody (Figure 2B).
The target of AT1413 is a novel sialylated CD43 epitope (CD43s). (A) IP with biotin-labeled, sortase-tagged AT1413 of THP-1 or Jurkat lysate. Imperial Coomassie-stained gel. (B) Western blot analysis of the AT1413 and AT1002 immunoprecipitates of THP-1 and Molm13 lysates with Mem59 mouse-anti-human CD43 antibody. A vertical line has been inserted to indicate the repositioned marker lane. (C) Deglycosylation of THP-1 cells with neuraminidase (sialidase) abrogated binding of antibodies AT1413, Mem59, DF-T1, and 84-3C1 in a dose-dependent manner. Clone L10 does not target a sialylated epitope of CD43.36 (D) Staining of THP-1 and Jurkat cells with AT1413 and with the commercially available CD43-specific antibodies DF-T1, L10, and Mem59. (E) Competition experiment with AT1413 and commercially available CD43-specific antibodies. THP-1 cells were incubated with indicated antibodies, biotinylated AT1413 and streptavidin PECy7. AT1413 binding to THP-1 target cells was not affected by preincubation of the cells with commercially available CD43 antibodies, but was inhibited in a dose-dependent manner when THP-1 cells were preincubated with unlabeled AT1413. (F) Immunoprecipitation with AT1413 of truncated variants of THP-1 expressed FLAG-tagged CD43. Immunoblotting with FLAG antibody revealed binding of AT1413 to CD43 mutants A-F and no binding to mutants H-K. Truncations were performed as indicated in supplemental Figures 3 and 4. Ctr, control with GFP-transduced THP-1 cells. (G) AT1413 (2.5 µg/mL) binding of the human cell line THP-1 and the murine AML cell line WEHI-3b. (H) Western blot analysis of the AT1413 and AT1002 immunoprecipitates of the mouse AML cell line WEHI-3b lysate with anti-mouse CD43 antibody.
The target of AT1413 is a novel sialylated CD43 epitope (CD43s). (A) IP with biotin-labeled, sortase-tagged AT1413 of THP-1 or Jurkat lysate. Imperial Coomassie-stained gel. (B) Western blot analysis of the AT1413 and AT1002 immunoprecipitates of THP-1 and Molm13 lysates with Mem59 mouse-anti-human CD43 antibody. A vertical line has been inserted to indicate the repositioned marker lane. (C) Deglycosylation of THP-1 cells with neuraminidase (sialidase) abrogated binding of antibodies AT1413, Mem59, DF-T1, and 84-3C1 in a dose-dependent manner. Clone L10 does not target a sialylated epitope of CD43.36 (D) Staining of THP-1 and Jurkat cells with AT1413 and with the commercially available CD43-specific antibodies DF-T1, L10, and Mem59. (E) Competition experiment with AT1413 and commercially available CD43-specific antibodies. THP-1 cells were incubated with indicated antibodies, biotinylated AT1413 and streptavidin PECy7. AT1413 binding to THP-1 target cells was not affected by preincubation of the cells with commercially available CD43 antibodies, but was inhibited in a dose-dependent manner when THP-1 cells were preincubated with unlabeled AT1413. (F) Immunoprecipitation with AT1413 of truncated variants of THP-1 expressed FLAG-tagged CD43. Immunoblotting with FLAG antibody revealed binding of AT1413 to CD43 mutants A-F and no binding to mutants H-K. Truncations were performed as indicated in supplemental Figures 3 and 4. Ctr, control with GFP-transduced THP-1 cells. (G) AT1413 (2.5 µg/mL) binding of the human cell line THP-1 and the murine AML cell line WEHI-3b. (H) Western blot analysis of the AT1413 and AT1002 immunoprecipitates of the mouse AML cell line WEHI-3b lysate with anti-mouse CD43 antibody.
CD43 is a highly O-glycosylated protein,28 and the commercially available CD43 antibodies Mem59, DF-T1, and 84-3C1 bind to sialylated epitopes of CD43.29-31 Removing all α2-3-N-acetylneuramic acids (sialic acids) from THP-1 cells by preincubating the cells with neuraminidase abrogated binding of all antibodies except L10 (that is directed against the same peptide as 84-3C1, but binds anon-sialylated epitope31 ) in a dose-dependent manner (Figure 2C). Thus, AT1413 targets an epitope that is sialylated, similar to the epitopes recognized by most commercially available CD43 antibodies. However, whereas AT1413 and the commercially available CD43 antibodies bound THP-1 cells, AT1413 did not bind the T ALL cell line Jurkat, in contrast to the other CD43 antibodies (Figure 2D). In addition, none of the commercially available CD43 antibodies competed with binding of AT1413 to THP-1 cells (Figure 2E), whereas they could inhibit binding of each other, as described previously.31 Together, these data indicate that AT1413 recognized a unique epitope that is not targeted by other CD43 antibodies.
To more specifically identify the binding epitope of AT1413, we generated 10 FLAG-tagged truncated variants of CD43 that were expressed in THP1 cells (supplemental Figure 3A). Immunoprecipitation with AT1413 of lysates of THP1 cells transduced with truncation variants A-K revealed strong binding to variants A-F, weak binding to variant G, and no binding to variants H-K, as shown in an immunoblot with a FLAG-specific antibody (Figure 2F). Full-length endogenous and truncated CD43 immunoprecipitation was confirmed in a CD43 immunoblot (supplemental Figure 3B). Thus, the epitope of AT1413 is located between amino acids 133 and 165 of the CD43 protein (supplemental Figure 4). Mem59 and DF-T1 demonstrated strong binding to truncated variants A-C and no binding to variants D-K, identifying the epitope of these CD43 antibodies to be at a different location, between amino acids 59 and 82 (supplemental Figure 5). Of note, CD43s is a conserved epitope that is also expressed on murine AML cells (Figure 2G). Immunoprecipitation of the murine AML cell line WEHI-3b with AT1413 confirmed CD43s as the target (Figure 2H).
CD43s is overexpressed on AML and MDS patient-derived leukemic blasts
Thus, AT1413 is an antibody that targets a unique, sialylated epitope on CD43 that is expressed on malignant myeloid cell lines. To further evaluate the breadth and specificity of AT1413, we tested binding of this antibody to bone marrow and peripheral blood samples obtained from patients with AML and MDS. The first patient to be tested was patient 101, the allogeneic HSCT recipient of whom AT1413 was obtained. Viable AML blasts of this patient had been frozen and stored at diagnosis and AT1413 showed clear binding to these leukemic blasts. (Figure 3A). We then tested binding of AT1413 to leukemic blasts obtained from 60 randomly selected, newly diagnosed patients with MDS and AML, and found that AT1413 bound to all samples tested (Figure 3B; Table 2). All World Health Organization 2008 AML subtypes32 were represented in this patient cohort (although as a result of the relatively small sample size not in the same proportions as published33 ), including patients with high-risk MDS and patients with extramedullary AML (myeloid sarcoma; Figure 3C). Interestingly, in a patient diagnosed with therapy-related AML several years after intensive chemotherapy for multiple myeloma, AT1413 clearly distinguished myeloid leukemic blasts from multiple myeloma cells (Figure 3D). In a direct comparison with bone marrow obtained from 6 newly diagnosed patients with AML, we confirmed that binding of AT1413 to leukemic blasts was stronger than binding to nonleukemic granulocytes, monocytes, and lymphocytes of the same patient (Figure 3E).
CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.
CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.
AT1413 induces ADCC and CDC of AML blasts, but not nonmalignant cells in vitro
We then tested the in vitro capacity of this antibody to induce target cell killing by ADCC and CDC, as described previously.34 Incubation of the AML cell line SH2 with AT1413 and human peripheral blood mononuclear cells or rabbit complement-induced ADCC and CDC, respectively (Figure 4A). Endothelial cells (HAEC or HUVEC) and granulocytes, which bound AT1413, albeit to a lesser extent than AML cells, were not killed when incubated with AT1413 and peripheral blood mononuclear cells (Figure 4B). Moreover, when we incubated SH2 cells with AT1413 and whole blood from a healthy individual, SH2 cells were killed, but healthy peripheral blood polymorphonuclear cells were not affected (Figure 4C).
AT1413 induces ADCC and CDC of malignant myeloid cells in vitro. (A) AT1413 (open squares) induced ADCC and CDC of the AML cell line SH2 with EC50s of 1.1 nM (0.16 μg/mL) and 12.4 nM (1.86 μg/mL), respectively. Control antibody, AT1002 (red dots). (B) AT1413 (blue bars) induced ADCC of AML cells (SH2), but not of HAEC, HUVEC, and granulocytes. Control antibody, AT1002 (red bars). (C) Labeled SH2 cells were incubated with whole blood from a healthy individual and with AT1413 or rituximab. AML cells but not mononuclear cells were killed. As a control experiment, CD20+ Ramos cells were incubated with healthy whole blood and AT1413 or rituximab. PMN, polymorphonuclear cells.
AT1413 induces ADCC and CDC of malignant myeloid cells in vitro. (A) AT1413 (open squares) induced ADCC and CDC of the AML cell line SH2 with EC50s of 1.1 nM (0.16 μg/mL) and 12.4 nM (1.86 μg/mL), respectively. Control antibody, AT1002 (red dots). (B) AT1413 (blue bars) induced ADCC of AML cells (SH2), but not of HAEC, HUVEC, and granulocytes. Control antibody, AT1002 (red bars). (C) Labeled SH2 cells were incubated with whole blood from a healthy individual and with AT1413 or rituximab. AML cells but not mononuclear cells were killed. As a control experiment, CD20+ Ramos cells were incubated with healthy whole blood and AT1413 or rituximab. PMN, polymorphonuclear cells.
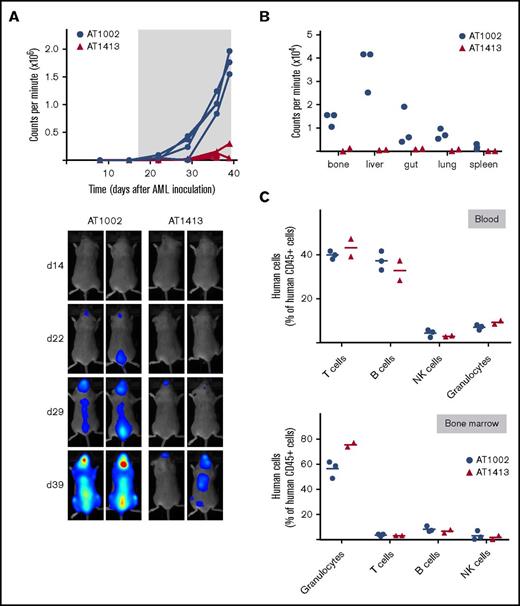
AT1413 specifically eliminates human AML blasts in vivo
To test whether AT1413 affects tumor growth in vivo, we set up a mouse model for human AML in mice bearing human effector cells. Newborn female NSG mice were reconstituted with human hematopoietic stem cells (HIS mice27 ) and, after confirmation of engraftment, inoculated 5 months later with 1×107 luciferase-GFP transduced human AML cell line SH2 cells via tail vein injection. Starting on day 19 after SH2 inoculation, mice were treated biweekly with AT1413 (15 mg/kg IV) or control antibody (AT1002, 15 mg/kg IV) and killed at day 39. Tumor growth was strongly inhibited by AT1413, as measured by whole-body bioluminescence of the mice (Figure 5A). After mice were killed, mouse organs (bone, liver, gut, lung, and spleen) were analyzed individually for the presence of AML cells. Whereas the organs of control-antibody treated mice were heavily infiltrated with AML, no AML infiltration was observed in the organs of AT1413-treated mice (Figure 5B). Importantly, AT1413 treatment did not affect human, nonmalignant myeloid cells present in the tumor-bearing HIS mice, consistent with the observation that healthy myeloid cells bound by AT1413 were not killed in an ADCC assay in vitro. Proportions of human CD45+ cells, which includes human granulocytes, T cells, B cells, and NK cells, but not AML cells, were similar between AT1413 and control antibody-treated mice (Figure 5C). AT1413 bound weakly to mouse liver cells and mouse endothelial cells in FACs analysis (supplemental Figure 6), similar to what we observed for human cells (Figure 1D). However, injection of AT1413 did not induce significant adverse effects, except for a transient reduction in food intake and weight loss of mice after the first injection only (supplemental Figure 7). We confirmed the efficacy of AT1413 in nonhumanized NSG mice inoculated with luciferase-labeled SH2 cells (supplemental Figure 8).
Anti-AML effect of AT1413 in vivo. (A) HIS mice with human AML (luciferase-GFP transduced SH2) received biweekly treatment with AT1413 or control antibody AT1002 (15 mg/kg IV, indicated by asterisk). AML progression was measured by bioluminescence (CPM) after luciferase injection. (B) Bioluminescence of individual organs harvested after mice were sacrificed. (C) Human T cells, B cells, NK cells, and granulocytes, expressed as proportion of human CD45+ hematopoietic cells (excluding CD45dim AML cells) in blood and bone marrow of AT1413- and AT1002-treated mice.
Anti-AML effect of AT1413 in vivo. (A) HIS mice with human AML (luciferase-GFP transduced SH2) received biweekly treatment with AT1413 or control antibody AT1002 (15 mg/kg IV, indicated by asterisk). AML progression was measured by bioluminescence (CPM) after luciferase injection. (B) Bioluminescence of individual organs harvested after mice were sacrificed. (C) Human T cells, B cells, NK cells, and granulocytes, expressed as proportion of human CD45+ hematopoietic cells (excluding CD45dim AML cells) in blood and bone marrow of AT1413- and AT1002-treated mice.
Discussion
The development of novel forms of immunotherapy, other than allogeneic HSCT, for the treatment of AML would be a significant therapeutic advance. In search for novel AML/MDS-specific antigens that can be employed as targets for immunotherapy, we screened the B-cell repertoire of a patient with a durable remission after receiving an allogeneic HSCT for relapsed AML for antibodies that react with cell surface antigens expressed on AML cells. We isolated 5 B-cell clones producing such antibodies, and 1 of these antibodies, AT1413, recognized a novel sialylated epitope on CD43 (CD43s). CD43 is a heavily O-glycosylated mucin-like type I transmembrane protein that is present on the surface of most hematopoietic cells, including hematopoietic stem cells, but not on resting B cells and erythrocytes.35 Different CD43 glycoforms that can be coexpressed on 1 cell have been described, each with specific functions, including roles in activation, proliferation, migration, and apoptosis.36 Although in healthy individuals CD43 is exclusively expressed on hematopoietic cells, certain CD43 glycoforms are expressed by a number of nonhematologic tumors, including colon, lung, and breast carcinoma, where it affects growth, migration, metastasis, and interaction with the immune system, and hence prognosis.28 For example, the UN1/CD43 epitope is a specific CD43 glycoform expressed by T lymphocytes, thymocytes, and certain leukemic T-cell lines, and by certain solid tumor types.37,38
We identified CD43s, a novel CD43 glycoform that is overexpressed on AML cell lines and blasts of patients with AML. In fact, although the extent of expression is variable, CD43s is expressed on all World Health Organization 2008 classification types of AML, including MDS-RAEB I and MDS-RAEB II.
CD43s is also expressed by myeloid progenitor cells, monocytes, and granulocytes and weakly expressed by endothelial cells. CD43s is a conserved tumor epitope, as it is also expressed by murine AML cells. Engagement of AT1413 induced in vitro death of AML cells, but not of healthy cells, via ADCC and CDC. Strikingly, AT1413 was highly efficacious against malignant cells in nonhumanized NSG mice (supplemental Figure 8) and in a humanized HIS mouse AML model. The nonmalignant human myeloid cells in the latter mice were, however, not affected. In addition, no adverse effects were observed in the treated mice, with the exception of a transient weight loss after the first dosing. These data suggest this antibody may have therapeutic potential for the treatment of patients with AML. The reactivity of AT1413 with human endothelial cells may raise safety concerns; however, the binding is weak and AT1413 does not mediate ADCC or CDC against endothelial cells. In this respect, it is noteworthy that trastuzumab, a HER2/neu-specific antibody that is widely used in the treatment of HER2/neu overexpressing breast cancer interacts with cardiac endothelial cells but is safely applied in humans.39 Another important point with respect to safety is that although AT1413 is a highly mutated, antigen-experienced antibody, the patient from whom this antibody was derived did not experience vascular complications, cardiac issues, neutropenia, or pancytopenia after allogeneic HSCT. He did develop bronchiolitis obliterans syndrome and pulmonary dysfunction after syncytial respiratory virus infection, but is no longer on immunosuppressants.
In conclusion, we identified CD43s as an immunotherapeutic target that is overexpressed by all World Health Organization 2008 types of AML and MDS. AT1413 induced ADCC and CDC of AML blasts in vitro and in vivo. Although the efficacy of AT1413 in vivo needs to be confirmed in other models, these data suggest AT1413 has the potential to be employed as a naked antibody, alone or in combination with standard AML chemotherapeutic regimens including cytarabine, anthracyclines, or hypomethylating agents. Alternatively, AT1413 may be developed into an antibody–drug conjugate, and a bispecific T-cell engager, chimeric antigen receptor T cells, or CD43s may be used in a vaccine to generate autologous immune responses to the tumor.40 Such novel approaches to treat AML and MDS are highly anticipated, given the poor outcome of the majority of patients with AML or high-risk MDS.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are greatly indebted to the patients who participated in this study. They also thank Ludo Evers for chimerism analysis, and their colleagues at the Academic Medical Center's Department of Hematology Trial Office for the collection of AML samples and the Department of Pathology for supplying tissue microarrays.
This study was financially supported by an intramural grant of the AMC (M.A.G.), the Netherlands Organization for Scientific Research (M.D.H.), and the Dutch Cancer Foundation (M.D.H. and H.S.).
Authorship
Contribution: H.S. and M.D.H. conceptualized the study; J.V. and P.J.H. devised the methodology; M.A.G., G.d.J., M.K., E.Y., S.E.L., A.Q.B., K.W., and G.M. performed experiments; M.A.G. and M.D.H. wrote the original draft of the manuscript; M.A.G., G.d.J., M.K., P.J.H., P.M.v.H., H.S., and M.D.H. wrote, reviewed, and edited the manuscript; M.A.G., H.S., and M.D.H. were responsible for funding acquisition; P.J.H. and M.D.H. provided laboratory resources; and P.M.v.H., H.S., and M.D.H. supervised the study.
Conflict-of-interest disclosure: M.A.G., G.d.J., M.K., E.Y., S.E.L., G.M., A.Q.B., K.W., J.V., P.M.v.H., and H.S. are employees of AIMM Therapeutics, a company that develops monoclonal antibodies for prevention and treatment of infectious diseases and cancer.
Correspondence: Mette D. Hazenberg, Department of Hematology F4-224, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: m.d.hazenberg@amc.nl.
References
Author notes
G.d.J. and M.K. contributed equally to this study.




![CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/19/10.1182_bloodadvances.2017008342/3/m_advances008342f3-1.jpeg?Expires=1764967771&Signature=QwQCo5AkkFE~HGruD2jV-U3QttRbd2-zhyeVP377Bg3oZZMteZqLSZADN-6-yVDtbssrtX5qyEsYHF-W9woRvRcLwP-ShB76XZj~c984NeUizDGbNDp5HSjGAbyLvws1MqU4ZfgPrVi5PC453MKVk-pofwOIRPNdJRdD7Lt4jGiSXIJa7ekdStgXXrr3M42NaUdU5E~u894rLJ0L7owmH1VVmXSEnpcHRqgfTejzch1LODnoCB~6eUw52l2dTevzneLD8LjIhEkim0vgUujLbmhRUllsxGT2Xmtw8tZWMqPw1USjyZjg9CR3FEzhiDpH8yEWGWFGvz3uQ-h24jOVZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/19/10.1182_bloodadvances.2017008342/3/m_advances008342f3-2.jpeg?Expires=1764967771&Signature=OTPaoyy7kQpd~Bj27mFlBMX964MO38-aS6KWH9sfkgmPzneZDkFFn-b5h6X7WoskpfwyworLbcFkFWWg5lTWrjgW78jp9j-H1UMzcQQTuDYB0wWppI1TUN1H0FKFjKuGwX7JerWmLGFiMtCXCSxIcmf2hGDoxYJs~KG4ThlV6Tak6chEfvGsNr1Q2Gqk2kjoNWZ6YJf93SzxX2clDNeTnFu73twQOXAM0S6m1qJWtdNtaBPPByUjgEBU44zkRt4EMPYKgD8Job6V2nChF8Ti1O1dIfsSvUe~eEnH-N-9rqmumhUU~o3Navl0e4fb1MONNEesOohKMhdmIA81aKLhWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/19/10.1182_bloodadvances.2017008342/3/m_advances008342f3-3.jpeg?Expires=1764967771&Signature=O7Fi9U7pRR8QRq8purrqibPQ6l2UNx4RkZ3VxxI9tgWB2CP8xLx9bLautFFMUhCQaA76aA3-a15eQzb0Kf8c0RTI4A3x7-eLHQBeeYFqCRbbD9-js2iGUYRvP4WP-2P2OsUTg2u6l~yrjEWv35Dr2qlBHihHWlztLEYhq4iRdeaQ4ctngOkl2A58DGJMwom339HErRzKLvJdN8C8coHgZmKv93JuWWimoWGt7FZZ9necVsqvstf8uNKDvEPFOOiZqOyi621EBN0XJg3~zLqUIa0XRp7zeCZiYjSfVq-xW62aP3mFw2YFjmj2v57I06wod6iTGTZDVzSzPJ4D8vdCkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/19/10.1182_bloodadvances.2017008342/3/m_advances008342f3-1.jpeg?Expires=1765134097&Signature=crzCPigvoi86IjF8Du0GD5Oii4AV4PL4tbwgOc3jralu4eGBMY756sRVPNzU8WhvMrG-dClkqvpeVg5IZ~xpJB6Ib-d~HXDgWNylwzPJjRFqVcs9RpNny5F-s-bwkz1vZXyE8zIrVi4RoIrunnaWA3YaiRP3f4tR83sZX~lbbgAk5Rrp8i2hQeqZu9Roi3GuSF74p6cj1BjzZkLNSJKEb9mJJ6fZhvrdrm~TVRpLAH7gZVjdtVzXJE34m1LLQeFa4qeDY7ZNIWDKtstcHzBojas2m4vwysUH3rDHH~r6uMeSajlZQbbfoI76mgjdLhoaYdmNvlbqCGoTkLZ3s~Ts5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/19/10.1182_bloodadvances.2017008342/3/m_advances008342f3-2.jpeg?Expires=1765134097&Signature=nahyo~sNj~KuFJjPc9x1UP4SPBJCFQl66Wf5RIdfNrXOm4nwDVzRGXnY9zmcHlNOU9tzU9ku3aPePZnnP~G6SG2us2IsdFKTfa6AVscDle772bSgKslIuquwjx5adb0xIeEE25HEo8mwAhUkkLIVkU2kgAWrb9nRzlJgKqwWnztYhK-YfbwzmkV3Aobdi7rAsxc43Eg1a1x1ffJok21fFi2lKWAKOsL1Y1xd5V3hV-gPquTAsJQSF6U70j9WyWewVohqSFKuh67AvrhEU~Q8WDWT7RXHst95Q9IXZMYqzaZ511V8UjIHLOI3XoO2YgrnZcsGTOYskE5hjb0m7zIXvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD43s is overexpressed by myeloid malignancies. (A) AT1413 binding to CD34+ and CD38+ CD45dim AML blasts of patient 101. Bone marrow cells of this patient were isolated using a ficoll gradient and stored at diagnosis, precluding analysis of AT1413 interaction with nonmalignant granulocytes. (B) Representative examples of AT1413 binding to AML blasts obtained from newly diagnosed patients with AML or MDS (Table 2). (C) AT1413 binding to extramedullary AML of 2 patients (myeloid sarcoma [chloroma] of inguinal node [1] and skin [2]). Paraffin-embedded THP-1 and Jurkat cells were used as a positive and negative control, respectively. Biotin immunoreactivity of antibody shown with streptavidin-HRP and the peroxidase substrate DAB. Scale bars, 20 μm. (D) Bone marrow of a patient with concomitant multiple myeloma and therapy-related AML. (Left) Hematoxylin and eosin staining. Asterisk, malignant double-nucleated plasma cell; arrowheads, AML blasts. Original magnification ×100. (Right) AT1413 staining of CD45dim AML blasts; CD138+ multiple myeloma plasma cells do not interact with AT1413. (E) AT1413 binding to CD45dim blasts of patients with AML, and to a lesser extent to CD45+ granulocytes and monocytes and absence of binding to CD45+ lymphocytes. The fold increase MFI of AT1413 compared with the negative control is indicated in gray (AT1002, filled gray histogram). Bone marrow (BL-079, BL-092, BL-095, BL-096, BL-099) or blood (BL-091, BL-106) of patients with AML was freshly obtained and red blood cells lysed before FACs analysis. RAEB, refractory anemia with excess blasts.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/19/10.1182_bloodadvances.2017008342/3/m_advances008342f3-3.jpeg?Expires=1765134097&Signature=acueIbfTuvZZwEkEKHdVqoxdpoOu3dHJaCKtiQfavSOb1GssNQ3HAs7eXns~O6ujT92wqHJLI1CAcmGvaS00DM1V-E3F~yvhlNRv7Q0WM53snnRpr7LbhXjT3XXZrzEf0HsOKqAYwk~VfD9s8UJq5VWTJnc204U2ZUXxhWiI1uFBREGHOuVhWvMsKFX9tqchqnk1f6DwwbbGXvWNWdeoyUF0q9GlX8j7HweVzuxA1FrDCajxfI5Ba7RgS3NirCn5BjblX2v~zyQehgRRczvgGhlLuOI2tw-gdbjz5iENAIvhxQtLGo7JKdoukF5W6GLLTlMFIj~-0XXnzusWCa1p7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

