Key Points
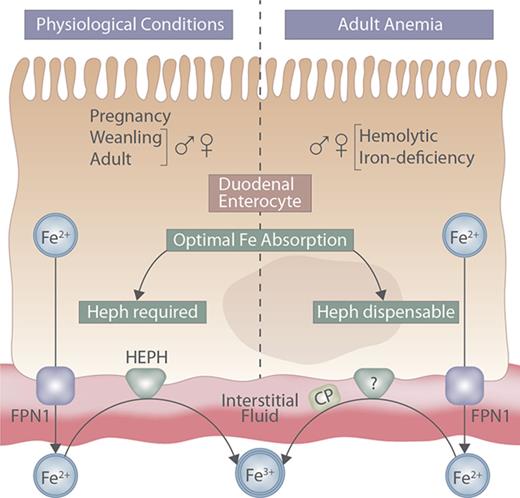
Intestinal Heph may be required to potentiate iron absorption during rapid growth and pregnancy, or when hypoxia stimulates erythropoiesis.
Here, the physiological conditions in which Heph is required are defined and other, complementary, intestinal ferroxidases are identified.
Abstract
Regulation of intestinal iron absorption is crucial to maintain body iron levels because humans have no regulated iron-excretory system. Elucidating molecular events that mediate intestinal iron transport is thus important for the development of therapeutic approaches to modify iron absorption in pathological states. The process of iron uptake into duodenal enterocytes is relatively well understood, but less is known about the functional coupling between the iron exporter ferroportin 1 and the basolateral membrane iron oxidase hephaestin (Heph). Initial characterization of intestine-specific Heph knockout (Hephint) mice demonstrated that adult male mice were mildly iron deficient; however, the specific role of intestinal Heph has not been determined in weanling mice, in female mice, or during physiological states which stimulate iron absorption. Furthermore, because ferroportin 1–mediated iron export from some tissues (eg, liver) is impaired in the absence of the Heph homolog, ceruloplasmin, we hypothesized that Heph is rate limiting for intestinal iron absorption, especially when iron demands increase. Our experimental approach was to assess various physiological parameters and iron (59Fe) absorption and tissue distribution in weanling, adult, and pregnant Hephint mice (and controls) under physiological conditions and in adult Hephint mice after dietary iron deprivation or acute hemolysis. Results demonstrate that intestinal Heph is essential for optimal iron transport in weanlings and adults of both sexes and during pregnancy, but not in adult mice with iron-deficiency or hemolytic anemia. Moreover, activation of unidentified, intestinal ferroxidases was noted, which may explain why intestinal Heph is not always required for optimal iron absorption.
Introduction
Iron functions physiologically in oxygen transport, energy production, cell growth and division, and regulation of gene expression. Many iron-dependent enzymes catalyze essential oxidation-reduction reactions.1 Maintaining body iron levels within the normal range is necessary to prevent iron deficiency and iron overload. Iron deficiency has numerous underlying causes, in addition to dietary insufficiency,2-4 and excess iron can potentiate the formation of highly reactive oxygen free-radicals.5 Importantly, humans do not possess a regulated excretory system for iron, and therefore, overall body iron balance is maintained by regulation of intestinal iron absorption.6 Elucidating the molecular mechanisms that facilitate dietary iron absorption may thus lead to the identification of therapeutic targets for the clinical management of diseases related to iron deficiency and iron overload.
Absorption of dietary nonheme ferric iron (Fe3+) across the brush-border membrane of duodenal enterocytes requires enzymatic reduction and subsequent import by divalent metal-ion transporter 1.7-10 At the basolateral membrane, export of ferrous iron (Fe2+) is mediated by the iron transporter ferroportin 1.11-13 Iron export by ferroportin 1 is functionally coupled to iron oxidation by the basolateral membrane–anchored ferroxidase (FOX) hephaestin (Heph).14 Iron oxidation facilitates formation of holo- (diferric) transferrin, which delivers diet-derived iron to the liver via the portal blood circulation. Mice expressing a mutant form of Heph (sla) and Heph (whole-body) knockout (KO) mice are iron deficient and anemic early in life, but only mildly iron deficient as adults.15 It has thus been proposed that additional oxidases exist, including unidentified intestinal FOXs, which complement Heph function in adult mice.15,16 Unfortunately, investigations using sla mice or Heph KO mice cannot definitively establish the specific impact of intestinal Heph on iron absorption, because impaired Heph activity in other tissues14,17 could secondarily influence intestinal iron transport. Furthermore, intestine-specific ablation of Heph in adult, male mice results in mild iron deficiency15 ; however, the influence of intestinal Heph on iron absorption in female mice, and during physiological conditions and postnatal developmental stages, which lead to increased intestinal iron absorption, has not been clarified. The aim of the current study was thus to test the hypothesis that intestinal Heph is required for optimal iron absorption in mice, especially when iron demand is increased by rapid growth, dietary iron deprivation, acute hemolysis, or pregnancy. To test this postulate, intestine-specific Heph KO male and female mice, and control littermates, were studied. Our results demonstrate that intestinal Heph is essential for optimal iron absorption in male and female weanling and adult mice, and in pregnant mice under physiological conditions, but that it is not necessary for the upregulation of iron transport in adult mice of either sex subjected to dietary iron restriction or acute hemolytic stress.
Methods
Animal procedures
Intestine-specific Heph KO (Hephint) mice (C57BL/6) were described previously.15 Female “floxed” mice (Hephfl/fl) were crossed with male Hephint mice (Hephint/y [villin-Cre positive]). Expected progeny were 25% Hephfl/fl (female littermate control), 25% Hephint/int (female KO), 25% Hephfl/y (male littermate control), and 25% Hephint/y (male KO). Animal care procedures were approved by the University of Florida Institutional Animal Care and Use Committee. For some studies, 5- to 6-week-old mice were fed an AIN-93G–based adequate-iron diet (50 ppm iron; Harlan-Teklad; TD.130018) or a low-iron diet (3-5 ppm iron) (TD.120105) for 5 to 6 weeks. For other experiments, 10- to 12-week-old adult mice were injected intraperitoneally with phosphate-buffered saline (PBS) or a hemolytic agent, phenylhydrazine (PHZ; Sigma-Aldrich; Cat. #P26252; 60 mg/kg body weight),18-21 followed by an iron absorption study 5 days later. Weanling mice used in some experiments were 21 to 23 days of age. Studies on pregnant mice were performed on days 18 to 20 of gestation.
Genotyping of experimental mice
Mice were genotyped at weaning by polymerase chain reaction (PCR) analysis of DNA extracted from tail snips (ZR Genomic DNA-Tissue MiniPrep kit; Zymo Research, Irvine, CA; Cat. #D3051), as previously described.15 Briefly, a duplex PCR reaction was carried out in which the Cre sequence was amplified with 1 primer set, and the T-cell receptor sequence was amplified with another primer set (Table 1). Because all mice had loxP sites flanking the Heph gene, Cre-positive mice were Hephint KOs. The T-cell receptor amplification was a positive control for the PCR reaction. Apex HotStart Master Mix (Genesee Scientific, San Diego, CA; Cat. #42-198) was used for PCR analysis. PCR reaction conditions were as follows: 95°C for 15 minutes, then 35 cycles of 94°C for 30 seconds, 64°C for 1 minute, and 72°C for 1.5 minute, followed by 72°C for 3 minutes. PCR products were run on agarose gels and visualized under UV light.
Hematological status and tissue mineral levels
Hemoglobin (Hb) and hematocrit (Hct) levels were quantified using standard methods.22 Serum and liver nonheme iron content were assessed using a standard colorimetric method.23,24 Inductively coupled plasma mass spectrometry (NexION 300X; Perkin Elmer, Waltham, MA) was used to determine liver and serum total iron and copper concentrations.
Iron absorption
Iron absorption and utilization were determined as described previously.25 Briefly, mice were fasted for 15 to 16 hours and then gavaged with an 59Fe-HCl solution containing 2.5 µCi of 59Fe (Perkin Elmer; Cat. #NEZ037500UC) diluted into 0.2 mL of PBS containing 0.5 M ascorbate, 0.15 M NaCl, and 5 µg FeSO4. Six hours later, mice were given food and water. Mice were euthanized 24 hours after gavage, and blood samples were collected. Radioactivity in each mouse (whole-body), in the entire gastrointestinal tract (unabsorbed iron), and in blood and tissues was quantified by gamma counting (2480 Wizard2; Perkin Elmer). The percentage of the 59Fe dose absorbed was calculated as: ([counts per minute (cpm) in the carcass plus blood minus cpm in the gastrointestinal tract]/[total cpm administered by gavage] × 100).
FOX activity assays
Duodenal enterocytes were isolated as previously described.26 FOX activity in enterocytes was quantified with a colorimetric ferrozine assay,22 using 200 μg of total protein. Data were plotted as Δabsorbance/Δtime (dA/dt) and then quantified as area under the curve (AUC). Results are expressed as 1/AUC (AUC−1), so the values would be positive (because this is a substrate disappearance assay), making it easier to understand the data presentation. Serum ceruloplasmin (Cp) activity was quantified using an amine oxidase assay.22
RNA isolation and quantitative PCR
Total RNA was isolated with RNAzolRT (MRC, Inc, Cincinnati, OH). RNA (1 µg) was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA; Cat. #1708891). Complementary DNA was subsequently amplified by PCR using SYBR green master mix (Bio-Rad) and oligonucleotide primers (Table 1) in a Bio-Rad iCycler C1000. Primers were initially validated by running standard-curve reactions, and melt curves were routinely performed. Cyclophilin A and ribosomal protein S18 were used as internal controls. The 2−ΔΔCt analysis method was used to calculate fold changes in messenger RNA (mRNA) expression.27
Western blot analysis
Proteins isolated from duodenal enterocytes were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. Membranes were blocked in 5% nonfat milk and then incubated with a 1:100 dilution of anti-Heph antiserum (Santa Cruz Biotechnology, Dallas, TX; Cat. #sc-49970) for 16 to 20 hours at 4°C. Subsequently, membranes were washed and then incubated with a 1:5000 dilution of anti-goat secondary antibody for 2 hours. After incubation with a chemiluminescence solution, immunoreactive bands were visualized with a FluorChem instrument. The intensity of immunoreactive bands was normalized to β-actin.
Statistical analysis
Analyses were performed using GraphPad Prism (v 7.0) and Jmp (v 12.2) software. Two- or 3-way analyses of variance (ANOVAs) were used to ascertain interactions between independent (genotype, sex, diet, age, and hemolytic stress) and dependent variables (Hb levels, iron absorption and utilization, and FOX activity). If a significant 2- or 3-way interaction was noted, then multiple pairwise comparisons were made by Tukey's honest significance difference (HSD) post-hoc test. When comparing the means between 2 groups, Student unpaired t test was used. P < .05 was considered statistically significant. Details of the specific statistical approaches used are provided in the figure legends.
Results
Phenotypical characterization and iron absorption under physiological conditions
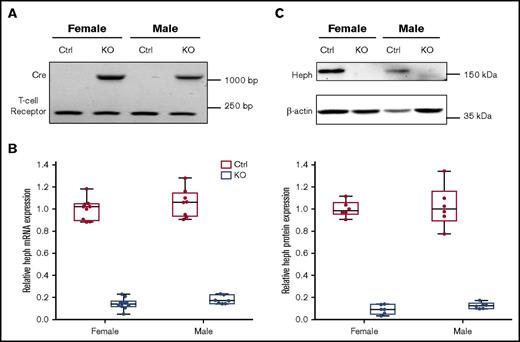
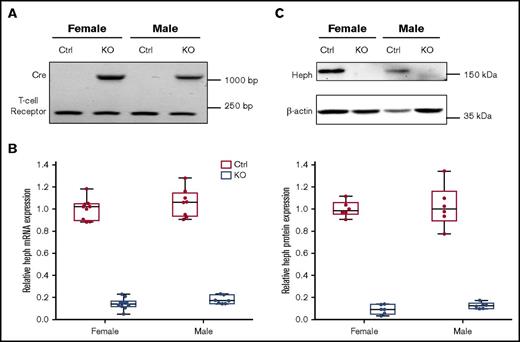
Typical genotyping results are shown in Figure 1A. We verified significant reduction in Heph mRNA (83% in male mice, 86% in female mice) and protein (87% in male mice, 91% in female mice) expression in Cre-positive mice (Figure 1B-C). Hephint mice were viable, fertile, and apparently normal by appearance. To assess overall health, various biologic parameters were assessed in adult mice under physiological conditions (Tables 2 and 3). Male mice were larger, but no genotype effect on body weight was noted. No differences in Hb or Hct levels were noted regardless of genotype or sex. Serum iron (nonheme and total) content was also similar when comparing Hephint mice to controls. Liver iron (nonheme and total) concentrations were, however, lower in Hephint mice of both sexes, as compared with controls, and hepatic hepcidin expression was decreased (most notably in female mice) (Figure 2). Adult Hephint mice are thus iron deficient. Furthermore, intestinal 59Fe absorption was higher in female mice, but genotype did not influence this parameter in either sex. Moreover, enterocyte FOX activity was higher in the Hephint mice of both sexes, despite greatly reduced Heph expression. These experiments revealed notable sex differences in iron metabolism. Also, because iron absorption was not upregulated in Hephint mice, as would have been expected due to low hepcidin expression, intestinal Heph must be required for the stimulation of iron absorption that is normally associated with iron deficiency.
Confirmation of intestinal KO of Heph in adult experimental mice. Genotypes of mice were determined by PCR amplification of the Cre and T-cell receptor sequences from genomic DNA isolated from tail tips followed by agarose gel electrophoresis. The amplicon at ∼1100 bp is the Cre transgene, and the band at ∼230 bp is the T-cell receptor (which was used as a positive control) (A). Expression of Heph mRNA in duodenal enterocytes was assessed by quantitative reverse transcription (qRT)-PCR and normalized to the expression of cyclophilin A (which did not vary significantly between samples) (B). n = 6 mice per group, with duplicate technical replicates. KO of intestinal Heph was also confirmed at the protein level by western blotting (C). The blot shown is representative of 6 independent experiments with similar results. Data are shown as box-and-whisker plots and were analyzed by 2-way ANOVA (B-C). A genotype main effect was noted in regards to Heph mRNA and protein expression (P < .001 for both). No sex main effects or 2-way interactions were noted. Ctrl, control.
Confirmation of intestinal KO of Heph in adult experimental mice. Genotypes of mice were determined by PCR amplification of the Cre and T-cell receptor sequences from genomic DNA isolated from tail tips followed by agarose gel electrophoresis. The amplicon at ∼1100 bp is the Cre transgene, and the band at ∼230 bp is the T-cell receptor (which was used as a positive control) (A). Expression of Heph mRNA in duodenal enterocytes was assessed by quantitative reverse transcription (qRT)-PCR and normalized to the expression of cyclophilin A (which did not vary significantly between samples) (B). n = 6 mice per group, with duplicate technical replicates. KO of intestinal Heph was also confirmed at the protein level by western blotting (C). The blot shown is representative of 6 independent experiments with similar results. Data are shown as box-and-whisker plots and were analyzed by 2-way ANOVA (B-C). A genotype main effect was noted in regards to Heph mRNA and protein expression (P < .001 for both). No sex main effects or 2-way interactions were noted. Ctrl, control.
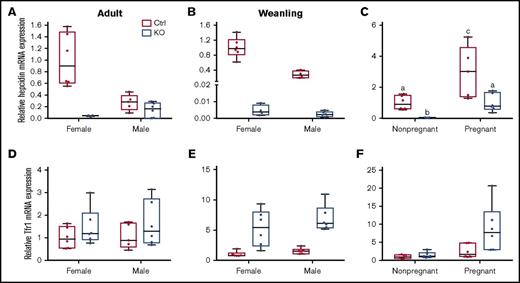
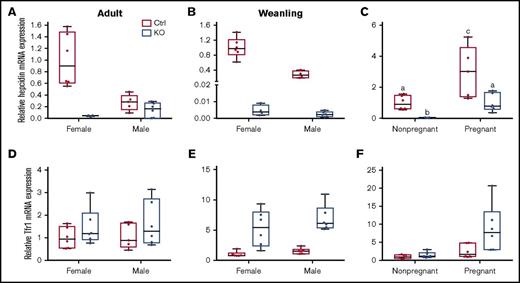
Hepatic hepcidin and Tfr1 mRNA expression in weanling and adult mice of both sexes and genotypes, and in pregnant mice of both genotypes. qRT-PCR was performed to assess hepcidin (A-C) and Tfr1 (D-F) mRNA expression levels in liver samples from adult (A,D), weanling (B,E), and pregnant or nonpregnant female (C,F) mice. Homogeneity of variances was assessed by Levene’s test. Because there was no homogeneity of variance in these data sets (except for Tfr1 expression levels in adults), data were log10 transformed prior to statistical analysis. For ease of interpretation, however, the nontransformed data are shown in the figure. Data are presented as box-and-whisker plots and were analyzed by 2-way ANOVA. Data from experimental genes were normalized to the expression of Rps18. A significant genotype main effect was noted for hepcidin mRNA expression in adult mice (P = .0011) (A). Sex (P = .0013) and genotype (P < .0001) main effects were noted for hepcidin mRNA expression in weanling mice (B). A significant 2-way interaction was noted for pregnancy × genotype (P = .0009), and significant sex (P < .0001) and genotype (P = .0001) main effects were additionally noted for hepcidin mRNA expression in pregnant mice (as compared to nonpregnant females of a similar age) (C). Because a significant 2-way interaction was noted, multiple pairwise comparisons were made by Tukey's HSD post-hoc test; letters atop bars indicate statistically significant differences (P < .05). Tfr1 expression data from adult mice (D) revealed no significant main effects or interactions. In weanling mice, significant sex (P = .0279) and genotype main effects (P < .0001) were noted in regards to Tfr1 mRNA expression (E). The main effects of pregnancy (P = .0001) and genotype (P = .0053) on Tfr1 mRNA expression were significant when pregnant mice were compared to nonpregnant female mice (F). n = 5-6 mice per group, except for the adult, control female group, which had 3 mice, and the weanling, KO female group, which had 4 mice. Each PCR reaction was run in duplicate and Ct values were averaged.
Hepatic hepcidin and Tfr1 mRNA expression in weanling and adult mice of both sexes and genotypes, and in pregnant mice of both genotypes. qRT-PCR was performed to assess hepcidin (A-C) and Tfr1 (D-F) mRNA expression levels in liver samples from adult (A,D), weanling (B,E), and pregnant or nonpregnant female (C,F) mice. Homogeneity of variances was assessed by Levene’s test. Because there was no homogeneity of variance in these data sets (except for Tfr1 expression levels in adults), data were log10 transformed prior to statistical analysis. For ease of interpretation, however, the nontransformed data are shown in the figure. Data are presented as box-and-whisker plots and were analyzed by 2-way ANOVA. Data from experimental genes were normalized to the expression of Rps18. A significant genotype main effect was noted for hepcidin mRNA expression in adult mice (P = .0011) (A). Sex (P = .0013) and genotype (P < .0001) main effects were noted for hepcidin mRNA expression in weanling mice (B). A significant 2-way interaction was noted for pregnancy × genotype (P = .0009), and significant sex (P < .0001) and genotype (P = .0001) main effects were additionally noted for hepcidin mRNA expression in pregnant mice (as compared to nonpregnant females of a similar age) (C). Because a significant 2-way interaction was noted, multiple pairwise comparisons were made by Tukey's HSD post-hoc test; letters atop bars indicate statistically significant differences (P < .05). Tfr1 expression data from adult mice (D) revealed no significant main effects or interactions. In weanling mice, significant sex (P = .0279) and genotype main effects (P < .0001) were noted in regards to Tfr1 mRNA expression (E). The main effects of pregnancy (P = .0001) and genotype (P = .0053) on Tfr1 mRNA expression were significant when pregnant mice were compared to nonpregnant female mice (F). n = 5-6 mice per group, except for the adult, control female group, which had 3 mice, and the weanling, KO female group, which had 4 mice. Each PCR reaction was run in duplicate and Ct values were averaged.
Iron absorption in adult mice with iron-deficiency anemia (IDA)
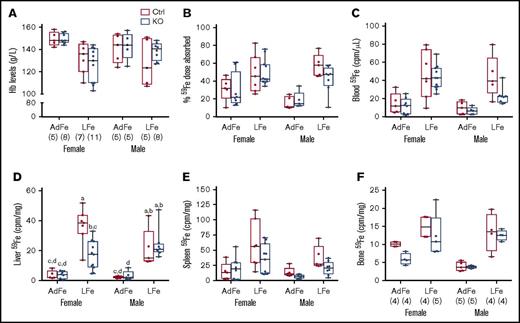
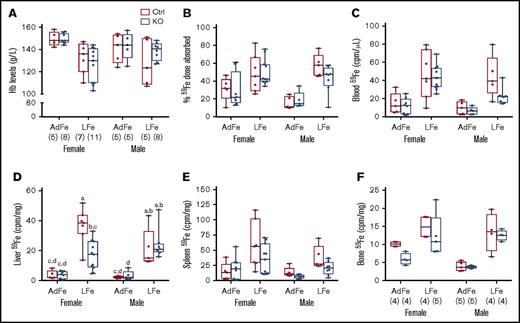
Chronic iron deprivation impairs erythropoiesis, thus causing anemia, and stimulates intestinal iron absorption. Therefore, we sought to determine whether intestinal Heph is required for the upregulation of iron absorption in mice fed a low-iron diet. Dietary iron restriction decreased blood Hb levels, enhanced intestinal iron absorption, and increased tissue 59Fe accumulation (Figure 3), but importantly, none of these parameters varied by genotype. Moreover, hepatic 59Fe accumulation was lower in female Hephint mice (than in controls) after consumption of the low-iron diet, despite apparently normal intestinal iron absorption (Figure 3D). Radiotracer iron uptake by the liver may not, however, accurately reflect overall hepatic iron levels (which are likely similar [and low] in all experimental mice given the comparable levels of anemia). Collectively, these observations demonstrate that intestinal Heph is not required to support the increased iron absorption associated with IDA in adult mice.
Intestinal Heph is not required for the enhancement of iron absorption after dietary iron restriction. Young, adult mice were placed on iron-adequate (AdFe) or low-iron (LFe) diets for 5 to 6 weeks, and then iron absorption was measured after oral gavage of 59Fe. Blood Hb levels were determined at euthanization (A). Iron absorption (percent) and tissue iron accumulation (cpm) are also shown (B-F). Data are shown as box-and-whisker plots and were analyzed by 3-way ANOVA. A significant diet main effect was noted for all measured parameters (P < .006 for all). A sex main effect was also noted for blood 59Fe levels (P = .0385). A diet × sex interaction was noted for Hb levels (P = .0311). Genotype × sex and 3-way interactions were also noted for liver 59Fe accumulation (P = .0261 for both). Because a significant 3-way interaction was noted for the latter, differences between individual groups were assessed with Tukey's HSD post-hoc test and are noted in panel D; letters atop bars indicate statistically significant differences (P < .05). The number of mice in each group is indicated in parentheses beneath panel A and is the same for data shown in all panels, unless otherwise noted. Also, note that the iron absorption data from control animals are presented in numerical form in Table 1, but all assays were done simultaneously with adults of both sexes and genotypes.
Intestinal Heph is not required for the enhancement of iron absorption after dietary iron restriction. Young, adult mice were placed on iron-adequate (AdFe) or low-iron (LFe) diets for 5 to 6 weeks, and then iron absorption was measured after oral gavage of 59Fe. Blood Hb levels were determined at euthanization (A). Iron absorption (percent) and tissue iron accumulation (cpm) are also shown (B-F). Data are shown as box-and-whisker plots and were analyzed by 3-way ANOVA. A significant diet main effect was noted for all measured parameters (P < .006 for all). A sex main effect was also noted for blood 59Fe levels (P = .0385). A diet × sex interaction was noted for Hb levels (P = .0311). Genotype × sex and 3-way interactions were also noted for liver 59Fe accumulation (P = .0261 for both). Because a significant 3-way interaction was noted for the latter, differences between individual groups were assessed with Tukey's HSD post-hoc test and are noted in panel D; letters atop bars indicate statistically significant differences (P < .05). The number of mice in each group is indicated in parentheses beneath panel A and is the same for data shown in all panels, unless otherwise noted. Also, note that the iron absorption data from control animals are presented in numerical form in Table 1, but all assays were done simultaneously with adults of both sexes and genotypes.
Iron absorption in adult mice with hemolytic anemia
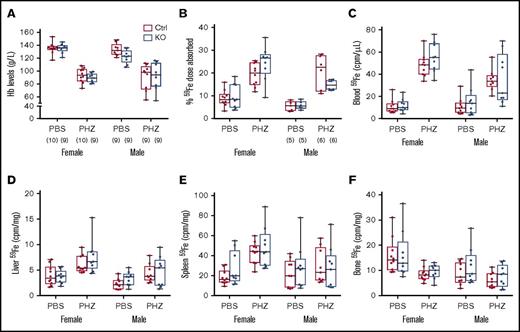
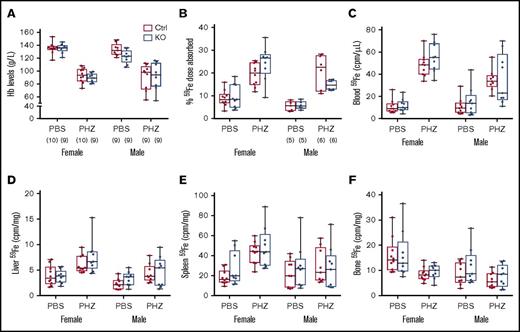
Hemolysis increases erythropoietic demand and stimulates intestinal iron transport. We thus sought to determine whether intestinal Heph is necessary for the upregulation of iron absorption in adult mice in response to acute hemolytic stress. Experimentally, a common way to stimulate erythropoiesis is by injecting mice with a hemolytic agent (eg, PHZ). PHZ treatment decreased blood Hb levels and enhanced iron absorption in mice of both sexes, but these parameters were not influenced by genotype (Figure 4). PHZ treatment also increased 59Fe accumulation in blood, liver, and bone, but again, no genotype differences were noted. These experimental findings suggest that intestinal Heph is not required to upregulate iron absorption during hemolytic anemia in adult male and female mice.
Intestinal Heph is not essential for the enhancement of iron absorption after induction of acute hemolysis. Adult mice were injected with the hemolytic agent (PHZ) to stimulate erythropoiesis. Five days later, iron absorption and utilization were determined. Hb levels were measured from blood samples taken at euthanization (A). Iron (59Fe) absorption (B) and tissue distribution (C-F) were quantified by well-established methods. Data are shown as box-and-whisker plots and were analyzed by 3-way ANOVA. No significant 3-way interactions were noted. A PHZ main effect was noted for all measured parameters (P < .0001 for all). A sex main effect was noted for 59Fe absorption (P = .0068) and for iron accumulation in blood, liver, and bone (P < .011 for all), but no genotype main effects were observed for these parameters. PHZ × sex interactions were noted for blood (P = .0016) and spleen (P = .0107) 59Fe accumulation, and a genotype × sex interaction was observed for 59Fe absorption (P = .0419). The number of mice in each group is indicated in parentheses beneath panel A and is the same for the data shown in all panels, unless otherwise indicated.
Intestinal Heph is not essential for the enhancement of iron absorption after induction of acute hemolysis. Adult mice were injected with the hemolytic agent (PHZ) to stimulate erythropoiesis. Five days later, iron absorption and utilization were determined. Hb levels were measured from blood samples taken at euthanization (A). Iron (59Fe) absorption (B) and tissue distribution (C-F) were quantified by well-established methods. Data are shown as box-and-whisker plots and were analyzed by 3-way ANOVA. No significant 3-way interactions were noted. A PHZ main effect was noted for all measured parameters (P < .0001 for all). A sex main effect was noted for 59Fe absorption (P = .0068) and for iron accumulation in blood, liver, and bone (P < .011 for all), but no genotype main effects were observed for these parameters. PHZ × sex interactions were noted for blood (P = .0016) and spleen (P = .0107) 59Fe accumulation, and a genotype × sex interaction was observed for 59Fe absorption (P = .0419). The number of mice in each group is indicated in parentheses beneath panel A and is the same for the data shown in all panels, unless otherwise indicated.
Iron and copper status, FOX activity, and iron absorption in weanling mice
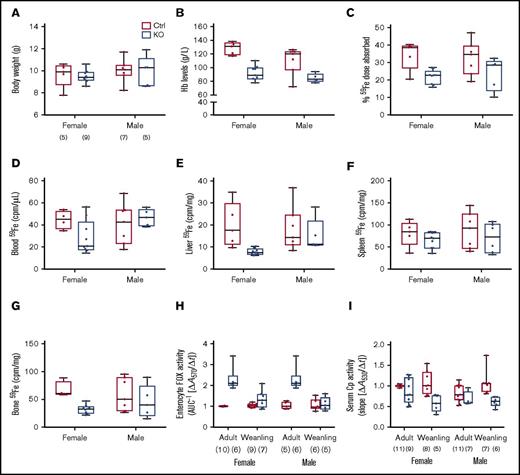
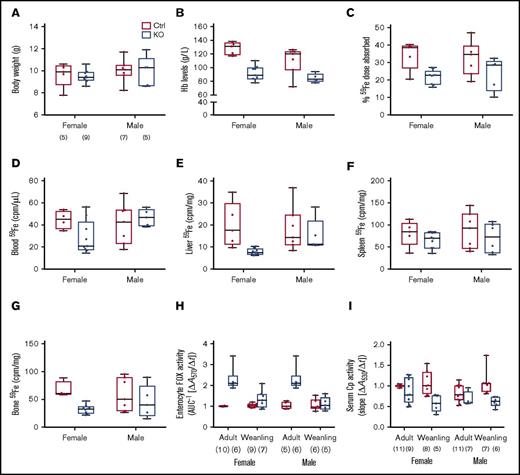
Sla and Heph (whole-body) KO mice are anemic during the rapid postnatal growth period, and intestinal iron absorption is impaired15,28,29 ; however, the specific contribution of intestinal Heph at this developmental stage has not been established. Various physiological parameters and iron absorption were thus assessed in weanling mice of both sexes and genotypes. Final body weights were the same irrespective of sex or genotype (Figure 5A). Serum Hb levels (Figure 5B) and hepatic iron stores (Table 3) were lower in male and female Hephint mice. Hepatic hepcidin expression was also dramatically reduced (Figure 2B), and Tfr1 expression was increased (Figure 2E). Weanling Hephint mice are thus iron deficient and anemic. Furthermore, iron absorption (Figure 5C) and liver (Figure 5E) and bone (Figure 5G) 59Fe accumulation were also lower in Hephint mice. Intestinal Heph thus appears to be essential for optimal iron absorption in the weanling period.
Intestinal iron absorption is impaired in weanling Hephint/intand Hephint/ymice. Iron absorption and tissue iron accumulation were quantified in weanling mice, by our standard protocol. Body weights at euthanization (A), blood HB (B), iron absorption (C), and tissue iron accumulation (D-G) in experimental mice are shown. Data are presented as box-and-whisker plots and were analyzed by 2-way ANOVA. No significant 2-way interactions were noted. A sex main effect was noted for Hb levels (P = .0183). Genotype main effects were noted for Hb levels (P < .0001), iron absorption (P = .0022), and liver (P = .0298) and bone (P = .0101) 59Fe accumulation. The number of mice in each group is indicated beneath panel A and is the same for data shown in panels B-G. Moreover, enterocyte FOX (H) and serum Cp (I) activities were assessed in weanling mice and also in adults. Data are shown as means ± SD and were analyzed by 3-way ANOVA. Age and genotype main effects were noted for enterocyte FOX activity, as was an age × genotype interaction (all P < .0001). For serum Cp activity, a genotype main effect (P < .0001) and an age × genotype interaction (P < .005) were noted. No significant 3-way interactions were noted for either of these parameters. Also, note that the enterocyte FOX and serum Cp data from adult animals are presented in numerical form in Table 1, but all assays were done simultaneously with adults and weanling mice of both genotypes. The number of mice in each group is shown in parentheses below panels H and I.
Intestinal iron absorption is impaired in weanling Hephint/intand Hephint/ymice. Iron absorption and tissue iron accumulation were quantified in weanling mice, by our standard protocol. Body weights at euthanization (A), blood HB (B), iron absorption (C), and tissue iron accumulation (D-G) in experimental mice are shown. Data are presented as box-and-whisker plots and were analyzed by 2-way ANOVA. No significant 2-way interactions were noted. A sex main effect was noted for Hb levels (P = .0183). Genotype main effects were noted for Hb levels (P < .0001), iron absorption (P = .0022), and liver (P = .0298) and bone (P = .0101) 59Fe accumulation. The number of mice in each group is indicated beneath panel A and is the same for data shown in panels B-G. Moreover, enterocyte FOX (H) and serum Cp (I) activities were assessed in weanling mice and also in adults. Data are shown as means ± SD and were analyzed by 3-way ANOVA. Age and genotype main effects were noted for enterocyte FOX activity, as was an age × genotype interaction (all P < .0001). For serum Cp activity, a genotype main effect (P < .0001) and an age × genotype interaction (P < .005) were noted. No significant 3-way interactions were noted for either of these parameters. Also, note that the enterocyte FOX and serum Cp data from adult animals are presented in numerical form in Table 1, but all assays were done simultaneously with adults and weanling mice of both genotypes. The number of mice in each group is shown in parentheses below panels H and I.
Enterocyte FOX and serum Cp activities were also assessed in weanlings, as we hypothesized that other FOXs could be activated when Heph activity was diminished. We also compared these parameters with adult mice to determine potential differences between these developmental stages. Interestingly, enterocyte FOX activity was higher in Hephint mice of both ages, but the magnitude of increase was less in the weanlings (Figure 5H). Conversely, serum Cp activity was lower in Hephint mice of both ages, but the decrease was more pronounced in weanling mice (Figure 5I). Consistent with this, serum copper was lower in adult and weanling Hephint mice (Table 3). These observations suggest that: (1) enterocyte FOXs partially compensate for the lack of intestinal Heph in adult mice under physiological conditions, but that these non-Heph FOXs are either not expressed or are otherwise insufficient to maintain adequate iron absorption during the weanling period; and (2) decreases in serum Cp activity, particularly in weanling mice, may contribute to the impairment of iron absorption.
Iron and copper status, FOX activity, and iron absorption in pregnant mice
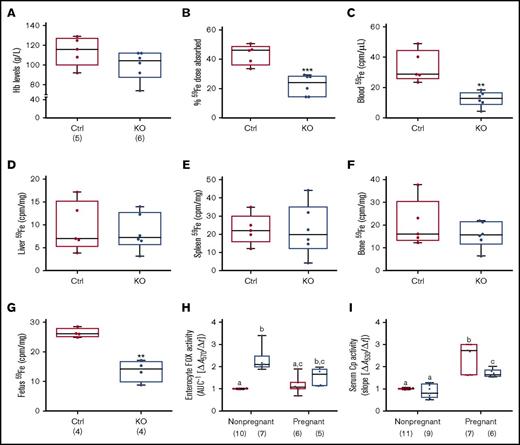
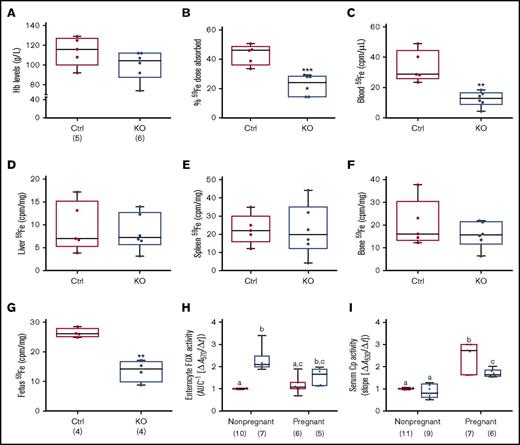
Iron absorption is enhanced during pregnancy due to increased demand,30-34 but whether intestinal Heph is required for this increase in iron transport is unknown. Late-term, pregnant mice of both genotypes were thus used to elucidate the role of Heph in maternal iron absorption. Serum iron did not vary by genotype (Table 3), but there was a trend toward decreased Hb levels in pregnant Hephint female mice (P = .1465) (Figure 6A). Moreover, hepatic iron stores were lower in pregnant Hephint female mice, as compared with pregnant controls (Table 3). Iron absorption was also impaired in pregnant KO female mice (Figure 6B), and consistent with this, 59Fe levels in the blood (Figure 6C) and in the developing fetuses were lower (Figure 6G). These observations thus support the postulate that intestinal Heph is required for optimal iron absorption during pregnancy.
Intestinal Heph is necessary for optimal iron absorption and iron delivery to the developing fetuses during pregnancy. Iron absorption and utilization were assessed in pregnant mice. Nonpregnant females of a similar age were used as controls. Hb levels were measured at euthanization (A). Iron absorption (B), tissue utilization (D-F), and delivery to the fetuses (G) were also quantified. Data are presented as means ± SD and were analyzed by Student unpaired t test (**P < .01; ***P < .001). The number of mice in each group is indicated beneath panel A and is the same for all data shown in panels B-G. Moreover, enterocyte FOX (H) and serum Cp (I) activities were assessed in pregnant and nonpregnant mice. Data are presented as box-and-whisker plots and were analyzed by 2-way ANOVA. For enterocyte FOX activity, a genotype main effect (P < .0001) and a 2-way pregnancy × genotype interaction (P = .0326) were noted. For serum Cp activity, pregnancy (P < .0001) and genotype (P = .0005) main effects were noted as well as a significant 2-way pregnancy × genotype interaction (P = .0061). When significant 2-way interactions were noted, multiple pairwise comparisons were made by Tukey's HSD post-hoc test; letters atop bars indicate statistically significant differences (P < .05). Also, note that enterocyte FOX and serum Cp data from nonpregnant female animals are also presented in numerical form in Table 1, but all assays were done simultaneously in pregnant and nonpregnant mice of both genotypes. The number of mice in each group is shown in parentheses below panels H and I.
Intestinal Heph is necessary for optimal iron absorption and iron delivery to the developing fetuses during pregnancy. Iron absorption and utilization were assessed in pregnant mice. Nonpregnant females of a similar age were used as controls. Hb levels were measured at euthanization (A). Iron absorption (B), tissue utilization (D-F), and delivery to the fetuses (G) were also quantified. Data are presented as means ± SD and were analyzed by Student unpaired t test (**P < .01; ***P < .001). The number of mice in each group is indicated beneath panel A and is the same for all data shown in panels B-G. Moreover, enterocyte FOX (H) and serum Cp (I) activities were assessed in pregnant and nonpregnant mice. Data are presented as box-and-whisker plots and were analyzed by 2-way ANOVA. For enterocyte FOX activity, a genotype main effect (P < .0001) and a 2-way pregnancy × genotype interaction (P = .0326) were noted. For serum Cp activity, pregnancy (P < .0001) and genotype (P = .0005) main effects were noted as well as a significant 2-way pregnancy × genotype interaction (P = .0061). When significant 2-way interactions were noted, multiple pairwise comparisons were made by Tukey's HSD post-hoc test; letters atop bars indicate statistically significant differences (P < .05). Also, note that enterocyte FOX and serum Cp data from nonpregnant female animals are also presented in numerical form in Table 1, but all assays were done simultaneously in pregnant and nonpregnant mice of both genotypes. The number of mice in each group is shown in parentheses below panels H and I.
Enterocyte FOX and serum Cp activities were also assessed in pregnant mice of both genotypes and compared with nonpregnant female mice of a similar age. Enterocyte FOX activity did not vary by genotype in pregnant Hephint mice (Figure 6H), unlike in nonpregnant female mice (where it was higher in the KOs). Serum Cp activity was higher in pregnant mice of both genotypes, but the increase was less in Hephint mice. Consistent with this, liver and serum copper levels were also higher in pregnant Hephint and control mice. These findings thus suggest that: (1) non-Heph FOXs partially compensate for the lack of Heph in nonpregnant female mice, but are insufficient to support optimal iron transport during pregnancy; and (2) increased Cp activity may support enhanced intestinal iron absorption during pregnancy, but less significant increases in Cp activity in pregnant Hephint mice may have contributed to the noted impairment of iron transport.
Discussion
Mice lacking Heph (whole-body KOs) or expressing a mutant form of Heph (sla mice) display moderate IDA during the postnatal growth period, but the anemia resolves as mice mature.15 Although these mice have reduced hepatic iron stores as adults, indicative of mild iron deficiency, they can assimilate adequate amounts of dietary iron to achieve normal adult body size and maintain adequate hematological parameters. Furthermore, initial characterization of mice with greatly reduced Heph expression in the intestine (same as the mice used in this study) revealed an even milder iron-deficient phenotype in adulthood.15 One possible explanation for these observations is that iron requirements are lower in adults (than in growing mice), so enzymatic oxidation of iron is less important. Dissolved oxygen in the interstitial fluids or other FOXs could provide sufficient oxidizing equivalents to prevent the development of more severe iron deficiency in adult Hephint mice. However, because ablation (or mutation) of Heph results in IDA during the rapid postnatal growth period when iron demand is higher, it is a logical postulate that an oxidase (Heph and/or a different FOX) is required for adequate iron absorption during physiological conditions in which intestinal iron transport is stimulated. This investigation was thus undertaken to assess the requirement of intestinal Heph for optimal iron absorption in anemic/hypoxic adult mice (caused by dietary insufficiency or acute hemolysis), in weanling mice, and during pregnancy. We further sought to determine whether the activities of other unidentified intestinal FOXs or serum Cp were altered in Hephint mice, perhaps providing insight into potential compensatory mechanisms when intestinal Heph expression is diminished.
We first assessed the iron-related phenotype of Hephint mice and quantified iron absorption under physiological conditions. These experiments demonstrated that intestinal Heph is not required to maintain normal serum Hb, Hct, and iron (total and nonheme) levels in adult mice of both sexes. Male and female Hephint mice, however, had lower total and nonheme hepatic iron concentrations and decreased hepatic hepcidin expression (consistent with the initial characterization of these mice15 ). This phenotype thus reflects what has been termed Stage I Iron Depletion or “Early Negative Iron Balance,”35,36 in which storage iron is decreased but circulating iron levels and iron delivery to the erythron are normal. Impaired iron absorption is the most plausible explanation for the noted depletion of liver iron stores. Although 24-hour 59Fe radiotracer uptake was similar in controls and Hephint mice of both sexes, it is possible that decreased transport rates could only be detected at earlier time points. Nonetheless, even if transport rates varied, bulk flow of iron was not different between genotypes. More importantly, given the observed suppression of hepatic hepcidin expression, intestinal iron absorption would be expected to increase (especially in female mice). These data demonstrate that basal iron transport capacity is similar between genotypes, but that the lack of intestinal Heph impairs the ability of Hephint mice to appropriately upregulate iron absorption when hepcidin expression is low. More significant iron deficiency may have been prevented by the induction of non-Heph FOXs in duodenal enterocytes. We thus conclude that intestinal Heph is required for enhancing iron absorption and replenishing hepatic iron stores in Stage I Iron Depletion.
The next logical question related to whether intestinal Heph was required for the upregulation of iron transport in adult mice during states of more significant iron deficiency, which impairs erythropoiesis, resulting in anemia with concurrent hypoxia. This condition, which is the most severe form of iron depletion, has been termed Stage IV Iron Deficiency,35,36 and can be modeled in rodents by feeding them a low-iron diet. Dietary iron deprivation suppresses hepatic hepcidin expression and stimulates iron absorption. The next experiments were thus designed to examine iron absorption in adult control and Hephint mice with IDA. As anticipated, low-iron feeding of male and female mice decreased blood Hb levels and increased iron absorption; however, unexpectedly, neither of these parameters were influenced by genotype. Furthermore, another condition that causes anemia and stimulates intestinal iron absorption is acute hemolysis, which can be modeled in mice by using a hemolytic agent, PHZ. We thus sought to test the function of intestinal Heph in mice with hemolytic anemia. PHZ treatment caused significant anemia and also stimulated iron absorption, with both of these parameters being unaffected by sex or genotype. Noted increases in iron absorption in anemic, adult mice presumably occurred due to depression of hepatic hepcidin expression37 and Hif2α-mediated increases in the expression of intestinal iron transporters.38-40 We thus conclude that intestinal Heph is required in adult mice to upregulate iron transport during Stage I Iron Depletion (as described in the previous paragraph), but not during Stage IV Iron Deficiency caused by dietary iron deprivation or acute hemolysis. The most plausible explanation for these observations is that other FOXs are activated during iron-deficiency and hemolytic anemia, possibly compensating for the lack of intestinal Heph. We have, in fact, previously reported that Heph (whole-body) KO mice express alternative intestinal FOXs,26 and also that Cp activity is enhanced during IDA in rats.22
We next sought to assess the impact of intestinal Heph KO on iron absorption in weanling mice, a developmental stage when iron demand is high.41,42 Given the previously noted IDA in young sla and whole-body Heph KO mice,15 we postulated that Hephint mice would be anemic and that intestinal iron absorption would be blunted. Indeed, intestinal iron absorption was impaired in weanling Hephint mice of both sexes, likely explaining the noted decrements in serum Hb and iron, liver iron, and hepatic hepcidin expression. Importantly, enterocyte (non-Heph) FOX activity did not increase in weanling Hephint mice (as it did in adult Hephint mice), and a decrement in serum Cp activity was noted, both being factors that could contribute to the decrease in dietary iron assimilation. It is puzzling that lack of intestinal Heph impairs iron absorption in weanling mice, yet assimilation of dietary iron is unaffected by diminution of Heph expression during diet-induced and hemolytic anemia in adults. Conceivably, iron requirements are greater in weanling mice than in adult, anemic mice, so Heph FOX activity could be functionally more important in weanlings. Alternatively, non-hepcidin-related mechanisms that increase intestinal iron absorption in anemic/hypoxic adult mice (eg, Hif2α-mediated increases in iron transporter expression) may not be operative in weanling Hephint mice. Additional experimentation is required to understand the differential influence of intestinal Heph during various physiological/developmental states that stimulate iron absorption.
Last, we examined the role of intestinal Heph in pregnancy, another condition that increases iron requirements and stimulates intestinal iron absorption. Lack of intestinal Heph expression impaired iron absorption, depleted hepatic iron stores, and depressed hepcidin mRNA expression during late-term pregnancy. Iron delivery to the developing fetuses was also diminished, suggesting that fetal iron deficiency may contribute to the noted IDA in weanling Hephint mice. Furthermore, serum copper levels and Cp activity increased in pregnant mice of both genotypes, but the magnitude of the increase was less in Hephint mice than in controls. Lower Cp activity could be one factor that contributed to the diminution of iron transport in pregnant Hephint mice.
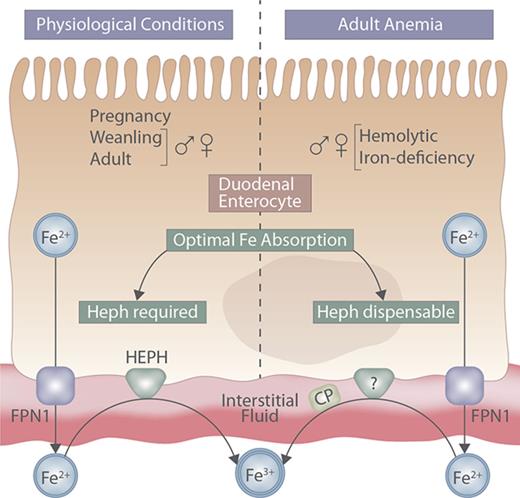
In summary, this investigation demonstrates that intestinal Heph is sometimes, but not always, required for adequate assimilation of dietary iron. Here, previous investigations have been extended to define the physiological role of intestinal Heph in male and female adult and weanling mice, in pregnant mice, and in adult mice of both sexes subjected to low-iron and hemolytic stress. Intestinal Heph is required to maintain iron homeostasis in weanlings and adults of both sexes and in pregnant mice under physiological conditions. Conversely, however, intestinal Heph is not necessary for the upregulation of intestinal iron transport in adult male and female mice with iron deficiency or hemolytic anemia. An imperative for future inquiry is to identify the intestinal FOXs in adult mice that compensate for the lack of Heph, because these could emerge as potential therapeutic targets to modulate iron absorption during perturbations of iron homeostasis (which commonly occur in adult humans). Moreover, this investigation has provided mechanistic insight into the process of intestinal iron absorption during the rapid postnatal growth period and during pregnancy, 2 physiological states that are commonly associated with iron deficiency in humans.
Acknowledgments
This investigation was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grants R01 DK074867 and R01 DK109717), and the Office of Dietary Supplements (J.F.C.) and supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia (G.J.A.).
Authorship
Contribution: C.D., J.-H.H., and J.F.C. designed the investigation; C.D. and J.-H.H. performed the experiments; and C.D., J.-H.H., S.G., C.D.V., G.J.A., and J.F.C. analyzed and interpreted the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.D. is Nutrition and Dietetics Department, Namık Kemal University, Tekirdag, Turkey.
Correspondence: James F. Collins, Food Science and Human Nutrition Department, University of Florida, 572 Newell Dr, FSHN Bldg, #441, PO Box 110370, Gainesville, FL 32611; e-mail: jfcollins@ufl.edu.