Key Points
Nine of 10 MMUD HCTs have at least equivalent outcomes as 10 of 10 MUD HCTs using TLI-ATG with low rates of GVHD and nonrelapse mortality.
MMUD HCT using TLI-ATG is well suited for patients with lymphoid malignancies given the low relapse rates without increased GVHD.
Abstract
Many patients lack a fully HLA-matched donor for hematopoietic cell transplantation (HCT), and HLA mismatch is typically associated with inferior outcomes. Total lymphoid irradiation and antithymocyte globulin (TLI-ATG) is a nonmyeloablative conditioning regimen that is protective against graft-versus-host disease (GVHD), and we hypothesized that the protective effect would extend beyond HLA-matched donors. We report outcomes for all consecutively transplanted patients at Stanford University from December 2001 through May 2015 who received TLI-ATG conditioning and HCTs from 8 to 9 out of 10 HLA-mismatched unrelated donors (MMUDs, N = 72) compared with 10 out of 10 HLA-matched unrelated donors (MUDs, N = 193). The median age of the patients was 60 years with a median follow-up of 2 years, and there was a similar distribution of lymphoid and myeloid malignancies in both cohorts. There were no significant differences between MMUD and MUD cohorts in overall survival (46% vs 46% at 5 years, P = .86), disease-free survival (38% vs 28% at 5 years, P = .25), nonrelapse mortality (17% vs 12% at 2 years, P = .34), acute GVHD grades III-IV (6% vs 3% at day +100, P = .61), or chronic GVHD (39% vs 35% at 5 years, P = .49). There was a trend toward less relapse in the MMUD cohort (45% vs 60% at 5 years, hazard ratio: 0.71, P = .094), which was significant for patients with lymphoid malignancies (29% vs 57% at 5 years, hazard ratio: 0.55, P = .044). Achieving full donor chimerism was strongly associated with lower relapse rates. TLI-ATG conditioning may overcome the traditionally poorer outcome associated with HLA-mismatched donors and may be particularly well suited for patients with lymphoid malignancies who lack HLA-matched donors.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for high-risk or refractory hematologic malignancies, but many patients lack a fully HLA-matched donor. For these patients, alternative donor sources include HLA-mismatched unrelated donors (MMUDs), HLA-haploidentical donors, or umbilical cord blood. In the setting of myeloablative HCT, increasing levels of HLA mismatch are associated with higher rates of graft-versus-host disease (GVHD) and nonrelapse mortality (NRM) and poorer overall survival (OS).1,2 Most studies that used reduced-intensity conditioning (RIC) have shown similarly inferior outcomes with MMUDs compared with HLA-matched unrelated donors (MUDs).3,4 However, the specific conditioning regimen used appears to modify the impact of HLA mismatch on posttransplant outcomes. In particular, RIC regimens containing T-cell depleting antibodies, such as alemtuzumab or antithymocyte globulin, may have a lower risk of GVHD and NRM in the setting of MMUD HCT.5,6
Total lymphoid irradiation and antithymocyte globulin (TLI-ATG) is a nonmyeloablative conditioning regimen associated with low rates of acute and chronic GVHD.7,8 The proposed mechanism involves skewing of residual host T-cell subsets to favor regulatory interleukin-4 (IL-4)–producing natural killer T cells that promote the expansion of donor CD4+CD25+FoxP3+ regulatory T cells.9-13 Previous studies that used TLI-ATG conditioning have demonstrated rates of acute GVHD grades II-IV of 5% to 10% and chronic GVHD of 18% to 28%,7,8 which are considerably lower than other RIC regimens, where rates of acute and chronic GVHD range from 20% to 65% and 46% to 73%, respectively.14-18 Despite the low risk of GVHD, graft-versus-tumor (GVT) reactions are maintained as demonstrated by conversion from partial to complete remissions after transplantation and tumor responses outside of the field of lymphoid irradiation.7,8 The safety and antitumor activity of allogeneic HCT with TLI-ATG conditioning have been demonstrated for a variety of lymphoid and myeloid malignancies, including acute and chronic leukemias,7,8 myelodysplastic syndromes and myeloproliferative neoplasms,19 and relapsed and refractory Hodgkin and non-Hodgkin lymphomas after failure of autologous HCT.20
Previous studies of TLI-ATG conditioning primarily report outcomes from patients who received transplants from HLA-matched donors. We hypothesized that the protective effect of TLI-ATG conditioning against GVHD would extend beyond matched donors, but that with mismatching, there would be at least as potent GVT reactions as with matched donors. We therefore compared outcomes in patients with high-risk and refractory lymphoid and myeloid malignancies who received TLI-ATG conditioning and allogeneic HCTs from MMUDs to those with MUDs.
Methods
Patients
We included all consecutively transplanted patients at Stanford University Hospital from 1 December 2001 through 31 May 2015 who received TLI-ATG conditioning and allogeneic HCTs from 10 out of 10 MUDs (N = 193) or 8 to 9 out of 10 MMUDs (N = 72), including 70 patients matching in 9 out of 10 antigens or alleles (HLA-A, -B, -C, -DRB1, and -DQB1) and 2 patients matching in 8 out of 10 antigens or alleles. HLA-DPB1 typing was also available for 12 patients with MMUDs. Killer cell immunoglobulin-like receptor (KIR) ligand matching was evaluated for all patients mismatching at HLA-B or -C loci. All patients provided informed consent in accordance with the Declaration of Helsinki and were enrolled in transplant protocols approved by the Stanford University Institutional Review Board or on treatment plans. The censoring date was the last clinic visit before 31 May 2016, allowing for a minimum follow-up of 12 months for all patients. The patients enrolled had diagnoses of malignant lymphoid or myeloid diseases and were ineligible for conventional myeloablative conditioning due to advanced age or comorbidities. Patients with a left ventricular ejection fraction <30% and uncontrolled congestive heart failure, pulmonary diffusion capacity <35%, Karnofsky performance status <50%, decompensated cirrhosis, or who were pregnant were excluded. Disease status at the start of conditioning was assigned according to consensus guidelines.21,22
Transplant regimen
TLI-ATG conditioning was administered as previously described.7,8 In brief, rabbit antithymocyte globulin (Thymoglobulin; Sanofi Genzyme, Boston, MA) was infused IV at 1.5 mg/kg per day for 5 consecutive days, beginning on day −11 before HCT. Total lymphoid irradiation was administered at a dose of 0.8 Gy/day from day −11 to day −7 and from day −4 to day −2 with 2 additional fractions of 0.8 Gy delivered on day −1 for a total dose of 8 Gy. In accordance with a protocol amendment, patients treated after 1 May 2009 received 1.2 Gy (rather than 0.8 Gy) fractions, scheduled as above, for a total dose of 12 Gy. The radiation fields used have been previously described.7,23 All patients received unmanipulated granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cells on day 0 and received cyclosporine and mycophenolate mofetil for GVHD prophylaxis. In the absence of GVHD, cyclosporine was tapered to discontinuation between days +100 and +180, and mycophenolate mofetil was tapered to discontinuation between days +42 and +96.7,8 All patients were monitored for cytomegalovirus (CMV) and Epstein-Barr virus (EBV) viremia with serum polymerase chain reaction assays, and all patients received bacterial, viral, and fungal prophylaxis according to institutional guidelines. Any CMV copy number >600/mL was treated with valganciclovir induction and maintenance, and EBV copy numbers >10 000/mL were treated with rituximab.
Evaluation of donor chimerism
DNA genotyping of polymorphic markers encoding short tandem repeats was used to quantify donor chimerism in all patients.24 Donor chimerism was evaluated in whole blood and cell subsets by using immunomagnetic beads (Dynal Biotech/Invitrogen, Waltham, MA) coated with monoclonal antibodies against CD3, CD15, CD19, and CD56. Full donor chimerism was defined as achievement of ≥95% donor CD3+ cells in the peripheral blood by day +90. Primary graft failure was defined as failure to surpass 5% donor CD3+ cells, and mixed chimerism was defined as donor CD3+ cells ranging from 5% to 94%.25 Donor lymphocyte infusions were given at the discretion of the attending physician to treat overt relapse or impending graft rejection, but were not used to convert stable mixed chimerism to full donor chimerism.
Study endpoints
The study endpoints included OS, disease-free survival (DFS), and the cumulative incidences of relapse, NRM, and acute and chronic GVHD. OS was defined as the time to death from any cause after transplant, and DFS was defined by the first observation of relapse, progression, or death. Relapse included progressive disease for patients who had measurable disease at the time of transplant. NRM was defined as death from any cause other than disease progression or relapse. GVHD was graded by using current consensus criteria for acute, late acute, and chronic GVHD.26,27 GVHD-free/relapse-free survival (GFRS) was defined as the absence of grade III-IV acute GVHD, chronic GVHD requiring systemic therapy, relapse, or death in the first year post-HCT as previously described.28
Statistical analyses
For time-to-event analyses, Kaplan-Meier curves were used to estimate the probabilities of OS, DFS, and the cumulative incidences of relapse, NRM, and acute and chronic GVHD. Log-rank tests were used to detect differences between groups. Fisher’s exact test was performed to determine the correlation between achieving full donor chimerism and the likelihood of relapse. All P values are two-tailed, and P < .05 was considered significant. Statistical analyses were performed using GraphPad software (San Diego, CA).
Results
Patient and disease characteristics
The baseline characteristics of the 72 patients with MMUDs and 193 patients with MUDs are summarized in Table 1. The median age at the time of transplant was 60 years for both cohorts (range: 21-76 years) with a median follow-up of 2 years (range: 0.1-12.5 years). There were no significant differences between the MMUD and MUD cohorts in underlying diagnoses: acute myeloid leukemia (AML), 32% vs 31%; non-Hodgkin lymphoma: 22% vs 29%; chronic lymphocytic leukemia, 17% vs 17%; myelodysplastic syndrome and myeloproliferative neoplasms, 11% vs 14%; Hodgkin lymphoma, 8% vs 5%; chronic myeloid leukemia, 7% vs 2%; and acute lymphoblastic leukemia, 3% vs 3%. The majority of patients in both cohorts were classified as having intermediate-risk (47% vs 52%) or high-risk disease (42% vs 37%) per the HCT disease risk index.29 The HCT comorbidity index was ≥3 in 24% of patients in the MMUD cohort and 22% of patients in the MUD cohort.
Patient and disease characteristics
| . | Mismatched unrelated donor (N = 72) . | Matched unrelated donor (N = 193) . |
|---|---|---|
| Age and follow-up (range), y | ||
| Median age at time of transplantation | 60 (21-74) | 60 (23-76) |
| Median follow-up | 1.9 (0.2-10.1) | 2.1 (0.1-12.5) |
| Diagnosis | ||
| Lymphoid malignancies | 36 (50) | 104 (54) |
| Non-Hodgkin lymphoma | 16 (22) | 56 (29) |
| Diffuse large B-cell lymphoma | 5 (7) | 17 (9) |
| Follicular lymphoma | 4 (6) | 7 (4) |
| Mantle cell lymphoma | 2 (3) | 24 (12) |
| Other B-cell lymphoma* | 0 (0) | 3 (2) |
| T-cell lymphoma† | 5 (7) | 5 (3) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 12 (17) | 33 (17) |
| Classical Hodgkin lymphoma | 6 (8) | 10 (5) |
| Pre-B acute lymphoblastic leukemia | 2 (3) | 5 (3) |
| Prior autologous transplantation | 9/36 (25) | 34/104 (33) |
| Myeloid malignancies | 36 (50) | 89 (46) |
| Acute myeloid leukemia | 23 (32) | 59 (31) |
| De novo acute myeloid leukemia | 15 (21) | 40 (21) |
| Secondary acute myeloid leukemia‡ | 8 (11) | 19 (10) |
| Myelodysplastic syndrome and myeloproliferative neoplasms | 8 (11) | 27 (14) |
| Chronic myeloid leukemia | 5 (7) | 3 (2) |
| Prior autologous transplantation | 2/36 (6) | 0/89 (0) |
| Prior allogeneic transplantation | 1/36 (3) | 2/89 (2) |
| Disease risk index | ||
| Low risk | 5 (7) | 14 (7) |
| Intermediate risk | 34 (47) | 101 (52) |
| High risk | 30 (42) | 72 (37) |
| Very high risk | 3 (4) | 6 (3) |
| Comorbidity index | ||
| HCT-CI score 0 | 25 (35) | 78 (40) |
| HCT-CI score 1 | 16 (22) | 44 (23) |
| HCT-CI score 2 | 14 (19) | 28 (15) |
| HCT-CI score ≥3 | 17 (24) | 43 (22) |
| Sex mismatch | ||
| Male donor, male recipient | 23 (32) | 70 (36) |
| Male donor, female recipient | 14 (19) | 50 (26) |
| Female donor, male recipient | 22 (31) | 41 (21) |
| Female donor, female recipient | 13 (18) | 32 (17) |
| CMV serologic status | ||
| Donor and/or recipient seropositive | 54 (75) | 130 (67) |
| Donor and recipient seronegative | 18 (25) | 63 (33) |
| Disease status at the start of conditioning | ||
| Lymphoid malignancies | N = 36 | N = 104 |
| Complete remission | 16 (44) | 51 (49) |
| Partial remission | 16 (44) | 46 (44) |
| Stable disease or progressive disease | 4 (11) | 7 (7) |
| Myeloid malignancies | N = 36 | N = 89 |
| First complete remission | 22 (61) | 62 (70) |
| Second complete remission | 6 (17) | 8 (9) |
| Disease status beyond second complete remission | 8 (22) | 19 (21) |
| . | Mismatched unrelated donor (N = 72) . | Matched unrelated donor (N = 193) . |
|---|---|---|
| Age and follow-up (range), y | ||
| Median age at time of transplantation | 60 (21-74) | 60 (23-76) |
| Median follow-up | 1.9 (0.2-10.1) | 2.1 (0.1-12.5) |
| Diagnosis | ||
| Lymphoid malignancies | 36 (50) | 104 (54) |
| Non-Hodgkin lymphoma | 16 (22) | 56 (29) |
| Diffuse large B-cell lymphoma | 5 (7) | 17 (9) |
| Follicular lymphoma | 4 (6) | 7 (4) |
| Mantle cell lymphoma | 2 (3) | 24 (12) |
| Other B-cell lymphoma* | 0 (0) | 3 (2) |
| T-cell lymphoma† | 5 (7) | 5 (3) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 12 (17) | 33 (17) |
| Classical Hodgkin lymphoma | 6 (8) | 10 (5) |
| Pre-B acute lymphoblastic leukemia | 2 (3) | 5 (3) |
| Prior autologous transplantation | 9/36 (25) | 34/104 (33) |
| Myeloid malignancies | 36 (50) | 89 (46) |
| Acute myeloid leukemia | 23 (32) | 59 (31) |
| De novo acute myeloid leukemia | 15 (21) | 40 (21) |
| Secondary acute myeloid leukemia‡ | 8 (11) | 19 (10) |
| Myelodysplastic syndrome and myeloproliferative neoplasms | 8 (11) | 27 (14) |
| Chronic myeloid leukemia | 5 (7) | 3 (2) |
| Prior autologous transplantation | 2/36 (6) | 0/89 (0) |
| Prior allogeneic transplantation | 1/36 (3) | 2/89 (2) |
| Disease risk index | ||
| Low risk | 5 (7) | 14 (7) |
| Intermediate risk | 34 (47) | 101 (52) |
| High risk | 30 (42) | 72 (37) |
| Very high risk | 3 (4) | 6 (3) |
| Comorbidity index | ||
| HCT-CI score 0 | 25 (35) | 78 (40) |
| HCT-CI score 1 | 16 (22) | 44 (23) |
| HCT-CI score 2 | 14 (19) | 28 (15) |
| HCT-CI score ≥3 | 17 (24) | 43 (22) |
| Sex mismatch | ||
| Male donor, male recipient | 23 (32) | 70 (36) |
| Male donor, female recipient | 14 (19) | 50 (26) |
| Female donor, male recipient | 22 (31) | 41 (21) |
| Female donor, female recipient | 13 (18) | 32 (17) |
| CMV serologic status | ||
| Donor and/or recipient seropositive | 54 (75) | 130 (67) |
| Donor and recipient seronegative | 18 (25) | 63 (33) |
| Disease status at the start of conditioning | ||
| Lymphoid malignancies | N = 36 | N = 104 |
| Complete remission | 16 (44) | 51 (49) |
| Partial remission | 16 (44) | 46 (44) |
| Stable disease or progressive disease | 4 (11) | 7 (7) |
| Myeloid malignancies | N = 36 | N = 89 |
| First complete remission | 22 (61) | 62 (70) |
| Second complete remission | 6 (17) | 8 (9) |
| Disease status beyond second complete remission | 8 (22) | 19 (21) |
Data are presented as n (%) unless otherwise indicated.
Other B-cell non-Hodgkin lymphoma includes 1 patient with nodal marginal zone lymphoma and 1 patient with lymphoplasmacytic lymphoma.
T-cell non-Hodgkin lymphoma includes 5 patients with angioimmunoblastic T-cell lymphoma, 2 patients with subcutaneous panniculitis-like T-cell lymphoma, 2 patients with peripheral T-cell lymphoma not otherwise specified, and 1 patient with anaplastic large cell lymphoma.
Secondary acute myeloid leukemia includes patients with an antecedent myelodysplastic syndrome or myeloproliferative neoplasm or therapy-related acute myeloid leukemia.
HLA and KIR ligand mismatching
HLA and KIR ligand mismatching for the 72 patients with MMUDs are summarized in Table 2. Seventy of 72 patients (97%) had a mismatch at a single antigen or allele (9/10 match), including 40% with mismatches at HLA-A, 15% at HLA-B, 17% at HLA-C, 4% at HLA-DRB1, and 21% at HLA-DQB1. Two patients had mismatches at 2 major antigens or alleles (8/10 match). HLA-DPB1 typing was available in 12 patients with MMUDs, 6 of whom had a nonpermissive mismatch at 1 allele (10/12 match) and 2 of whom had nonpermissive mismatches at both alleles (9/12 match). Of note, both patients with a 9 of 12 match developed primary graft failure as did 2 patients with a 10 of 12 match. No patients with an 11 of 12 match developed graft failure. Of 23 patients mismatching at HLA-B or -C loci, 6 patients had KIR ligand mismatch in the GVH direction, and 2 out of 6 of these patients had disease relapse.
HLA and KIR ligand mismatches
| . | n (%) . |
|---|---|
| HLA-MMUDs (N = 72) | |
| Single antigen or allele mismatch (9/10 match) | 70 (97) |
| Mismatch at HLA-A | 29 (40) |
| Mismatch at HLA-B | 11 (15) |
| Mismatch at HLA-C | 12 (17) |
| Mismatch at HLA-DRB1 | 3 (4) |
| Mismatch at HLA-DQB1 | 15 (21) |
| Two antigen or allele mismatch (8/10 match) | 2 (3) |
| Mismatch at HLA-A and HLA-C | 1 (1) |
| Mismatch at HLA-C and HLA-DQB1 | 1 (1) |
| Patients with available HLA-DPB1 typing (N = 12), n/N | |
| No nonpermissive HLA-DPB1 mismatch (11/12 match) | 4/12 |
| Nonpermissive HLA-DPB1 mismatch at 1 antigen (10/12 match) | 6/12 |
| Nonpermissive HLA-DPB1 mismatch at both antigens (9/12 match) | 2/12 |
| KIR ligand mismatch (N = 8), n/N | |
| KIR ligand mismatch in graft-versus-host direction | 6/8 |
| KIR ligand mismatch in host-versus-graft direction | 2/8 |
| . | n (%) . |
|---|---|
| HLA-MMUDs (N = 72) | |
| Single antigen or allele mismatch (9/10 match) | 70 (97) |
| Mismatch at HLA-A | 29 (40) |
| Mismatch at HLA-B | 11 (15) |
| Mismatch at HLA-C | 12 (17) |
| Mismatch at HLA-DRB1 | 3 (4) |
| Mismatch at HLA-DQB1 | 15 (21) |
| Two antigen or allele mismatch (8/10 match) | 2 (3) |
| Mismatch at HLA-A and HLA-C | 1 (1) |
| Mismatch at HLA-C and HLA-DQB1 | 1 (1) |
| Patients with available HLA-DPB1 typing (N = 12), n/N | |
| No nonpermissive HLA-DPB1 mismatch (11/12 match) | 4/12 |
| Nonpermissive HLA-DPB1 mismatch at 1 antigen (10/12 match) | 6/12 |
| Nonpermissive HLA-DPB1 mismatch at both antigens (9/12 match) | 2/12 |
| KIR ligand mismatch (N = 8), n/N | |
| KIR ligand mismatch in graft-versus-host direction | 6/8 |
| KIR ligand mismatch in host-versus-graft direction | 2/8 |
Engraftment and chimerism
Primary graft failure occurred in 10% of patients in the MMUD cohort and 7% of patients in the MUD cohort, with a higher rate of graft failure observed among patients with superimposed nonpermissive HLA-DPB1 mismatching as indicated above. Anti-HLA donor-specific antibodies were not detected in any patients with primary graft failure. Full donor CD3+ chimerism was achieved in 65% and mixed chimerism in 25% of patients by day +90 in the MMUD cohort compared with 55% and 38%, respectively, in the MUD cohort. Full donor chimerism was achieved more rapidly in the MMUD cohort compared with the MUD cohort, but did not differ by day +60 or day +90 (supplemental Figure 1).
Survival, NRM, GVHD, and relapse
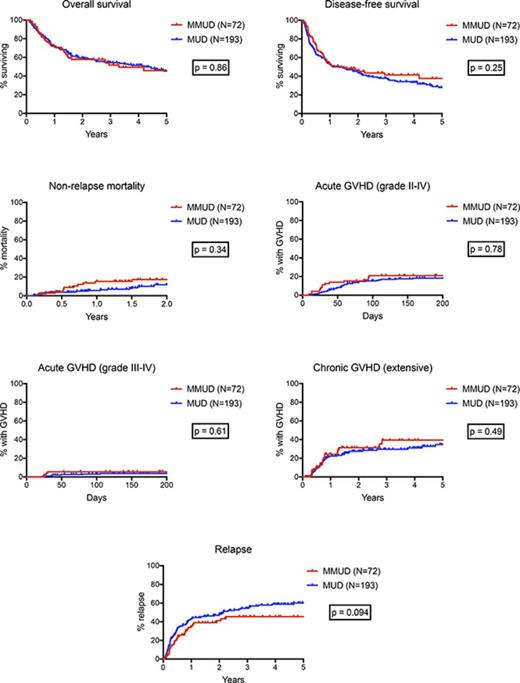
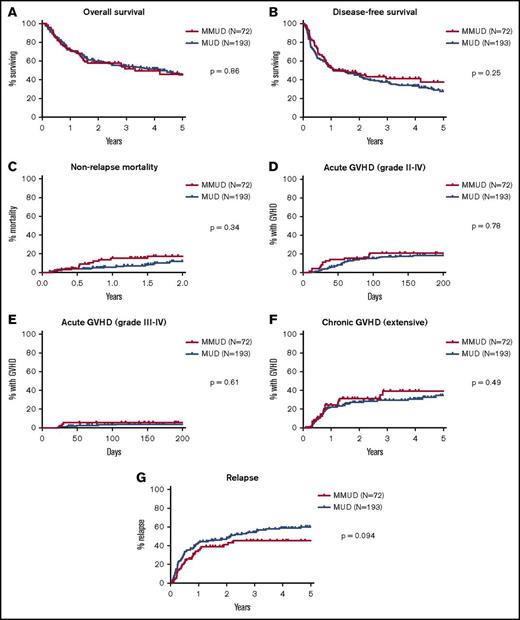
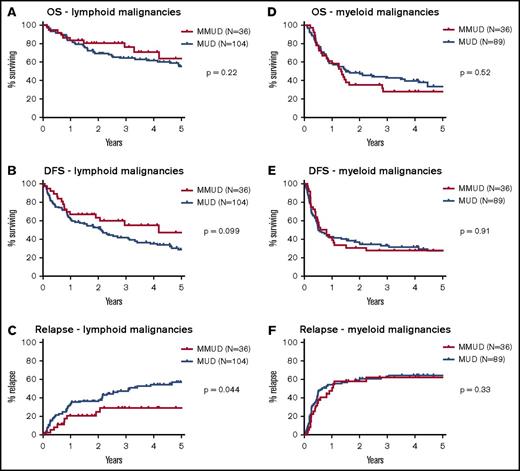
Posttransplant outcomes, including OS, DFS, NRM, acute and chronic GHVD, and relapse for both MMUD and MUD cohorts are shown in Figure 1. There were no significant differences between the MMUD and MUD cohorts in OS (58% vs 60% at 2 years, 46% vs 46% at 5 years, P = .86) or DFS (46% vs 44% at 2 years, 38% vs 28% at 5 years, P = .25). NRM at 2 years was not significantly different (17% vs 12%, P = .34), nor was the cumulative incidence of acute GVHD at day +100 (grades II-IV: 21% vs 15%, P = .78; grades III-IV: 6% vs 3%, P = .61) or chronic GVHD (31% vs 27% at 2 years, 39% vs 35% at 5 years, P = .49). There was a trend toward a lower rate of relapse in the MMUD cohort (40% vs 48% at 2 years, 45% vs 60% at 5 years, hazard ratio: 0.71, P = .094). For patients with lymphoid malignancies, the cumulative incidence of relapse was significantly lower in the MMUD group (25% vs 38% at 2 years, 29% vs 57% at 5 years, hazard ratio: 0.55, P = .044), and there was a trend toward improved DFS (63% vs 52% at 2 years, 47% vs 28% at 5 years, hazard ratio: 0.64, P = .099, Figure 2). For patients with myeloid malignancies, there was no significant difference in relapse between the MMUD and MUD cohorts. Excluding the 15 patients mismatched at HLA-DQB1, there were still no significant differences in OS, DFS, NRM, or acute or chronic GVHD between patients matching at 7 of 8 vs 8 of 8 antigens or alleles (supplemental Figures 2 and 3).
Survival, NRM, GVHD, and relapse. (A-G) Kaplan-Meier estimates of OS, DFS, and the cumulative incidence of NRM, acute GVHD grades II-IV and grades III-IV, extensive chronic GVHD, and relapse. Outcomes for the 72 patients with HLA-MMUDs are shown in red and for the 193 patients with HLA-MUDs are shown in blue. P values were calculated by using log-rank tests.
Survival, NRM, GVHD, and relapse. (A-G) Kaplan-Meier estimates of OS, DFS, and the cumulative incidence of NRM, acute GVHD grades II-IV and grades III-IV, extensive chronic GVHD, and relapse. Outcomes for the 72 patients with HLA-MMUDs are shown in red and for the 193 patients with HLA-MUDs are shown in blue. P values were calculated by using log-rank tests.
Survival, DFS, and relapse for lymphoid and myeloid malignancies. Kaplan-Meier estimates of OS, DFS, and the cumulative incidence of relapse are shown for all patients with (A-C) lymphoid malignancies and (D-F) myeloid malignancies. Outcomes are shown in red and for patients with HLA-MMUDs and in blue for patients with HLA-MUDs. P values were calculated by using log-rank tests.
Survival, DFS, and relapse for lymphoid and myeloid malignancies. Kaplan-Meier estimates of OS, DFS, and the cumulative incidence of relapse are shown for all patients with (A-C) lymphoid malignancies and (D-F) myeloid malignancies. Outcomes are shown in red and for patients with HLA-MMUDs and in blue for patients with HLA-MUDs. P values were calculated by using log-rank tests.
Impact of chimerism
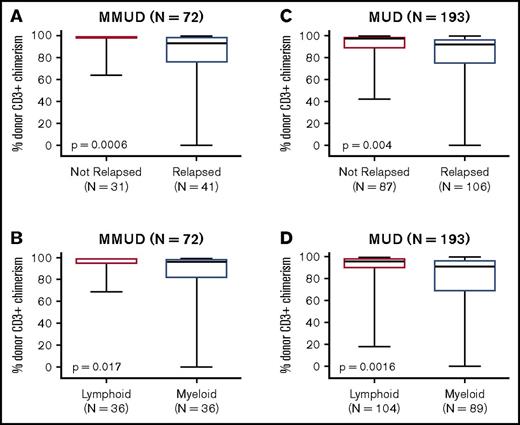
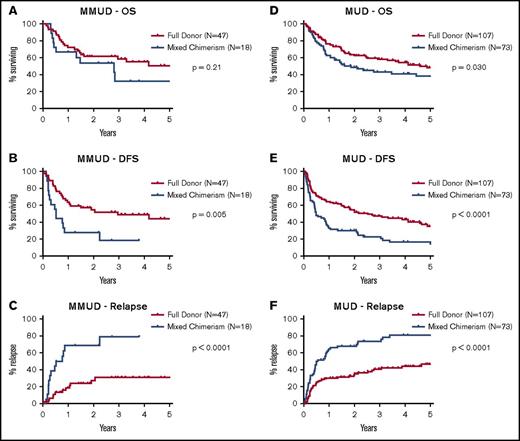
Achieving a high level of donor CD3+ chimerism was strongly associated with a reduced risk of disease relapse. Among patients without disease relapse, peak donor CD3+ chimerism by day +90 was significantly higher than for patients with relapse (mean: 96% vs 76% for the MMUD cohort, P = .0006; mean: 90% vs 81% for the MUD cohort, P = .004, Figure 3). Peak donor CD3+ chimerism was also significantly higher for patients with lymphoid malignancies than those with myeloid malignancies (mean: 96% vs 83% for the MMUD cohort, P = .017; mean: 89% vs 80% for the MUD cohort, P = .0016). In the MMUD cohort, the cumulative incidence of relapse at 2 years was 27% for patients who achieved full donor chimerism vs 69% for patients with mixed chimerism at day +90 (hazard ratio: 0.24, P < .0001, Figure 4). In the MUD cohort, the cumulative incidence of relapse at 2 years was 32% for patients who achieved full donor chimerism vs 68% for patients with mixed chimerism (hazard ratio: 0.39, P < .0001). Achieving full donor chimerism by day +90 was also strongly associated with superior DFS in both cohorts as well as an OS benefit in the larger MUD cohort.
Donor CD3+chimerism by day +90. Box-and-whisker plots indicate peak donor CD3+ chimerism by day +90 for the 72 patients with (A-B) HLA-MMUDs and (C-D)193 patients with HLA-MUDs. Data are stratified by relapse status (upper panels) and by underlying disease (bottom panels). The dark horizontal line indicates the median, box indicates the interquartile range, and whiskers indicate the range. P values were calculated by using Fisher’s exact test.
Donor CD3+chimerism by day +90. Box-and-whisker plots indicate peak donor CD3+ chimerism by day +90 for the 72 patients with (A-B) HLA-MMUDs and (C-D)193 patients with HLA-MUDs. Data are stratified by relapse status (upper panels) and by underlying disease (bottom panels). The dark horizontal line indicates the median, box indicates the interquartile range, and whiskers indicate the range. P values were calculated by using Fisher’s exact test.
Survival, DFS, and relapse stratified by chimerism status. Kaplan-Meier estimates of OS, DFS, and the cumulative incidence of relapse are shown for (A-C) 65 patients with HLA-MMUDs, excluding 7 patients with primary graft failure, and (D-F) 180 patients with HLA-MUDs, excluding 13 patients with graft failure. Data are stratified by chimerism status with outcomes shown in red for patients achieving full donor chimerism by day +90 and in blue for patients with mixed chimerism. P values were calculated by using log-rank tests.
Survival, DFS, and relapse stratified by chimerism status. Kaplan-Meier estimates of OS, DFS, and the cumulative incidence of relapse are shown for (A-C) 65 patients with HLA-MMUDs, excluding 7 patients with primary graft failure, and (D-F) 180 patients with HLA-MUDs, excluding 13 patients with graft failure. Data are stratified by chimerism status with outcomes shown in red for patients achieving full donor chimerism by day +90 and in blue for patients with mixed chimerism. P values were calculated by using log-rank tests.
CMV and EBV reactivation
We did not observe any significant differences in CMV or EBV reactivation between the MMUD and MUD cohorts. CMV viremia occurred in 51% of seropositive patients in the MMUD cohort vs 54% in the MUD cohort. In both groups, CMV reactivation typically occurred before day 30 and was typically low grade and responsive to valganciclovir. Less than 5% of patients in both cohorts developed clinical evidence of CMV infection (several cases of colitis and 1 case of esophagitis), which were responsive to ganciclovir and/or foscarnet in all cases. There was 1 case of EBV-positive posttransplant lymphoproliferative disease in each cohort, and both cases were responsive to treatment with rituximab. There were no fatalities due to CMV or EBV infection in either cohort.
Other patient outcomes
Additional patient outcomes are summarized in Table 3. In the MMUD cohort, 51% of patients were alive at the last follow-up, 42% were alive without relapse, 31% were off all immunosuppression, and GFRS was 21%. In the MUD cohort, 45% were alive, 30% were alive without relapse, 34% were off immunosuppression, and GFRS was 17%. In both MMUD and MUD cohorts, the major cause of death was relapse or disease progression (31% vs 39%). Other causes of death included acute GVHD (6% vs 2%), chronic GVHD (6% vs 4%), infections (3% vs 4%), and secondary malignancies (3% vs 2%).
Posttransplant outcomes
| . | MMUD (N = 72) . | MUD (N = 193) . |
|---|---|---|
| Alive | 37 (51) | 87 (45) |
| Median follow-up (range), y | 3.5 (1.0-10.1) | 4.3 (0.8-12.5) |
| Alive without relapse | 30 (42) | 58 (30) |
| Off immunosuppression | 22 (31) | 65 (34) |
| Alive without GVHD or relapse | 15 (21) | 32 (17) |
| Alive with relapse or disease progression | 7 (10) | 29 (15) |
| Median time to progression (range), y | 0.8 (0.3-2.1) | 1.3 (0.1-12.0) |
| Deceased | 35 (49) | 106 (55) |
| Death from relapse or disease progression | 22 (31) | 75 (39) |
| Death from other cause | 13 (18) | 31 (16) |
| Acute GVHD | 4 (6) | 4 (2) |
| Chronic GVHD | 4 (6) | 8 (4) |
| Infection | 2 (3) | 8 (4) |
| Secondary malignancy* | 2 (3) | 3 (2) |
| Other causes of death† | 1 (1) | 8 (4) |
| . | MMUD (N = 72) . | MUD (N = 193) . |
|---|---|---|
| Alive | 37 (51) | 87 (45) |
| Median follow-up (range), y | 3.5 (1.0-10.1) | 4.3 (0.8-12.5) |
| Alive without relapse | 30 (42) | 58 (30) |
| Off immunosuppression | 22 (31) | 65 (34) |
| Alive without GVHD or relapse | 15 (21) | 32 (17) |
| Alive with relapse or disease progression | 7 (10) | 29 (15) |
| Median time to progression (range), y | 0.8 (0.3-2.1) | 1.3 (0.1-12.0) |
| Deceased | 35 (49) | 106 (55) |
| Death from relapse or disease progression | 22 (31) | 75 (39) |
| Death from other cause | 13 (18) | 31 (16) |
| Acute GVHD | 4 (6) | 4 (2) |
| Chronic GVHD | 4 (6) | 8 (4) |
| Infection | 2 (3) | 8 (4) |
| Secondary malignancy* | 2 (3) | 3 (2) |
| Other causes of death† | 1 (1) | 8 (4) |
Data are presented as n (%) unless otherwise indicated.
Secondary malignancies include donor-derived myelodysplastic syndrome, diffuse large B-cell lymphoma, gastric adenocarcinoma, rectal adenocarcinoma, and thoracic sarcoma.
Other causes of death include pulmonary embolism, cerebrovascular accident, cardiac arrest, thrombotic thrombocytopenic purpura, and suicide.
Discussion
We report our 15-year experience with MMUD HCT with TLI-ATG conditioning and demonstrate at least equivalent outcomes as with MUDs and particularly favorable outcomes among patients with lymphoid malignancies. In our high-risk MMUD cohort with a median age of 60 years, TLI-ATG conditioning was associated with low rates of 2-year NRM (17%), acute GVHD grades II-IV (21%) and grades III-IV (6%), and chronic GHVD (31%). These outcomes compare favorably to other RIC and nonmyeloablative regimens. For example, nonmyeloablative conditioning with low-dose total body irradiation (TBI, 2 Gy) and fludarabine in a similar group of patients with a single antigen or allele mismatch was associated with a 2-year NRM rate of 47%, an acute GVHD grades II-IV rate of 69%, an acute grades III-IV rate 26%, and extensive chronic GVHD at a rate of 41%.30 The cumulative incidence of relapse was 40% at 2 years in our high-risk MMUD cohort compared with 28% in the low-dose TBI-fludarabine study.30 A previous multicenter randomized phase II study from Belgium comparing TLI-ATG to low-dose TBI-fludarabine in patients with HLA-matched donors showed similar outcomes with higher rates of NRM and GVHD in the TBI-fludarabine arm, higher rates of relapse in the TLI-ATG arm, and equivalent OS at 4 years.31
Our data also compare favorably to other RIC regimens incorporating posttransplant cyclophosphamide (PT CY) or bortezomib for GVHD prophylaxis. Gaballa et al32 used PT CY in combination with a RIC regimen of fludarabine, melphalan, and thiotepa in the setting of 9 of 10 MMUD HCT and haploidentical HCT and reported NRM rates at 1 year of 31% and 21%, respectively; acute GVHD grade II-IV at day +100 rates of 33% and 28%, respectively, and grade III-IV rates of 13% and 3%, respectively; chronic GVHD rates at 2 years of 19% and 24%, respectively; and relapse rates at 1 year of 25% and 19%, respectively. Compared with our TLI-ATG cohort, both PT CY cohorts were associated with higher rates of NRM despite the significantly older median age of our cohort (60 years vs 45 and 51 years).32 Relapse rates were higher in our TLI-ATG cohort, but our patients had higher disease risk index scores. The OS rate was similar between our TLI-ATG cohort (71% at 1 year) and the haploidentical PT CY cohort (70% at 1 year), both of which were higher than the MMUD PT CY cohort (60% at 1 year).32 Our outcomes with TLI-ATG are also very similar to those previously reported for the use of bortezomib and a RIC regimen of fludarabine and busulfan in the setting of MMUD HCT.33,34 A previous phase I/II trial of 45 patients treated with this regimen reported an NRM rate of 11% at 2 years, an acute GVHD grade II-IV rate of 22% and a grade III-IV rate of 7% at day +180, a chronic GVHD rate of 29% at 1 year, and a relapse rate of 38% at 2 years.34 Larger randomized studies are needed for a direct comparison of these various immunomodulatory approaches for patients lacking HLA-matched donors.
Although there were no significant differences in OS, DFS, NRM, or GVHD between our MMUD and MUD cohorts, we observed a trend toward lower relapse rates in the MMUD cohort, which likely reflects enhanced GVT reactions. The lower rate of relapse was particularly notable for patients with lymphoid malignancies and likely reflects the more indolent disease kinetics of chronic lymphocytic leukemia and follicular lymphoma compared with more aggressive myeloid malignancies like AML, which may outpace GVT reactions. Although we observed particularly favorable outcomes using MMUDs for lymphoid malignancies, a randomized trial comparing 9 out of 10 MMUDs to 10 out of 10 MUDs would be necessary before concluding that a 9 out of 10 MMUD should be preferred in this setting. KIR ligand mismatching may serve as another mechanism for enhanced GVT reactions in patients mismatched at HLA-B or -C loci. However, only 6 patients in this study had KIR ligand mismatch in the GVH direction, so we cannot draw any conclusions about the role of KIR alloreactivity in this study.
As with other RIC regimens, the main reason for treatment failure following TLI-ATG is relapse. In our study, disease relapse strongly correlated with failure to achieve full donor chimerism by day +90. An attractive strategy to decrease relapse rates is to preemptively convert patients with mixed chimerism to full donor chimerism. Previous studies have used donor lymphocyte infusions (DLIs) in this setting with successful conversion to full donor chimerism in the majority of patients but with substantial rates of acute GVHD occurring in up to 66% of patients.35-37 Our group has shown that specific lymphocyte subsets, such as CD8+CD44hi central and effector memory T cells, convert mixed to full donor chimerism and induce GVT reactions without causing GVHD.38 We recently administered CD8+ memory T-cell DLIs in patients with relapsed hematologic malignancies following allogeneic HCT and reported objective responses in the majority of patients while precipitating mild acute GVHD in only 1 of 15 patients.39 Our group is currently evaluating preemptive CD8+ memory T-cell DLI for patients with stable mixed chimerism who are at high risk for relapse.
Although a single antigen or allele mismatch was well tolerated in our cohort, we observed a high rate of graft failure among patients with superimposed nonpermissive HLA-DPB1 mismatching. Of the 7 patients in our MMUD cohort who developed graft failure, 4 of these patients had available HLA-DPB1 typing, all of whom had nonpermissive mismatching at 1 or both alleles leading to a 10 out of 12 or 9 out of 12 match, whereas no patients with an 11 out of 12 match had graft failure. Given the relatively small number of patients with available HLA-DPB1 typing, these data are observational only, but larger studies may further evaluate the relationship between graft failure and nonpermissive HLA-DPB1 mismatching in the setting of MMUD HCT. If this association is reproducible in larger studies, then it may be prudent to avoid nonpermissive HLA-DPB1 mismatching in the setting of MMUD HCT.
In conclusion, TLI-ATG conditioning may overcome the traditionally poorer outcome and higher risk of GVHD and NRM associated with MMUD HCT, and may be particularly well suited for patients with lymphoid malignancies who lack HLA-matched donors. Additional randomized studies are needed to determine whether a 9 out of 10 MMUD may be preferable to a 10 out of 10 MUD in specific patient populations. Larger randomized studies are also needed to compare various immunomodulatory approaches, including TLI-ATG, PT CY, and bortezomib, for patients lacking HLA-matched donors who are at high risk of GVHD. Mixed chimerism is strongly associated with a higher risk of relapse, and ongoing studies are investigating preemptive CD8+ memory T-cell DLI to increase donor T-cell chimerism, augment GVT reactions, and reduce relapse rates.
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by the National Institutes of Health, National Cancer Institute program project grant 4P01CA049605-27.
Authorship
Contribution: M.A.S. analyzed the data and wrote the manuscript; M.F.-V. and L.E.C. performed HLA and KIR ligand testing; O.Q. helped analyze the chimerism data; L.E. maintained the patient databases; S.A., L.J.J., E.H.M., D.B.M., L.S.M., R.S.N., J.A.S., W.-K.W., G.G.L., S.S., R.L., and A.R.R. provided clinical care; and R.L. and A.R.R. designed the study and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew R. Rezvani, Division of Blood and Marrow Transplantation, Department of Medicine, Stanford University Medical Center, 300 Pasteur Dr, Room H0115A, Stanford, CA 94305; e-mail: arezvani@stanford.edu.