Key Points
ZR2 yielded a complete response rate, 2-year progression-free and overall survival rate of 65.0%, 67.1%, and 82.4% among older DLBCL.
ZR2 efficacy depended on conventional type 1 dendritic cells activation, with T-cell clone expansion detected during long-term remission.
Visual Abstract
Older patients with diffuse large B-cell lymphoma (DLBCL) present unfavorable genetic and microenvironmental alterations. In this phase 2 trial, we assessed the efficacy and safety of zanubrutinib in combination with rituximab and lenalidomide (ZR2) in patients with de novo DLBCL aged ≥75 years. Forty patients were enrolled, and the primary end point was the complete response rate, which was 65.0% (95% confidence interval [CI], 48.3-78.9) at the end of induction treatment. The 2-year progression-free and overall survival rates were 67.1% (95% CI, 50.1-79.4) and 82.4% (95% CI, 66.5-91.2). The most common grades 3 and 4 hematologic adverse event (AE) was neutropenia (n = 14 [35.0%]). The most common grades 3 and 4 nonhematologic AEs were increased alanine transaminase (n = 5 [12.5%]) and aspartate transaminase levels (n = 5; 12.5%), and pulmonary infection (n = 5 [12.5%]). No events of atrial fibrillation were observed. Importantly, the efficacy of ZR2 was more dependent on tumor microenvironmental than genetic alterations, and was associated with upregulation of class I and II human leukocyte antigen and increased number and function of conventional type 1 dendritic cells. Preexisting expansion of intratumoral CD8+ T cells and treatment-induced clonal T-cell receptor (TCR) repertoire contributed to better clinical outcome. TCR sequencing of the peripheral blood mononuclear cell samples from patients with durable remission detected the expanded T-cell clones 3 years after treatment. These findings thus improve the understanding of the effect of T-cell immunological memory on ZR2-based immunotherapy, and support a paradigm shift toward mechanism-based targeted therapy of aggressive lymphoma. This trial was registered at www.clinicaltrials.gov as #NCT04460248.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the predominant subtype of non-Hodgkin lymphoma with various clinical, immunophenotypic, and genetic features.1 The incidence of DLBCL increases with age, with patients aged ≥75 years constituting ∼30% of DLBCL cases.2,3 Notably, age is a significant component of the well-established International Prognostic Index (IPI) and represents one of the most relevant risk factors for DLBCL.4 The comprehensive geriatric assessment (CGA) has now become a reliable method for evaluating the chemotherapy tolerance of older patients with DLBCL.5,6 R-miniCHOP (rituximab in combination with attenuated cyclophosphamide, doxorubicin, vincristine, and prednisone) is the standard of care in older patients without severe comorbidities, and shows a complete response (CR) rate of ∼60%, with 2-year progression-free survival (PFS) and overall survival (OS) rates of 47% and 59%, respectively.7-11 However, there is an unmet need for exploring new effective and safe approaches for older patients due to comorbidities and intolerance to standard immunochemotherapy.11,12
Older patients with DLBCL represent a distinct biological entity with unfavorable molecular characteristics, such as age-related oncogenic mutations of MYD88 and CD79B, and immunosuppressive tumor microenvironment with a decrease in cytotoxic activity, but an increase in exhausted T cells.12-14 Zanubrutinib is an irreversible selective Bruton tyrosine kinase (BTK) inhibitor and disrupts chronic B-cell receptor (BCR) signaling, leading to downregulation of the nuclear factor κB (NF-κB) pathway and lymphoma cell apoptosis.15,16 Inhibition of the BTK also promotes differentiation of monocyte-lineage dendritic cells (DCs) and enhances antitumor T-cell immunity.17 Lenalidomide induces NF-κB inactivation by targeting interferon regulatory factor 4 downstream of BCR/MYD88 cascade and also costimulates CD4+ and CD8+ T cells.18 Therefore, there remains great interest in using “chemotherapy-free” immunotherapy that dually targets genetic and microenvironmental alterations as first-line treatment in older patients with DLBCL.
In this study, we assessed the efficacy and safety of zanubrutinib with rituximab and lenalidomide (ZR2) in patients aged ≥75 years with de novo DLBCL. In addition, we performed a mechanistic analysis of treatment response using single-cell RNA sequencing (scRNA-seq).
Methods
Study design and ethics
This is a single-arm, open-label, phase 2 study (www.Clinicaltrials.gov identifier: NCT04460248) that enrolled patients aged ≥75 years with de novo DLBCL between 26 August 2020 and 9 August 2021. The study was approved by the Ethics Committee and Institutional Review Board of Shanghai Ruijin Hospital in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment.
Participants
Inclusion criteria included the following: aged ≥75 years; refused systemic chemotherapy or unfit or frail for standard chemotherapy as defined by CGA scale; measurable disease lesions ≥1.5 cm in both the longest and shortest dimensions; and life expectancy >3 months.6
Exclusion criteria were as follows: patients with a history of systemic or local treatment including chemotherapy for other tumors within 3 weeks before enrollment; uncontrollable cardiovascular, cerebrovascular, coagulative, connective tissue, or infectious diseases; neutrophil count <1.5 × 109/L, platelet count <80 × 109/L, alanine aminotransferase or aspartate aminotransferase levels >2× the upper limit of normal (ULN), alkaline phosphatase or bilirubin >1.5× ULN, creatinine >1.5× ULN; inability to adhere to the protocol; HIV positivity; and hepatitis B virus DNA positivity.
Study procedures
Pretreatment clinical investigations included complete blood count; serum biochemical detection and coagulation tests; viral panel; bone marrow biopsy; electrocardiography; echocardiography; and positron emission tomography–computed tomography. IPI was calculated and treatment responses were evaluated according to the 2014 Lugano classification, with cell-of-origin germinal center B-cell–like (GCB) or non-GCB subtypes identified by Hans classification.19,20 The cutoff values for BCL2 and MYC in BCL2/MYC double-expressor (DE) lymphoma were 50% and 40%, respectively.21
Treatment and dose modifications
During the induction phase, zanubrutinib was administered at a dosage of 160 mg orally twice a day throughout each 21-day treatment cycle, rituximab 375 mg/m2 intravenously on day 1, and lenalidomide 25 mg orally daily from days 2 to 11 from cycles 1 to 6. Patients who achieved CR or partial response (PR) after induction were given lenalidomide 25 mg orally daily from days 1 to 10 for every 21 days for a maximum of 2 years. Local radiotherapy was performed after cycle 6 with evidence of localized lesion.
Zanubrutinib and lenalidomide were discontinued for patients who had grade 3 or 4 nonhematologic adverse events (AEs), grade 3 or 4 neutropenia with infection or fever, or grade 4 hematologic AEs, until AEs were improved to grade 1 or normal levels. The drug dose of zanubrutinib and lenalidomide was reduced because of reoccurrence of drug-related AEs. Hematopoietic growth factors were allowed. Granulocyte colony-stimulating factor prophylaxis was given if grade ≥3 neutropenia was present in the previous cycles.
End points
The primary end point was the CR rate after 6 cycles or at the end of the induction treatment. The secondary end points were PFS (measured from diagnosis to the date of documentation of either disease progression or death) rate and OS (measured from diagnosis to the date of documentation of death) rate at 2 years. AEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0. The exploratory end point was the analysis of potential prognosis biomarkers.
Sample size and statistical analysis
This study evaluated the CR rate of the ZR2 regimen using Simon 2-stage design with a 1-sided α of 0.05 and a power of 0.95. The null hypothesis assumed a CR rate of <30%. This refers to a previous study reporting that the ibrutinib-rituximab-lenalidomide (iR2) regimen led to a CR rate of ∼30% among patients with relapsed or refractory DLBCL.22 We conservatively assumed that the CR rate of ZR2 among older patients with DLBCL was 60% on the basis of R-miniCHOP data of patients aged >80 years with de novo DLBCL. Considering these calculations, a total of 34 patients were required. In stage 1, futility analysis was performed after 13 patients were enrolled. The trial would have been stopped early if ≤4 patients achieved CR. The remaining 21 patients were recruited in stage 2 because the futility criteria were not met. The null hypothesis would be rejected if ≥15 CRs were observed. Considering a dropout rate of 20%, 40 patients were needed. Sample size was calculated using the PASS software, Version 11.0.10 (NCSS, Kaysville, UT).
A 2-tailed unpaired t test was used for comparing the means of continuous variables between 2 groups when the data followed normal distribution and had equal variances; otherwise, the Mann-Whitney U test was applied. The Fisher exact test was applied for nonordinal categoric variables. Survival estimates were calculated using the Kaplan-Meier method, with survival curves compared using the log-rank test, and hazard ratios and associated 95% confidence interval (CI) estimated using a univariate Cox proportional hazards model. Two-sided P values <.05 were considered significant. All analyses were performed using IBM SPSS Statistics software, Version 25.0 (IBM Corp, Armonk, NY).
DNA sequencing and genetic subtyping
Genomic DNA was extracted using the GeneRead DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) from formalin-fixed, paraffin-embedded tumor samples of 39 patients, after which the samples were categorized into different genetic subtypes using the LymphPlex algorithm.23
Collection of tumor samples and bioinformatics analysis
Eighteen tumor samples (including 1 paired sample at disease progression) were collected from 17 patients for scRNA-seq analysis. Collection of scRNA-seq data and bioinformatics analyses were detailed in the supplemental Methods, available on the Blood website. After quality control, 124 866 high-quality cells were obtained for subsequent analysis, with an average of 1006 genes and 6503 unique molecular identifiers for each cell (supplemental Figure 1A-C). We assessed and performed unsupervised clustering analysis, which revealed 7 major cell types according to canonical cell type markers, including B cells (nonmalignant and malignant), plasma cells, T cells, natural killer cells, myeloid cells, fibroblasts, and endothelial cells (supplemental Figure 1D-E).
Immunohistochemistry
Immunohistochemistry of tumor tissue was performed on 5-μm paraffin sections using antibodies against HLA-I molecules (1:50; catalog no. ab70328; Abcam) and HLA-II molecules (1:50; catalog no. ab55152; Abcam).
DC differentiation and cell treatment
CD14+ monocytes were isolated from healthy donors’ peripheral blood mononuclear cells (PBMCs) using a CD14+ selection kit (Miltenyi Biotec). For immature monocyte-derived DC (moDC) differentiation study, CD14+ cells (2 × 105/mL) were suspended in cell culture medium (10% heat-activated fetal bovine serum + 1% Penicillin-Streptomycin + 1‰ 2-mercaptoethanol + RPMI 1640) supplemented with recombinant human granulocyte-macrophage colony-stimulating factor (50 ng/mL) and interleukin-4 (25 ng/mL) in the presence of the tested drugs at various concentrations, as indicated, with the medium refreshed with cytokines and drugs every 3 days. Cells were harvested on day 6, the surface markers of DC maturation were characterized by flow cytometry using Cytek Aurora (Cytek Biosciences), and the data were analyzed using SpectroFlo (Cytek Biosciences).
TCR-seq and repertoire analysis
PBMC samples were collected from 7 nonresponders (at pretreatment and disease progression) and 22 responders (at pretreatment and the end of the induction treatment), including PBMC samples from 10 responders with durable remission 3 years after treatment. Genomic RNA was extracted from unsorted bulk PBMC samples using TransZol Up (TransGen Biotech). The Nanodrop One Spectrophotometer and Qubit 4 Fluorometer (Thermo Fisher Scientific) were used to assess RNA concentration and integrity. RNA was subsequently subjected to T-cell receptor (TCR) sequencing (TCR-seq) of TCR α- and β-chain complementarity-determining region 3 regions using MultipSeq Human TCR Research Assay (M62022, for Illumina; iGeneTech), and multiplex polymerase chain reaction with primers annealing to variable and joining segments, resulting in amplification of rearranged variable diversity joining segments. Concentration and insert size of RNA libraries were assessed using Qubit and Qsep100. The related bioinformatics analysis is detailed in the supplemental Methods.
Results
Patient characteristics
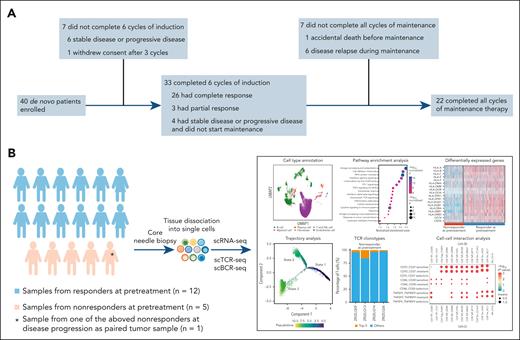
A total of 40 patients with de novo DLBCL were enrolled, including 27 (67.5%) men and 13 (32.5%) women. The patient flow diagram of the trial is shown in Figure 1A. Pretreatment tumor biopsies from 17 patients were available for scRNA-seq analysis. Among the 17 patients, 1 patient with a paired sample at disease progression was also included for scRNA-seq analysis (Figure 1B).
Flow diagram and flowchart related to scRNA-seq of the patients with DLBCL. (A) Patient flow diagram of the trial. (B) Flowchart of sample collection and analysis of scRNA-seq. UMAP, Uniform Manifold Approximation and Projection.
Flow diagram and flowchart related to scRNA-seq of the patients with DLBCL. (A) Patient flow diagram of the trial. (B) Flowchart of sample collection and analysis of scRNA-seq. UMAP, Uniform Manifold Approximation and Projection.
The median age of the patients was 78 years (range, 75-91), with 12 (30.0%) patients having an Eastern Cooperative Oncology Group score of ≥2. Twenty-six (65.0%) patients had a high-intermediate or high-risk IPI score. GCB subtype was identified in 17 (42.5%) patients; 13 (32.5%) patients were classified as having DE lymphoma, and 2 (5%) patients had rearrangements of MYC and BCL-6. All participants completed the CGA scales, and 6 (15.0%) patients were classified as fit, 16 (40.0%) as unfit, and 18 (45.0%) as frail. All the baseline characteristics are summarized in Table 1. Oncogenic gene mutations closely associated with DLBCL progression were sequenced in 39 patients, with 6 (15.4%) classified as BN2-like, 1 (2.6%) as EZB-like, 9 (23.1%) as MCD-like, 1 (2.6%) as N1-like, 11 (28.2%) as not otherwise specified, 4 (10.3%) as ST2-like, and 7 (17.9%) as TP53mut subtype.
Clinical and pathological characteristics of the patients
| . | All patients (N = 40) . |
|---|---|
| Median age (range), y | 78 (75-91) |
| Sex | |
| Male | 27 (67.5) |
| Female | 13 (32.5) |
| Eastern Cooperative Oncology Group score | |
| 0-1 | 28 (70.0) |
| ≥2 | 12 (30.0) |
| Ann Arbor stage | |
| I-II | 18 (45.0) |
| III-IV | 22 (55.0) |
| Serum lactate dehydrogenase | |
| Normal | 15 (37.5) |
| Elevated | 25 (62.5) |
| Extranodal sites | |
| 0-1 | 18 (45.0) |
| ≥2 | 22 (55.0) |
| IPI | |
| 1 | 2 (5.0) |
| 2 | 12 (30.0) |
| 3 | 11 (27.5) |
| 4-5 | 15 (37.5) |
| Cell of origin | |
| GCB-like | 17 (42.5) |
| Non–GCB-like | 23 (57.5) |
| BCL-2/MYC double expression | |
| Yes | 13 (32.5) |
| No | 27 (67.5) |
| BCL-2/BCL-6 and MYC translocation | |
| Yes | 2 (5.0) |
| No | 38 (95.0) |
| CGA | |
| Fit | 6 (15.0) |
| Unfit | 16 (40.0) |
| Frail | 18 (45.0) |
| . | All patients (N = 40) . |
|---|---|
| Median age (range), y | 78 (75-91) |
| Sex | |
| Male | 27 (67.5) |
| Female | 13 (32.5) |
| Eastern Cooperative Oncology Group score | |
| 0-1 | 28 (70.0) |
| ≥2 | 12 (30.0) |
| Ann Arbor stage | |
| I-II | 18 (45.0) |
| III-IV | 22 (55.0) |
| Serum lactate dehydrogenase | |
| Normal | 15 (37.5) |
| Elevated | 25 (62.5) |
| Extranodal sites | |
| 0-1 | 18 (45.0) |
| ≥2 | 22 (55.0) |
| IPI | |
| 1 | 2 (5.0) |
| 2 | 12 (30.0) |
| 3 | 11 (27.5) |
| 4-5 | 15 (37.5) |
| Cell of origin | |
| GCB-like | 17 (42.5) |
| Non–GCB-like | 23 (57.5) |
| BCL-2/MYC double expression | |
| Yes | 13 (32.5) |
| No | 27 (67.5) |
| BCL-2/BCL-6 and MYC translocation | |
| Yes | 2 (5.0) |
| No | 38 (95.0) |
| CGA | |
| Fit | 6 (15.0) |
| Unfit | 16 (40.0) |
| Frail | 18 (45.0) |
Values are represented as n (%) of patients unless otherwise indicated.
Patient disposition and dose intensity
Of the 40 patients enrolled, 7 did not complete 6 cycles of induction treatment, as 6 had stable disease (SD) or progressive disease (PD), and 1 withdrew consent after 3 cycles of treatment because of personal reasons. Furthermore, 33 (72.5%) patients completed 6 cycles of induction treatment, 22 (66.6%) of whom completed lenalidomide maintenance. Eleven patients did not complete all cycles of maintenance, including 4 patients who had SD or PD after induction, 1 patient who died in an accident, and 6 patients who relapsed during maintenance. The median dose intensity of zanubrutinib, rituximab, and lenalidomide during induction was 95.8% (interquartile range [IQR], 93.7%-97.7%), 100% (IQR, 100%-100%), and 97.0% (IQR, 95.7%-98.1%), respectively.
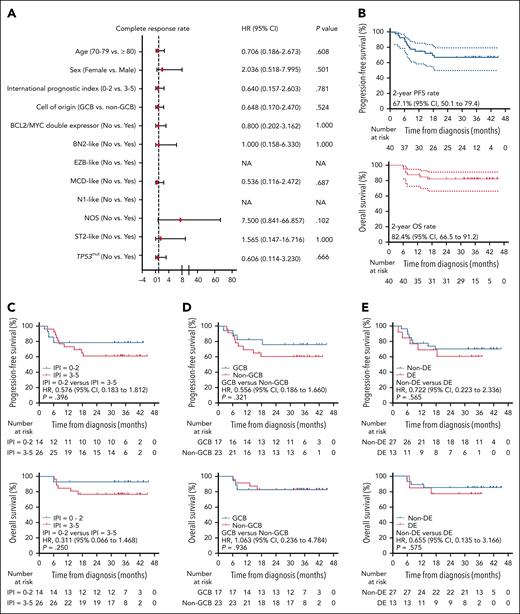
Treatment response and survival
All 40 patients received at least 1 response assessment in the induction phase. The CR rate at the end of induction was 65.0% (n = 26; 95% CI, 48.3-78.9), and the objective response rate was 75.0% (n = 30; 95% CI, 58.5-86.8). SD was observed in 5 (12.5%) patients and PD in 5 (12.5%) patients. A total of 4 patients achieved PR at the end of treatment, 3 of whom received radiotherapy. Among the 3 patients, 1 achieved PR after 3 cycles of induction, but subsequently withdrew consent, and received radiotherapy thereafter; the other 2 completed all 6 cycles and achieved PR, and then received radiotherapy and lenalidomide maintenance per protocol. IPI, cell of origin, DE or genetic subtypes were not associated with CR rate (Figure 2A). With a median follow-up of 34.8 months (range, 6.0-45.3), the 2-year PFS and OS rates were 67.1% (95% CI, 50.1-79.4) and 82.4% (95% CI, 66.5-91.2), respectively (Figure 2B). The 2-year PFS rates were 78.6% and 61.1% for patients with IPI of 0 to 2 and 3 to 5, respectively (P = .396); 76.0% and 60.6% for GCB and non-GCB subtypes (P = .321); and 60.6% and 70.0% for DE and non-DE subtypes (P = .565). The 2-year OS rates were 92.9% and 76.9% for patients with IPI of 0 to 2 and 3 to 5, respectively (P = .250); 82.4% and 82.6% for GCB and non-GCB subtypes (P = .936); and 76.9% and 85.0% for DE and non-DE subtypes (P = .575; Figure 2C-E).
First-line treatment efficacy and survival analysis of patients receiving ZR2. (A) Forest plot of CR rates for all patients. The 2-sided P values were calculated using the χ2 test. (B) PFS and OS survival curves of all patients. The 2-sided P values were calculated using the log-rank test. (C) PFS and OS survival curves stratified by IPI. The 2-sided P values were calculated using the log-rank test. (D) PFS and OS survival curves stratified by cell of origin. The 2-sided P values were calculated using the log-rank test. (E) PFS and OS survival curves stratified by DE lymphoma. The 2-sided P values were calculated using the log-rank test. HR, hazard ratio; NA, not available; NOS, not otherwise specified.
First-line treatment efficacy and survival analysis of patients receiving ZR2. (A) Forest plot of CR rates for all patients. The 2-sided P values were calculated using the χ2 test. (B) PFS and OS survival curves of all patients. The 2-sided P values were calculated using the log-rank test. (C) PFS and OS survival curves stratified by IPI. The 2-sided P values were calculated using the log-rank test. (D) PFS and OS survival curves stratified by cell of origin. The 2-sided P values were calculated using the log-rank test. (E) PFS and OS survival curves stratified by DE lymphoma. The 2-sided P values were calculated using the log-rank test. HR, hazard ratio; NA, not available; NOS, not otherwise specified.
AEs
Hematologic and nonhematologic AEs during induction treatment are summarized in Table 2. The most common grade 3 and 4 hematologic AE was neutropenia (n = 14 [35.0%]). The most common grade 3 and 4 nonhematologic AEs were increased alanine transaminase level (n = 5 [12.5%]), increased aspartate transaminase level (n = 5 [12.5%]), and pulmonary infection (n = 5 [12.5%]). No atrial fibrillation was observed.
AEs during induction of the patients (N = 40)
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Hematologic AEs | ||||
| Neutropenia | 4 (10.0) | 5 (12.5) | 6 (15.0) | 8 (20.0) |
| Anemia | 7 (17.5) | 3 (7.5) | 2 (5.0) | 0 (0.0) |
| Thrombocytopenia | 9 (22.5) | 2 (5.0) | 2 (5.0) | 0 (0.0) |
| Nonhematologic AEs | ||||
| Alanine transaminase elevation | 7 (17.5) | 3 (7.5) | 4 (10.0) | 1 (2.5) |
| Aspartate transaminase elevation | 8 (20.0) | 2 (5.0) | 4 (10.0) | 1 (2.5) |
| Pulmonary infection | 0 (0.0) | 0 (0.0) | 5 (12.5) | 0 (0.0) |
| Hyponatremia | 5 (12.5) | 1 (2.5) | 2 (5.0) | 0 (0.0) |
| Skin rash | 2 (5.0) | 1 (2.5) | 1 (2.5) | 0 (0.0) |
| Acute renal injury | 0 (0.0) | 0 (0.0) | 1 (2.5) | 0 (0.0) |
| Hypokalemia | 11 (27.5) | 1 (2.5) | 1 (2.5) | 0 (0.0) |
| Hypoalbuminemia | 16 (40.0) | 2 (5.0) | 0 (0.0) | 0 (0.0) |
| Serum creatinine elevation | 7 (17.5) | 2 (5.0) | 0 (0.0) | 0 (0.0) |
| Hypocalcemia | 6 (15.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) |
| Total bilirubin elevation | 7 (17.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Fatigue | 2 (5.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) |
| Abdominal distension | 2 (5.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) |
| γ-Glutamyl transpeptidase elevation | 3 (7.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Hyperuricemia | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal hemorrhage | 0 (0.0) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal toxicity | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hematuria | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Hematologic AEs | ||||
| Neutropenia | 4 (10.0) | 5 (12.5) | 6 (15.0) | 8 (20.0) |
| Anemia | 7 (17.5) | 3 (7.5) | 2 (5.0) | 0 (0.0) |
| Thrombocytopenia | 9 (22.5) | 2 (5.0) | 2 (5.0) | 0 (0.0) |
| Nonhematologic AEs | ||||
| Alanine transaminase elevation | 7 (17.5) | 3 (7.5) | 4 (10.0) | 1 (2.5) |
| Aspartate transaminase elevation | 8 (20.0) | 2 (5.0) | 4 (10.0) | 1 (2.5) |
| Pulmonary infection | 0 (0.0) | 0 (0.0) | 5 (12.5) | 0 (0.0) |
| Hyponatremia | 5 (12.5) | 1 (2.5) | 2 (5.0) | 0 (0.0) |
| Skin rash | 2 (5.0) | 1 (2.5) | 1 (2.5) | 0 (0.0) |
| Acute renal injury | 0 (0.0) | 0 (0.0) | 1 (2.5) | 0 (0.0) |
| Hypokalemia | 11 (27.5) | 1 (2.5) | 1 (2.5) | 0 (0.0) |
| Hypoalbuminemia | 16 (40.0) | 2 (5.0) | 0 (0.0) | 0 (0.0) |
| Serum creatinine elevation | 7 (17.5) | 2 (5.0) | 0 (0.0) | 0 (0.0) |
| Hypocalcemia | 6 (15.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) |
| Total bilirubin elevation | 7 (17.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Fatigue | 2 (5.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) |
| Abdominal distension | 2 (5.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) |
| γ-Glutamyl transpeptidase elevation | 3 (7.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Hyperuricemia | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal hemorrhage | 0 (0.0) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal toxicity | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hematuria | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are represented as n (%) of patients.
scRNA-seq analysis of tumor samples at pretreatment and progression
The scRNA-seq analysis, paired with single-cell BCR sequencing and single-cell TCR-seq analysis, was conducted on tumor samples to investigate microenvironmental features related to ZR2 efficacy. The 17 pretreatment samples were categorized into 2 groups according to clinical efficacy: responders (including 11 patients with CR and 1 patient who achieved PR followed by local radiotherapy and remained disease-free to date) and nonresponders (including 1 patient with SD and 4 patients with PD).
Tumor human leukocyte antigen expression as a response biomarker
The B cells were first extracted for subclustering analysis, showing that nonmalignant B cells from different tumor samples clustered together as a universally shared cluster, whereas malignant B cells tended to cluster separately as tumor-private clusters or tumor-shared clusters (Figure 3A-C). Meanwhile, InferCNV analysis confirmed the identification of malignant B cells using interpatient heterogeneity, as malignant B cells presented more copy number variations than T cells or myeloid cells (Figure 3D-E).
Intertumoral heterogeneity of DLBCL associated with the efficacy of ZR2. (A) UMAP of malignant and nonmalignant B cells, with each cell colored for sample origin. (B) UMAP of malignant and nonmalignant B cells, with each cell colored for malignant and normal clusters. (C) UMAP of malignant and nonmalignant B cells, with each cell colored for sample subgroups according to efficacy and time point. Samples of responders (n = 12) and nonresponders (n = 5) were collected before treatment, with 1 paired sample collected from one of the nonresponders at disease progression. (D) The inferred single-cell CNVs are shown with single cells (rows) and chromosomal regions (columns) for each malignant cluster and reference immune cells. (E) The inferred CNV scores are shown for each malignant cluster and reference immune cells. (F) Upregulated pathways in malignant B cells of responders (n = 12) by gene set enrichment analysis compared with nonresponders (n = 5). Color and size of points indicate normalized P value of upregulated pathways in the 2 groups, respectively. (G) Heat map of differentially expressed genes in malignant B cells between responders (n = 12) and nonresponders (n = 5). The 2-sided P values were calculated using the Wilcoxon rank sum test. (H) Immunohistochemical assay of HLA-I and HLA-II in tumor samples of responders (n = 10) and nonresponders (n = 7). The 2-sided P values were calculated using the Student t test. CNV, copy number variation; NK, natural killer cell; UMAP, Uniform Manifold Approximation and Projection.
Intertumoral heterogeneity of DLBCL associated with the efficacy of ZR2. (A) UMAP of malignant and nonmalignant B cells, with each cell colored for sample origin. (B) UMAP of malignant and nonmalignant B cells, with each cell colored for malignant and normal clusters. (C) UMAP of malignant and nonmalignant B cells, with each cell colored for sample subgroups according to efficacy and time point. Samples of responders (n = 12) and nonresponders (n = 5) were collected before treatment, with 1 paired sample collected from one of the nonresponders at disease progression. (D) The inferred single-cell CNVs are shown with single cells (rows) and chromosomal regions (columns) for each malignant cluster and reference immune cells. (E) The inferred CNV scores are shown for each malignant cluster and reference immune cells. (F) Upregulated pathways in malignant B cells of responders (n = 12) by gene set enrichment analysis compared with nonresponders (n = 5). Color and size of points indicate normalized P value of upregulated pathways in the 2 groups, respectively. (G) Heat map of differentially expressed genes in malignant B cells between responders (n = 12) and nonresponders (n = 5). The 2-sided P values were calculated using the Wilcoxon rank sum test. (H) Immunohistochemical assay of HLA-I and HLA-II in tumor samples of responders (n = 10) and nonresponders (n = 7). The 2-sided P values were calculated using the Student t test. CNV, copy number variation; NK, natural killer cell; UMAP, Uniform Manifold Approximation and Projection.
To investigate the favorable features associated with the efficacy of ZR2, gene set enrichment analysis and direct comparison of differentially expressed genes were performed between malignant B cells from the nonresponders and the responders. Immunostimulatory pathways, namely antigen presentation, cell adhesion, and interferon gamma (IFN-γ) signaling, were significantly enriched in the responders (Figure 3F). We observed increased expression of HLA-I molecules (HLA-A, HLA-B, HLA-C, HLA-E, and HLA-F), and HLA-II molecules (HLA-DQB1, HLA-DRB1, HLA-DQA1, HLA-DRA, HLA-DQA1, and CD74; Figure 3G, all P < .05). Immunohistochemistry demonstrated significantly higher expressions of HLA-I and HLA-II among the responders (P = .001 and P = .036, respectively; Figure 3H).
cDC1s as a microenvironmental biomarker
Alterations in tumor microenvironment were then analyzed between the responders and the nonresponders. Myeloid cells showed remarkable heterogeneity, and 7 myeloid cell subclusters were identified after batch correction and unsupervised clustering (Figure 4A-B). A significantly increased percentage of conventional type 1 DCs (cDC1s) was observed among the responders (P = .048; Figure 4C; supplemental Figure 2A-B) with a higher expression of the antigen-presenting cell gene signature, as well as lower expression of M2-like and angiogenesis gene signature, suggesting enhanced antitumor ability (Figure 4D). Differentially expressed gene analysis showed that HLA molecules and their transcriptional regulators of HLA-I and HLA-II genes (NLRC5 and CIITA, respectively) were significantly increased among the responders. Meanwhile, upregulation of DC maturation markers (IDO1 and ITGB7), a development- and maintenance-associated factor (FLT3), an activation marker (CD83), and a key factor in antigen cross-presentation (MPEG1) was also observed among the responders, indicating higher antigen presentation ability (Figure 4E; supplemental Figure 2C). Consistently, pathways including antigen presentation, endocytosis, tumor necrosis factor α signaling via NF-κB, and response to IFN-γ were significantly enriched in cDC1s of the responders (Figure 4F).
Infiltrating myeloid cells of DLBCL associated with the efficacy of ZR2. (A) UMAP of myeloid cells, with each cell colored for specific cell types. (B) Heat map showing the expression of marker genes in myeloid cell types identified by scRNA-seq. (C) The comparison of percentages of myeloid cells per sample according to the efficacy of ZR2. The 2-sided P values were calculated using the Mann-Whitney U test. (D) Expression levels of 4 gene signatures (M1-like, M2-like, angiogenesis, antigen presentation signatures) across myeloid cell clusters. (E) Volcano plot showing differentially expressed genes of cDC1s between responders (n = 12) and nonresponders (n = 5). The 2-sided P values were calculated using the Wilcoxon rank sum test. (F) Upregulated pathways in cDC1s of responders (n = 12) by gene set enrichment analysis compared with nonresponders (n = 5). The color and size of points indicate normalized P value of upregulated pathways in the 2 groups, respectively. (G) Comparison of expression of cDC1 signature at baseline according to the efficacy of “chemotherapy-free” (ZR2 and iR2) treatment by bulk RNA sequencing. The 2-sided P values were calculated using the Mann-Whitney U test. (H) Kaplan-Meier curves of PFS of patients treated with “chemotherapy-free” (ZR2 and iR2) treatment according to the expression of cDC1 signature. The group cutoff was determined by progression of disease within 2 years in the “chemotherapy-free” cohort. P values were calculated using the log-rank test. pDC, plasmacytoid dendritic cell.
Infiltrating myeloid cells of DLBCL associated with the efficacy of ZR2. (A) UMAP of myeloid cells, with each cell colored for specific cell types. (B) Heat map showing the expression of marker genes in myeloid cell types identified by scRNA-seq. (C) The comparison of percentages of myeloid cells per sample according to the efficacy of ZR2. The 2-sided P values were calculated using the Mann-Whitney U test. (D) Expression levels of 4 gene signatures (M1-like, M2-like, angiogenesis, antigen presentation signatures) across myeloid cell clusters. (E) Volcano plot showing differentially expressed genes of cDC1s between responders (n = 12) and nonresponders (n = 5). The 2-sided P values were calculated using the Wilcoxon rank sum test. (F) Upregulated pathways in cDC1s of responders (n = 12) by gene set enrichment analysis compared with nonresponders (n = 5). The color and size of points indicate normalized P value of upregulated pathways in the 2 groups, respectively. (G) Comparison of expression of cDC1 signature at baseline according to the efficacy of “chemotherapy-free” (ZR2 and iR2) treatment by bulk RNA sequencing. The 2-sided P values were calculated using the Mann-Whitney U test. (H) Kaplan-Meier curves of PFS of patients treated with “chemotherapy-free” (ZR2 and iR2) treatment according to the expression of cDC1 signature. The group cutoff was determined by progression of disease within 2 years in the “chemotherapy-free” cohort. P values were calculated using the log-rank test. pDC, plasmacytoid dendritic cell.
Using bulk RNA sequencing data, higher cDC1 signature was also observed among the responders treated with ZR2 (n = 18) or iR2 (n = 18), and was associated with improved PFS (Figure 4G-H), but not related to efficacy of RCHOP (rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone)–based therapy (N = 328; supplemental Figure 2D-E).23,24 We examined the markers involved in DC maturation and activation (HLA-I, HLA-DR, CD80, CD40, and CD83) by flow cytometry and found that zanubrutinib and lenalidomide increased the expression of these markers in moDCs from healthy donors (supplemental Figure 2F).
Activated CD8+ T cells and clonal TCR repertoire following antigen presentation associated with favorable response to ZR2
A total of 20 infiltrating T-cell and natural killer–cell clusters were also identified in all samples after batch correction and unsupervised clustering (supplemental Figure 3A-D). Compared with the nonresponders, the percentage of CD8 effector T cell (Teff)_MCM5 cells was significantly increased among the responders (P = .048), and CD8 Teff_MKI67 cells showed the same tendency (P = .064; supplemental Figure 3E). Higher percentage of S and G2M phases of the cell cycle, suggesting proliferative features, was observed in CD8 Teff_MCM5 and CD8 Teff_MKI67 cells (supplemental Figure 3F-G). The clonotype size, represented by the number of cells expressing the same TCR sequence, was greater among the responders at the expense of lower diversity, indicating greater T-cell expansion among the responders (supplemental Figure 4A-D).
Overlaps of TCR clonotypes were observed predominantly between CD8+ subtypes and increased among the responders, specifically, between the CD8 Teff_MCM5 and CD8 Teff_MKI67 cells and CD8 exhausted T cell (Tex)_CCL4L2, CD8 Tex_LAG3, and CD8 Tex_XCL1 cells (supplemental Figure 4E). Of note, cytotoxicity and exhaustion scores were higher in CD8+ T cells from the responders (P = .008 and P < .001, respectively; supplemental Figure 4F). Moreover, using bulk RNA sequencing data, higher cytotoxicity and exhaustion scores were observed among the responders, with better prognosis in the “chemotherapy-free” cohort (supplemental Figure 4G-H), consistent with the scRNA-seq results but not observed in the RCHOP-based cohort (supplemental Figure 4I-J).
A flow cytometric analysis of a panel of T-cell populations showed that responders had a significantly higher proportion of CD3+HLA-DR+ T cells after treatment (P = .013; Figure 5A; supplemental Figure 5A). TCR-seq of the PBMC samples from these patients showed no significant difference in TCR α- and β-chain repertoire diversity between responders and nonresponders at baseline. However, significant increases in TCR α- and β-chain repertoire diversity were observed among the responders, indicating the activation of oligoclonal tumor-directed T-cell clones and individual T-cell clones capable of recognizing distinct antigens after ZR2 treatment (Figure 5B-D). Meanwhile, responders exhibited significantly greater T-cell expansion inferred by TCR-seq data following ZR2 treatment (TCR α chain: P = .024; TCR β chain: P = .018; Figure 5E-F), which could be due to enhanced antigen presentation by cDC1s. Of note, TCR-seq of the PBMC samples from 10 responders with durable remission 3 years after treatment was conducted, showing ZR2-induced expanded clones persisting at low abundance (Figure 5G; supplemental Figure 5B).
TCR diversity and clonal T-cell expansion related to the efficacy of ZR2. (A) Comparison of proportion of peripheral CD3+HLA-DR+ T cells before and after ZR2 induction in nonresponders (n = 7) and responders (n = 22). The 2-sided P values were calculated using the Mann-Whitney U test. (B) Comparison of TCR diversity of PBMC samples between nonresponders (n = 7) and responders (n = 22) at pretreatment. The 2-sided P values were calculated using the Mann-Whitney U test. (C) Change of TCR diversity of PBMC samples in nonresponders (n = 7) between the pretreatment and disease progression time points. The 2-sided P values were calculated using the Mann-Whitney U test. (D) Change of TCR diversity of PBMC samples in responders (n = 22) between pretreatment and end of induction therapy time points. The 2-sided P values were calculated using the Mann-Whitney U test. (E) Comparison of percentages of T-cell expansion clones in PBMC samples between nonresponders (n = 7) and responders (n = 22) at pretreatment. The 2-sided P values were calculated using the Mann-Whitney U test. (F) Comparison of percentages of T-cell expansion clones in PBMC samples between nonresponders (n = 7) at disease progression and responders (n = 22) at the end of induction therapy. The 2-sided P values were calculated using the Mann-Whitney U test. (G) Percentage of T-cell clones in PBMC samples from responders (n = 10) at the end of induction therapy. “Expanded/persisted” refers to T-cell clones that expanded by the end of induction therapy and still existed 3 years after treatment. “Not-expanded/persisted” refers to T-cell clones that did not expand by the end of induction therapy but still existed 3 years after treatment. “Expanded/not-persisted” refers to T-cell clones that expanded by the end of induction therapy but did not persist 3 years after treatment. “Not-expanded/not-persisted” refers to T-cell clones that did not expand at the end of induction therapy and did not persist 3 years after treatment.
TCR diversity and clonal T-cell expansion related to the efficacy of ZR2. (A) Comparison of proportion of peripheral CD3+HLA-DR+ T cells before and after ZR2 induction in nonresponders (n = 7) and responders (n = 22). The 2-sided P values were calculated using the Mann-Whitney U test. (B) Comparison of TCR diversity of PBMC samples between nonresponders (n = 7) and responders (n = 22) at pretreatment. The 2-sided P values were calculated using the Mann-Whitney U test. (C) Change of TCR diversity of PBMC samples in nonresponders (n = 7) between the pretreatment and disease progression time points. The 2-sided P values were calculated using the Mann-Whitney U test. (D) Change of TCR diversity of PBMC samples in responders (n = 22) between pretreatment and end of induction therapy time points. The 2-sided P values were calculated using the Mann-Whitney U test. (E) Comparison of percentages of T-cell expansion clones in PBMC samples between nonresponders (n = 7) and responders (n = 22) at pretreatment. The 2-sided P values were calculated using the Mann-Whitney U test. (F) Comparison of percentages of T-cell expansion clones in PBMC samples between nonresponders (n = 7) at disease progression and responders (n = 22) at the end of induction therapy. The 2-sided P values were calculated using the Mann-Whitney U test. (G) Percentage of T-cell clones in PBMC samples from responders (n = 10) at the end of induction therapy. “Expanded/persisted” refers to T-cell clones that expanded by the end of induction therapy and still existed 3 years after treatment. “Not-expanded/persisted” refers to T-cell clones that did not expand by the end of induction therapy but still existed 3 years after treatment. “Expanded/not-persisted” refers to T-cell clones that expanded by the end of induction therapy but did not persist 3 years after treatment. “Not-expanded/not-persisted” refers to T-cell clones that did not expand at the end of induction therapy and did not persist 3 years after treatment.
Longitudinal single-cell clonal dynamics related to response to ZR2
Longitudinal single-cell analysis was performed on malignant cells before treatment and at progression, combined with single-cell BCR analysis. The data showed that each transcriptional clone had different BCR clonotypes, indicating that different origins of malignant cells shared similar transcriptional features (supplemental Figure 6A). In addition, the transcriptional features were associated with the efficacy of ZR2, as obvious clonal dynamics were observed between samples before treatment and at progression (supplemental Figure 6B-C). The percentage of sensitive clones reduced, whereas that of the resistant clones remained unchanged and that of the selective clones markedly increased, becoming dominant (supplemental Figure 6D-E). Gene set enrichment analysis showed that the BCR signaling pathway, MAPK signaling pathway, apoptosis, and immunostimulatory pathways (such as antigen presentation, cell adhesion, and IFN-γ signaling) were significantly enriched in the sensitive clones, whereas tumor progression–related pathways (including cell cycle, MYC targets, glycolysis, and oxidative phosphorylation pathways) were upregulated in the resistant or selection clones (supplemental Figure 6F). Similar to the enriched genes in malignant cells of the responders, antigen presentation molecules were overexpressed in the sensitive clone; nevertheless, cell cycle–related genes (eg, MKI67, PCNV, CDK4, MCM7, HMGA1, and MYC) and metabolic genes involved in glucose metabolism (eg, LDHA and LDHB) were upregulated in the resistant or selection clones (supplemental Figure 6G). Consistently, the percentages of S and G2M phases were increased in the resistant or selection clones (supplemental Figure 6H-I).
Discussion
ZR2 is a new, effective, and safe “chemotherapy-free” immunotherapy, representing an alternative to immunochemotherapy for first-line DLBCL treatment. The 2-year PFS and OS rates were 67.1% and 82.4%, higher than 47% and 59% in R-miniCHOP and comparable to ibrutinib plus R-miniCHOP.11,25 Of 40 patients, 23 (>50%) achieved durable remission for over 2 years. Of note, the efficacy of ZR2 was probably more dependent on tumor microenvironmental than genetic alterations frequently observed in older patients with DLBCL, such as MYD88, CD79B, or PIM1.14 Antigen presentation by tumor cells is essential for T-cell activation, recognition, and killing of tumor cells within the tumor microenvironment. Previous studies showed that tumor cells could evade immune recognition by multiple mechanisms, particularly impaired expression of HLA molecules.26 HLA deficiency is associated with unfavorable response to immunotherapy with programmed cell death protein 1 inhibitors in solid tumors.27,28 In this study, lymphoma cells in the responders presented features of antigenic tumors with high expression of HLA-I and HLA-II, along with increased recruitment and activation of cDC1s, suggesting a novel microenvironment-dependent mechanism of treatment based on T-cell activation mediated by tumor cells and cDC1s. This underlies the sensitivity to immunotherapy with ZR2 rather than to RCHOP-based immunochemotherapy.
Although rare in immune populations, cDC1s are key antigen-presenting cells for activation of antitumor immunity because they are capable of antigen processing and priming of both CD4+ and CD8+ T cells.29 More recently, cDC1 has been considered as a major predictive factor for tumor sensitivity to immune checkpoint blockade.30,31 In this study, a higher percentage of cDC1s, as well as their upregulated antitumor functions, was associated with better response to immunotherapy with ZR2 than to RCHOP-based immunochemotherapy. Both BTK inhibitors and lenalidomide can promote DC maturation and activation.17,32-34 In vitro, we further confirmed that zanubrutinib plus lenalidomide enhanced maturation of moDCs from healthy donors compared with either single agent alone. These results support that alterations of the tumor microenvironment contribute to the efficacy of ZR2. The presence of cDC1s, linked to increased antigen presentation and T-cell activation, could thus be a potential biomarker of favorable response to immunotherapy in DLBCL.
Features of T-cell exhaustion were accompanied by features of cytotoxicity.35 Using TCR-seq analysis, we showed that proliferating CD8+ T cells had the same TCR clonotypes and similar features as exhausted T cells, suggesting that proliferative potential coexisted in exhausted T cells for producing progeny with exhaustion characteristics, which indicated potential antitumor ability by effective antigen presentation and chronic TCR stimulation.36-38 In solid tumors, several studies have noted that tumor-specific T cells, even with exhausted features, are able to induce strong autologous tumor recognition.39-41 TCR-seq on our PBMC samples revealed that increased TCR repertoire diversity and proliferation ability were almost exclusively observed among the responders, indicating that ZR2 functioned to induce the release of tumor neoantigens, contributing to the amplification and reinvigoration of preexisting intratumoral T cells, and to the induction of novel T-cell clones.42-44 Increased number and functions of cDC1s, along with upregulation of tumor HLA-I and HLA-II molecules, maximized the effect of antigen presentation, rendering the preexisting expansion of tumor-specific T-cell clones to a higher proliferative status. Meanwhile, novel T-cell clones were induced by ZR2 and persisted in PBMCs of the patients achieving durable remission, providing a mechanistic explanation for how the “chemotherapy-free” ZR2 regimen induced long-term protection from tumor relapse in DLBCL.
This study has certain limitations. This study was conducted in a single center, and correction for multiple hypotheses was not performed because of the small sample size. However, bulk RNA sequencing data from a larger cohort and TCR-seq data on paired samples were used to verify these findings. Moreover, a multicenter, prospective, randomized trial evaluating the efficacy of ZR2 is ongoing (NCT05179733).
In summary, this study, based on a promising “chemotherapy-free” regimen that is effective and safe in older patients with de novo DLBCL, provided an improved understanding of the effect of T-cell immunological memory on immunotherapy, and offers a rationale for reprograming the tumor microenvironment as a paradigm shift toward mechanism-based targeted therapy of aggressive lymphoma.
Acknowledgments
This study was supported, in part, by research funding from the National Key R&D Program of China (2022YFC2502600), the National Natural Science Foundation of China (82200199, 82300210, and 82370187), the Changjiang Scholars Program, the Shanghai Clinical Research Center for Cell Therapy (23J41900100), the Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20220018 and 20230013), the Clinical Research Plan of the Shanghai Hospital Development Center (SHDC2020CR1032B and SHDC2022CRD033), the Multicenter Clinical Research Project of the Shanghai Jiao Tong University School of Medicine (DLY201601), the Collaborative Innovation Center of Systems Biomedicine, and the Samuel Waxman Cancer Research Foundation.
Authorship
Contribution: W.-L.Z. conceptualized the study; P.-P.X., Y. Zhu, and Z.-Y.S. curated data; P.-P.X., Y. Zhu, and Z.-Y.S. performed formal analysis; Y. Zhu collected tumor and peripheral blood mononuclear cell samples; W.-L.Z. and P.-P.X. acquired funding; L.W., S.C., Y.Q., H.-M.Y., B.-s.O.-y. X.-F.J., B.L., Q.S., Y. Zhao, and Y.H. conducted investigations; P.-P.X., Y. Zhu, Z.-Y.S., and R.-J.M. developed methodology; W.-L.Z. administered the project, provided resources, and supervised the study; P.-P.X., Y. Zhu, and Z.-Y.S. wrote the original draft; and W.-L.Z. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wei-Li Zhao, Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics, National Research Center for Translational Medicine at Shanghai, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197, Ruijin Rd, Shanghai 200025, China; email: zhao.weili@yahoo.com.
References
Author notes
P.-P.X., Y. Zhu, Z.-Y.S., and L.W. contributed equally to this study.
Raw single-cell RNA sequencing (scRNA-seq), single-cell B-cell receptor sequencing, single-cell TCR sequencing (TCR-seq), bulk RNA-seq, and bulk TCR-seq data generated in this study have been deposited at the Genome Sequence Archive for Human database (accession number HRA007235). Published bulk RNA-seq data from previous studies were available in the National Omics Data Encyclopedia (https://www.biosino.org/node) under the projects OEP00003171 and OEP001143 for the iR2 (ibrutinib-rituximab-lenalidomide) cohort and the RCHOP (rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone)–based cohort, respectively.
Data are open to the scientific and medical community. Bioinformatic analysis tools used in the study are listed in the key resources table. Proposals requesting individual participant data that underlie the results reported in this article (after deidentification) can be sent to the corresponding author, Wei-Li Zhao (zhao.weili@yahoo.com). A steering committee involving all principal investigators will evaluate the request and make the decision before sending the database to any academic partners.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal