Approximately 40% of subjects with acute promyelocytic leukemia (APL) who are given arsenic develop neuropathy symptoms,1 which are generally mild and will resolve after completion of arsenic treatment. However, severe Guillain-Barre–like neuropathy occasionally occurs and can be crippling.2 We recently witnessed severe neurotoxicity during arsenic therapy in a subject with occult thiamine deficiency. In analogy to electrolyte disturbance potentiating the occurrence of arrhythmia during arsenic therapy,3 this might suggest potentiation of thiamine deficiency and arsenic in the genesis of neurotoxicity. The enzyme complex pyruvate dehydrogenase (PDH) in the glycolysis pathway can be one site where thiamine and arsenic interact.4 PDH requires thiamine as a cofactor for pyruvate decarboxylation, which is also the enzyme very sensitive to inhibition by arsenic at micromolar concentrations,5 a level achievable with arsenic therapy.6 Three weeks of oral arsenic in the rat can reduce liver PDH activity by a half.7 Thus, arsenic therapy can inhibit further the readily diminished PDH activity from thiamine deficiency and adversely affect tissue heavily dependent on carbohydrates for energy provision, such as neural tissue. During the era when arsenic was used as arsphenamine for the treatment of syphilis, a Canadian group had reported the temporal relationship of blood pyruvate elevation and encephalopathy during arsenic treatment in subjects with thiamine deficiency.8Those with thiamine deficiency had higher blood pyruvate elevation during therapy and were more prone to developing encephalopathy.
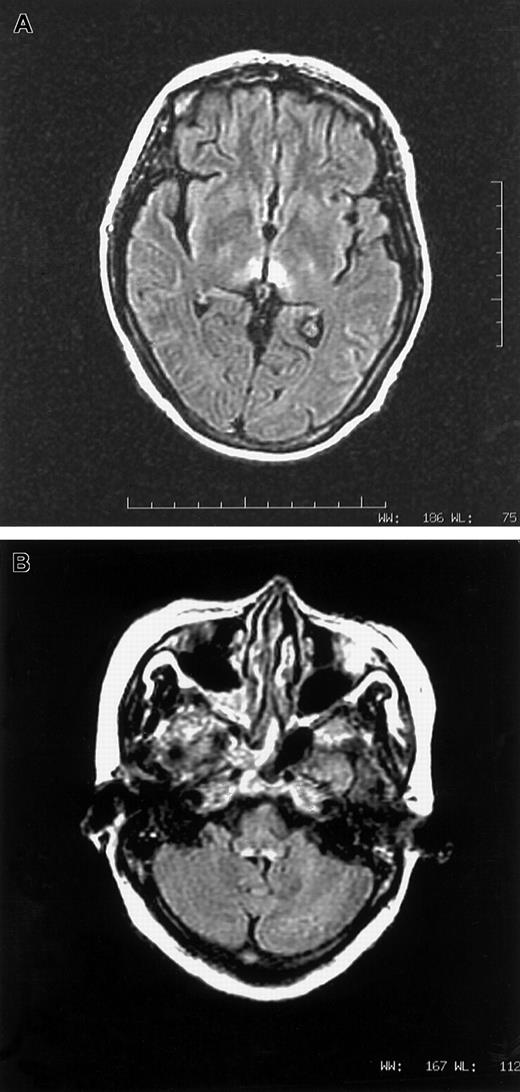
A vegetarian woman, aged 46, with relapsed APL, t(15:17), was given arsenic trioxide 10 mg/d for 28 days. Her first induction with all-trans retinoic acid and daunorubicin a year ago was uncomplicated. Medical history was unremarkable. In particular, there was no prior neurological disorder. Her diet was mainly refined rice and leafy vegetables, very sparse in meat and beans. Starting from day 17, she experienced nausea and vomiting. Pain and congestion were noted over the tongue, throat, and conjunctiva. On day 33, she complained of numbness over lower limbs. Confabulation was absent. On day 37, all 4 limbs were paralyzed and areflexic. Speech was inaudible and bulbar paralysis was noted. She sweated heavily and had a vesicular rash over her body. Cerebrospinal fluid showed white count, 1/μL; protein, 0.5 gm/L; and glucose, 6.7 mM. Studies for Venereal Disease Research Laboratory, bacteria, fungus, virus, and oligoclonal band were negative. Nerve conduction study detected generalized reduction of sensory action potential. Motor conduction was normal, while electromyogram showed active denervation. Drug review did not suggest a neurotoxic side effect from any supportive medication. With cranial magnetic resonance imaging (MRI), lesions consistent with Wernicke syndrome were seen over tectum, periaqueductal gray, and periventricular white matter of the third ventricle, both thalami, and the dorsal medulla (Figure1A,B). The low level of red cell (RBC) transketolase (24 μmol/min L; normal 45-90 μmol/min L), which increased by 32% after in vitro addition of thiamine pyrophosphate, confirmed thiamine deficiency. Parental thiamine 100 mg/d was given. The next day, power of the upper limbs dramatically improved and speech became audible. Over the next 5 days, upper limbs regained full power. As2O3 at 5 mg/d for 28 days as maintenance was given 5 weeks later, with oral thiamine. There was no deterioration in neurology. Lower limb power continuously improved. RBC transketolase during arsenic maintenance was normal, and MRI scan demonstrated complete resolution of all previous abnormalities.
(A,B) MRI images in axial fluid attenuated inversion recovery sequence showing T2-weighted hyperintense lesions surrounding third ventricle (1A) and dorsal aspect of medulla (1B).
(A,B) MRI images in axial fluid attenuated inversion recovery sequence showing T2-weighted hyperintense lesions surrounding third ventricle (1A) and dorsal aspect of medulla (1B).
Rapidly progressive neuropathy with a dose level of 10 mg/d and cumulative dose of 280 mg As2O3 is unusual. Moreover, recovery of APL subjects from severe neuropathy had been slow in previous arsenic trials.1 2 The rapid improvement in our subject after thiamine administration would not signify a pure arsenic toxicity but would support a contributory role of thiamine deficiency in development of an early severe neurotoxicity during arsenic administration. We have no idea yet whether thiamine or its deficiency has any role in the more frequent but milder form of sensory neuropathy. Yet, we recommend an adequate intake of thiamine during arsenic therapy, and we suggest thiamine deficiency be considered when severe neurotoxicity during arsenic is encountered.
We would like to thank Dr Y. L. Kwong and Dr E. S. K. Ma of Queen Mary Hospital, University of Hong Kong, and Dr S. H. Ng of Tuen Mun Hospital for assistance in patient care and for advice.
This work did not receive research grant or any other financial support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal